Abstract
In the last decade, there has been a marked resurgence of syphilis in the United States despite the availability of effective treatments and previously reliable prevention strategies. The majority of cases are among the population of men who have sex with men (MSM); however, there has also been a recent increase among premenopausal women, coinciding with a concerning rise of congenital cases. The resurgence of syphilis can be largely attributed to changing social and behavioral factors, especially among young MSM. The biological association of syphilis with human immunodeficiency virus (HIV) transmission and acquisition is particularly alarming because of the increased individual and healthcare burden. In addition, some individual actions and public health efforts that are meant to reduce the risk of acquiring HIV may actually lead to risk compensation that facilitates the transmission of syphilis. Untreated syphilis is associated with detrimental health outcomes; therefore, both effective prevention strategies and treatment of this systemic disease have important short-term and long-term public health implications. This article offers a review of social and behavioral factors contributing to the current resurgence and recommendations for reducing syphilis incidence through medical and public health prevention strategies.
Keywords: Illicit drug abuse, HIV coinfection, HIV risk compensation, men who have sex with men, sexual behavior, syphilis incidence, sexually transmitted infection
Introduction
Syphilis is a sexually transmitted infection (STI) caused by the bacteria Treponema pallidum, which has impacted human health throughout history. Recently, incidence rates have been steadily increasing throughout the United States.1 This resurgence has taken place despite well-established treatment and preventive approaches, which may indicate a lack of utilization or implementation of these programs. Changes in frequency of many of the contributing social and behavioral risk factors such as illicit drug use and high-risk sexual behavior suggest that perceptions of the high risks of acquisition and the potential adverse outcomes from syphilis are not prevalent in much of the population.2 However, untreated syphilis carries potentially life-altering health consequences including neurological complications, hearing loss, blindness, and an increased likelihood of contracting other STIs, such as HIV.1 In addition, infection of pregnant females can lead to congenital syphilis in their unborn babies, resulting in miscarriage, stillbirth, birth defects, and/or infant death.1 Although deaths from syphilis in the adult population are rare,3,4 the case-fatality rate of babies born with congenital syphilis is 6.5%5 The adverse health outcomes experienced by these patients impose a significant economic burden on the US healthcare system with an estimated $39 million per year in direct medical costs (in 2010 US dollars).6
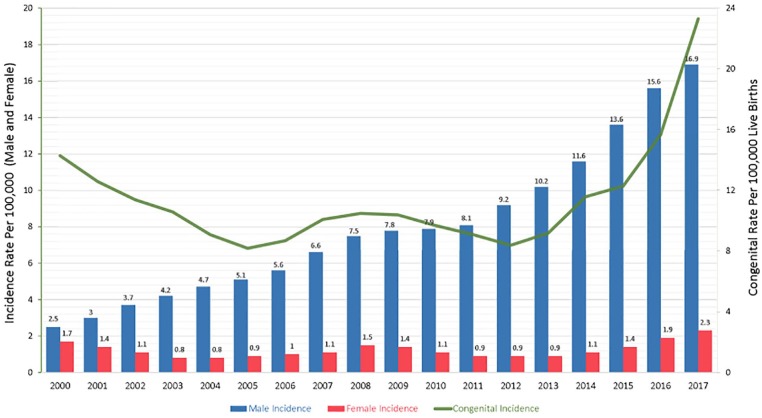
Since 2000, there has been a steady increase in syphilis incidence across many population groups. In 2000, there were 5973 reported primary and secondary (P&S) cases (2.12 per 100 000) while in 2017, there were 30 644 P&S cases (9.5 per 100 000) reported, a 413% increase (Figure 1).7 According to 2017 data released from the Centers for Disease Control and Prevention (CDC), the rates of syphilis are particularly high in the male population (16.9 per 100 000 males) compared to the female population (2.3 per 100 000 females).7 Syphilis rates are the highest among men who have sex with men (MSM), accounting for 82% of male syphilis cases.8,9 Of the reported cases of syphilis among MSM, almost half are also coinfected with HIV.1 Among females, syphilis incidence rates began increasing in 2013 with an increase of 156% occurring between 2013 and 2017.7 It is also important to note that the rising incidence of syphilis in the female population has, not surprisingly, affected the incidence of congenital syphilis. In 2017, there were 918 cases of reported congenital syphilis (incidence rate of 23.3 cases per 100 000 live births), which is a 153% increase from 2013 when there were 362 reported cases (incidence rate of 9.2 cases per 100 000 live births).7 In a media release from the CDC in September 2017, the explosion of cases of syphilis and other STDs was noted to be outpacing our ability to respond, calling for an urgent need for improved prevention.
