Abstract
t(14;20)(q32;q11)/IGH-MAFB is a rare chromosomal abnormality in plasma cell myeloma (PCM), accounting for 1-2% of PCM cases. Patients with this translocation may have a poor prognosis. However, the clinicopathological features and response to novel agents have not been well clarified. We present a 63-year-old Japanese female with PCM positive for t(14;20). The tumor responded well to a proteasome inhibitor, bortezomib, and the patient achieved complete remission. Six months after remission, tumor relapse was noted in the left cerebellum and the right frontal lobe of the cerebrum. After whole brain radiation therapy, the tumor masses decreased in size. The patient was followed up with best-care support, but died of the disease 29 months after the initial PCM diagnosis. t(14;20)-positive PCM responded well to bortezomib at the time of the initial treatment. The CNS tumor involvement, which is rare in PCM, may be associated with the clinical aggressiveness of the t(14;20)-positive form of this myeloma.
Keywords: plasma cell myeloma, t(14; 20)(q32; q11)/IGH-MAFB, CNS
INTRODUCTION
Plasma cell myeloma (PCM) is a fatal proliferation of neoplastic plasma cells producing monoclonal immunoglobulins. At the time of diagnosis, extramedullary lesions are found in approximately 7% of patients, whereas another 6% may develop such lesions later in their disease course.1 However, the central nervous system (CNS) is a rare location for extramedullary involvement, found in approximately 1% of PCM patients.2 The translocation, t(14;20)(q32;q11) involving IGH and MAFB gene loci, is a rare chromosomal abnormality in PCM. Patients with this translocation usually have a poor prognosis and may be resistant to novel agents for PCM.3-6 However, the clinical course of t(14;20)-positive PCM patients has not been well clarified due to its rarity. In this report, we present a t(14;20)-positive PCM patient who responded well to a proteasome inhibitor, bortezomib, but later developed tumor relapse in the CNS and died of the disease.
CASE REPORT
A 63-year-old Japanese female patient with an unremarkable medical history presented to our hospital with the complaint of general fatigue persisting for three months. No hepatosplenomegaly or lymphadenopathy was identified and the physical examination was otherwise unremarkable. She was found to have localized pneumonia, mild anemia, and moderate hyperproteinemia. The laboratory data at the initial presentation are shown in Table 1. Serum electrophoresis revealed a homogeneous peak in the gamma fraction, which was identified as the IgA-lambda type by immunofixation assay. The patient also had a high IgA concentration (3665 mg/dL; reference range, 90-450 mg/dL), and reduced concentrations of IgM (26 mg/dL; 60-250 mg/dL) and IgG (363 mg/dL; 800-1800 mg/dL). Serum-free light chain analysis revealed increased lambda-type (529 mg/L; 5.7-26.3 mg/L) and decreased kappa-type (2.6 mg/L; 3.3-19.4 mg/L) levels. The β2-microglobulin level was high (7.9 mg/L; reference range; 1-1.9 mg/L) and the serum calcium level was normal (8.9 mg/dL; 8.7-10.3 mg/dL). Radiological examinations demonstrated no lytic bone lesions.
Table 1. Laboratory findings on admission.
| Peripheral blood | WBC | 6820/μL↓ |
|---|---|---|
| Neut | 31.0%↓ | |
| Lymph | 43.0% | |
| Atypical-Lymp | 22.0% | |
| Mono | 2.0%↓ | |
| Eosi | 2.0% | |
| Baso | 0.0% | |
| RBC | 197×104/μL↓ | |
| Hb | 6.4g/dL↓ | |
| Plt | 10.5×104/μL↓ | |
| Urinalysis | pH | 5.5 |
| Specific gravity | 1.010 | |
| Protein | (-) | |
| Occult blood | (1+) | |
| Biochemistry | T.P | 8.5g/dL↑ |
| Alb | 2.6g/dL↓ | |
| AST | 36U/L↑ | |
| ALT | 15U/L | |
| LDH | 170U/L(0.74N) | |
| ALP | 127U/L | |
| g-GTP | 41U/L | |
| T-Bil | 0.3mg/dL | |
| BUN | 10.4mg/dL | |
| Cr | 0.97mg/dL↑ | |
| Uric acid | 4.2mg/dL | |
| Ca | 8.9mg/dL | |
| CRP | 0.5mg/dL↑ | |
| Immunoserological findings | IgG | 363mg/dL↓ |
| IgA | 3665mg/dL↑ | |
| IgM | 26mg/dL↓ | |
| IgD | ≤0.6mg/dL | |
| Serum b2MG | 7.9mg/L↑ | |
| Immunoelectrophoresis (specific antiserum) | IgA/lambda-positive | |
| Immunoelectrophoresis (urine) | IgA/lambda-positive | |
↑denotes a level above the upper limit of the reference range, and ↓ denotes a level below the lower limit of the reference range.
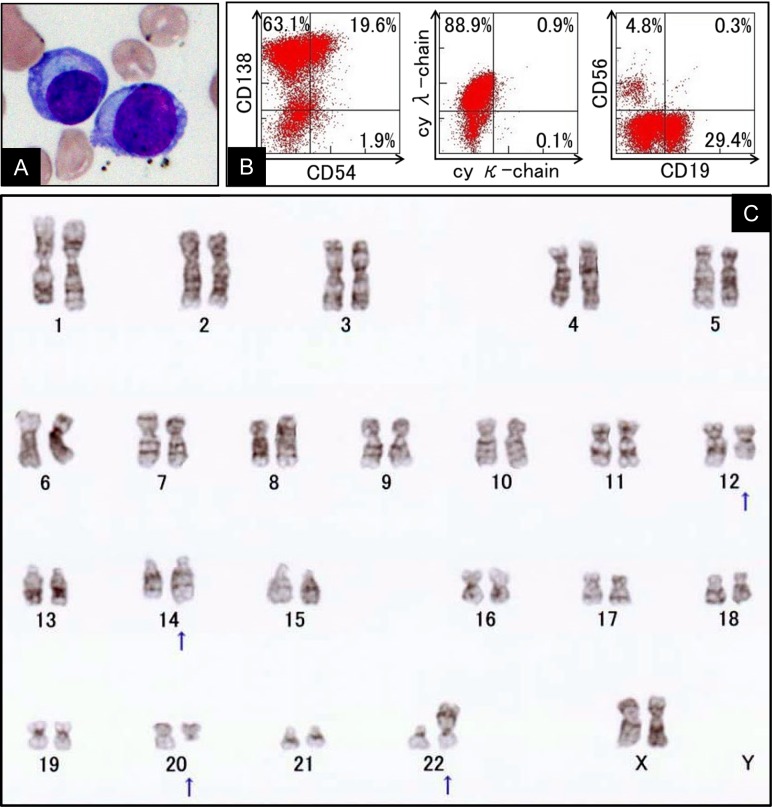
Morphological examination of the peripheral blood smear revealed the presence of atypical plasmacytic cells (22% in 6820/μL of WBC, Figure 1A). Moderate diffuse infiltration of plasma cells (82.4% of marrow cells) was noted in the bone marrow aspirate. A small number of plasma cells exhibited atypical multinucleated forms (≤3 nuclei). Flow cytometry analysis of the bone marrow specimen demonstrated an increased number of cells that were positive for CD138 and cy lambda chain, but negative for CD56 (Figure 1B). Mitotic figures were obtained for cytogenetic analysis using the bone marrow biopsy tissue, and the t(14;20)(q32;q11.2) translocation was detected in the tumor (Figure 1C). Based on these findings, the patient was diagnosed with t(14;20)/IGH-MAFB-positive PCM and characterized as having stage III disease according to the revised International Staging System of PCM.7
Fig. 1.
Morphological findings of atypical plasmacytic cells in the peripheral blood (A). Analysis of marrow tumor cells from the present case using flow cytometry at disease onset. The tumor cells were gated using CD38-positive cells (B). Cytogenetic features of bone marrow cells (C). Altered karyotype by conventional G-banding (arrows): 46, XX, add(12)(q13), t(14;20)(q32;q11), add(22)(p11.2).
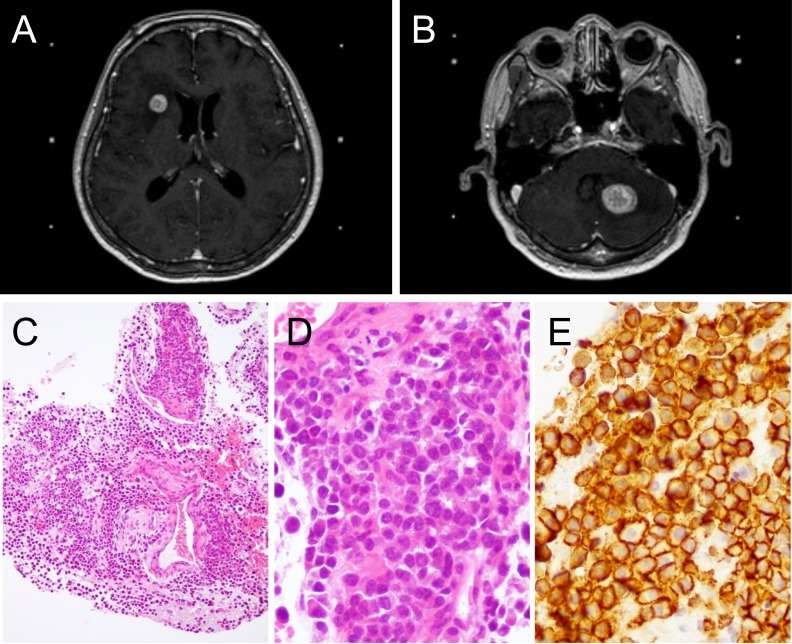
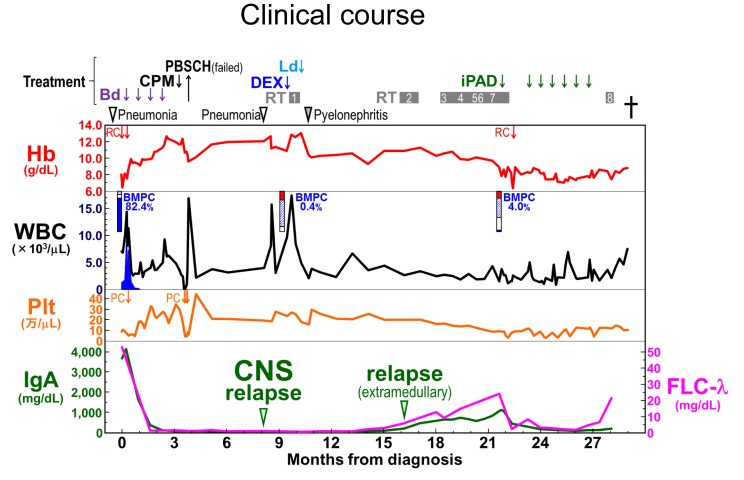
After the administration of four cycles of a proteasome inhibitor (bortezomib) and dexamethasone, the patient achieved complete remission. For autologous stem cell transplantation, peripheral blood stem cells were collected after the administration of high-dose cyclophosphamide. However, the amount of stem cells was insufficient and the transplantation was not carried out. Six months after the complete response, the patient complained of mild headache. Head CT and MRI studies revealed tumor masses in the left cerebellum and right frontal lobe of the cerebrum (Figure 2A, B). Brain biopsy was performed. Pathologically, the atypical plasmacytic cells with an increased N/C ratio were positive for CD138 and lambda light chain, but negative for kappa light chain and CD56 (Figure 2C-E),8,9 and a diagnosis of PCM relapse in the CNS was made. After whole-brain radiation therapy, the tumor masses decreased in size. The patient was placed on a lenalidomide-dexamethasone regimen, but acute pyelonephritis developed. Tumor relapse was noted in several organs and salvage treatment was not effective. The patient died of the disease 29 months after the initial PCM diagnosis. Autopsy revealed bronchopneumonia in both lungs, and PCM lesions in the bone marrow, thoracic lymph nodes, right atrium, right lung, and right psoas. No PCM lesion was found in the CNS. The clinical course of the patient is summarized in Figure 3.
Fig. 2.
Plasma cell myeloma relapse in the central nervous system. T1-weighted axial magnetic resonance images of relapsed tumors in the right frontal lobe of the cerebrum (A) and in the left cerebellum (B). The brain biopsy shows infiltration of myeloma cells with an increased N/C ratio (C and D). The tumor cells are positive for CD138 (E).
Fig. 3.
The clinical course. PBSCH, peripheral blood stem cell harvest; Ld, lenalidomide and dexamethasone; CPM, high-dose cyclophosphamide; DEX, high-dose dexamethasone; iPAD, bortezomib, doxorubicin, and dexamethasone; Bd, bortezomib and dexamethasone; WBRT, whole-brain radiation therapy; RT, radiation therapy; Hb, hemoglobin; RC, red blood cell transfusion; WBC, white blood cell; BMPC, bone marrow plasma cell; Plt, platelet; PC, platelet transfusion; CNS, central nervous system; FLC, serum free light chain. WBRT1, RT to the whole brain; RT2, to the right pelvis and the right thigh; RT3, to the left thigh, RT4, to the right periscapular and upper arm; RT5, to the right calf; RT6, to the right upper arm; RT7, to the right chest wall; and RT8, to the left calf.
DISCUSSION
A molecular cytogenetic classification of PCM has been proposed by the International Myeloma Working Group, and the genetic categories serve as major indicators of prognosis and form the basis for risk stratification.10 Seven recurrent oncogenes are associated with the IGH gene on 14q32: CCND1 on 11q13, MAF on 16q23, FGFR3/NSD2 on 4p16.3, CCND3 on 6p21, MAFB on 20q11, MAFA on 8q24, and CCND2 on 12p13.10,11 Among these oncogenes, t(14;20)/IGH-MAFB is one of the most infrequent translocations in primary PCM cases, accounting for less than 2%.12-14 Another research group estimated the frequency to be 0.9%.3 t(14;20) was reported to be associated with a poor prognosis. PCM patients with t(14;20) often develop primary plasma cell leukemia and extramedullary disease.4 These t(14;20)-positive PCM features are consistent with those in our patient, who exhibited atypical plasma cells in the peripheral blood at diagnosis and tumor relapse in the CNS.
Proteasome inhibitor-based regimens may not improve survival for patients with t(14;20).15,16 The molecular mechanism of this resistance has not been well clarified. Recently, Qiang et al. reported that MAFB protein provides PCM cells with an intrinsic resistance to proteasome inhibitors via the abrogation of proteasome inhibitor-induced apoptosis and activation of the caspase family.6 However, our patient responded well to the initial treatment including bortezomib. The therapeutic significance of proteasome inhibitors in t(14;20)-positive PCM needs further investigation.
Involvement of the CNS in PCM is a rare complication accounting for approximately 1% of primary PCM cases.2 The prognosis of these patients is poor. Jurczyszyn et al. carried out a multicenter retrospective study that included 172 adult patients with CNS PCM (22% were diagnosed with CNS involvement at the time of the initial PCM diagnosis and 78% at relapse/progression).17 Although the therapies targeted the CNS in most patients, the median overall survival from the onset of CNS involvement was 7 months. They also reported chromosomal abnormalities: del13q in 39% of the PCM cases, del17p in 23%, t(4;14) in 12%, and t(11;14) in 7%.17 It is currently unknown whether t(14;20) is specifically associated with CNS involvement. However, considering the clinical aggressiveness of t(14;20)-positive PCM, the CNS involvement in our patient may have been associated with t(14;20).
In PCM patients with CNS involvement, the efficacy of systemic treatment or the use of novel agents, including proteasome inhibitors and immunomodulatory drugs, is controversial. Katodritou et al. reported that these novel agents did not improve the post-CNS-PCM survival in a study involving 2,408 newly diagnosed symptomatic PCM patients.18 On the other hand, Chen et al. reported that long-term survival in CNS PCM can be achieved by combination therapy including multi-dosing intrathecal chemotherapy, radiation, and immunomodulatory agents.19 For our patient, the administration of immunomodulatory agents (lenalidomide) was not sufficient and its efficacy for CNS tumors was not evaluated. Based on the autopsy findings in our case, the CNS was tumor free, suggesting that radiotherapy was effective for local control of PCM involving the CNS.
In summary, we reported a rare t(14;20)-positive PCM patient in whom tumor relapse in the CNS developed six months after complete remission was achieved by proteasome inhibitor treatment. The accumulation of more cases is needed to establish the most appropriate therapeutic regimens against t(14;20)-positive PCM.
ACKNOWLEDGMENTS
TM and AI contributed equally to this study. Informed consent was obtained, and the study was approved by the Nagoya City University Internal Review Board. This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (15K08351 to T. Murase and H. Inagaki, 16K09855 to M. Ri, and 16K07179 to S. Iida). This study was also partly supported by a grant from the National Cancer Center Research and Development Fund (26-A-4), and from the Practical Research for Innovative Cancer Control from Japan Agency for Medical Research and Development, AMED (15ck0106077h0002).
Footnotes
CONFLICT OF INTEREST: IH received honoraria from BMS, Takeda, and Celgene Co., Ltd., and research funding from BMS, Kyowa-Kirin, and Chugai Co., Ltd.. SI received honoraria from Celgene, Janssen, Ono, Takeda, Daiichi-Sankyo, and Bristol-Myers Squibb Co., Ltd., and research funding from Celgene, Janssen, Ono, Takeda, Daiichi-Sankyo, MSD, Bristol-Myers Squibb, Novartis, Chugai, Abbvie, Kyowa-Hakko Kirin, and Gilead Co., Ltd.. The remaining authors declare no competing financial interests.
REFERENCES
- 1.Varettoni M, Corso A, Pica G, et al. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010; 21: 325-330. 10.1093/annonc/mdp329 [DOI] [PubMed] [Google Scholar]
- 2.Fassas ABT, Muwalla F, Berryman T, et al. Myeloma of the central nervous system: association with high-risk chromosomal abnormalities, plasmablastic morphology and extramedullary manifestations. Br J Haematol. 2002; 117: 103-108. 10.1046/j.1365-2141.2002.03401.x [DOI] [PubMed] [Google Scholar]
- 3.Vekemans MC, Lemmens H, Delforge M, et al. The t(14;20)(q32;q12): a rare cytogenetic change in multiple myeloma associated with poor outcome. Br J Haematol. 2010; 149: 901-904. 10.1111/j.1365-2141.2010.08113.x [DOI] [PubMed] [Google Scholar]
- 4.Zhan F, Huang Y, Colla S, et al. The molecular classification of multiple myeloma. Blood. 2006; 108: 2020-2028. 10.1182/blood-2005-11-013458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd KD, Ross FM, Chiecchio L, et al. NCRI Haematology Oncology Studies Group A novel prognostic model in myeloma based on co-segregating adverse FISH lesions and the ISS: analysis of patients treated in the MRC Myeloma IX trial. Leukemia. 2012; 26: 349-355. 10.1038/leu.2011.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiang YW, Ye S, Huang Y, et al. MAFb protein confers intrinsic resistance to proteasome inhibitors in multiple myeloma. BMC Cancer. 2018; 18: 724. 10.1186/s12885-018-4602-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: A report from International Myeloma Working Group. J Clin Oncol. 2015; 33: 2863-2869. 10.1200/JCO.2015.61.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohbayashi K, Taniwaki M, Ninomiya M, et al. A xeno-transplantable plasma cell leukemia line with a split translocation of the IgH gene. Cancer Genet Cytogenet. 2003; 144: 31-35. 10.1016/S0165-4608(02)00862-2 [DOI] [PubMed] [Google Scholar]
- 9.Narita T, Inagaki A, Kobayashi T, et al. t(14;16)-positive multiple myeloma shows negativity for CD56 expression and unfavorable outcome even in the era of novel drugs. Blood Cancer J. 2015; 5: e285. 10.1038/bcj.2015.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009; 23: 2210-2221. 10.1038/leu.2009.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest. 2012; 122: 3456-3463. 10.1172/JCI61188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanamura I, Iida S, Akano Y, et al. Ectopic expression of MAFB gene in human myeloma cells carrying (14;20)(q32;q11) chromosomal translocations. Jpn J Cancer Res. 2001; 92: 638-644. 10.1111/j.1349-7006.2001.tb01142.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart AK, Bergsagel PL, Greipp PR, et al. A practical guide to defining high-risk myeloma for clinical trials, patient counseling and choice of therapy. Leukemia. 2007; 21: 529-534. 10.1038/sj.leu.2404516 [DOI] [PubMed] [Google Scholar]
- 14.McKenna RW, Kyle RA, Kuehl WM, et al. Plasma cell myeloma. In: Swerdlow SH, Campo E, Harris NL, et al. (eds): WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed, Lyon, IARC, World Health Organization. 2017; pp. 243-248. [Google Scholar]
- 15.Nair B, van Rhee F, Shaughnessy JD, Jr, et al. Superior results of Total Therapy 3 (2003-33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006-66 with VRD maintenance. Blood. 2010; 115: 4168-4173. 10.1182/blood-2009-11-255620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in multiple myeloma linked to bortezomib in total therapy 3: comparison with total therapy 2. Br J Haematol. 2008; 140: 625-634. 10.1111/j.1365-2141.2007.06921.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurczyszyn A, Grzasko N, Gozzetti A, et al. Central nervous system involvement by multiple myeloma: A multi-institutional retrospective study of 172 patients in daily clinical practice. Am J Hematol. 2016; 91: 575-580. 10.1002/ajh.24351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katodritou E, Terpos E, Kastritis E, et al. Lack of survival improvement with novel anti-myeloma agents for patients with multiple myeloma and central nervous system involvement: the Greek Myeloma Study Group experience. Ann Hematol. 2015; 94: 2033-2042. 10.1007/s00277-015-2484-y [DOI] [PubMed] [Google Scholar]
- 19.Chen CI, Masih-Khan E, Jiang H, et al. Central nervous system involvement with multiple myeloma: long term survival can be achieved with radiation, intrathecal chemotherapy, and immunomodulatory agents. Br J Haematol. 2013; 162: 483-488. 10.1111/bjh.12414 [DOI] [PubMed] [Google Scholar]