Figure 1.
Incidence of primary, secondary, and congenital syphilis rates 2000-2017.
Adapted from the CDC’s Sexually Transmitted Disesease Surveillance Report.7
Figure 2.
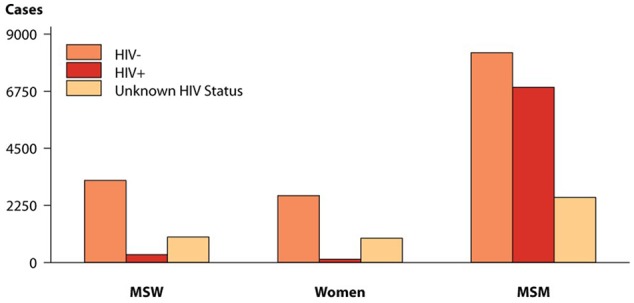
Primary and Secondary Syphilis-Reported Cases by Sex, Sexual Behavior, and HIV Status, United States, 2017.
Obtained from the CDC STD Surveillance website (https://www.cdc.gov/std/stats17/figures/46.htm).
MSW indicates men who have sex with women; MSM, men who have sex with men.
This article will explore the recent resurgence of syphilis by reviewing the associated complex and multifaceted contributing factors. Particular attention will be given to high-risk sexual behaviors, such as unprotected intercourse and having multiple partners, and the role illicit drug use and HIV risk compensation has on these behaviors. Finally, this article will offer recommendations for prevention of syphilis transmission in the United States.
Methods
Based on the broad nature of this literature review, several separate searches were conducted through the US National Library of Medicine. The primary search of literature took place from November 2017 to March 2018 and the search criteria included “syphilis outbreaks,” “syphilis history,” “risk compensation HIV,” “condomless sex,” “MSM STI,” “seroadaptive,” “multiple partners syphilis,” “sexual networks,” “drug use STI,” and “syphilis HIV co-infection.” Articles were included that were peer reviewed and published within the past 10 years. Articles were excluded that were not primary sources or contained limitations that deemed them as not applicable to the scope of this article (based on relevance to the topic of this review) or articles that did not contain the full text. In addition, articles were excluded that did not use the US population. A total of 78 sources were reviewed for this article.
Assessment of Contributing Factors
The current resurgence of syphilis cannot be attributed to a single factor, but a complex interplay of social and behavioral factors which allows T pallidum to circulate throughout the population. The inherent difficulties associated with human behavioral change and the associated stigma with STIs make reversing the current syphilis trend a challenging public health endeavor.
MSM
Men who have sex with men (MSM) are disproportionally affected by syphilis, accounting for the majority of cases in recent years.10 A study by Pathela et al11 compared the rates of P&S syphilis infections in MSM with MSW (men who have sex with women) using data from the New York City public health department. From 2005 to 2008, there were 2678 cases (707 cases per 100 000) of reported P&S syphilis among the MSM group, compared with 334 cases (4.8 cases per 100 000 people) among the MSW group (a case rate ratio difference of 147 fold). During this time period, syphilis cases increased by 80% among MSM and 35% in MSW.11 Several biological and social/behavioral reasons explain the association between MSM and STI acquisition.
From a biological standpoint, anal intercourse, which is more common among MSM, carries a significantly higher likelihood of transmitting syphilis (and other STIs) compared with vaginal intercourse.12 Although contact investigations done in the 1940s indicated similar syphilis incidence between heterosexual contacts (58%) and homosexual contacts (49%),13 these studies have many potential errors and were completed during a time of more pervasive anti-homosexuality. More recent studies consider that syphilis transmission is affected by many factors and transmission rate estimates change based on sexual contact type (ie, penile-anal, penile-oral, and penile-vaginal).14,15 This is, in part, due to the greater likelihood of epithelial abrasions and tears to the rectum (especially during traumatic sex, such as “fisting”) because it lacks the self lubricating capability and elasticity of the vagina.12,16,17 In addition, the rectum is highly vascularized, which creates an accessible pathway to the bloodstream.16 These aspects are advantageous for a systemic pathogen like T pallidum because loss of epithelial integrity allows for microbial invasion.16 Therefore, it should not be surprising that condomless anal intercourse has been identified as one of the greatest risk factors for STI acquisition among MSM.16
Evolving behavioral trends have also played an important role in the transmission of syphilis in MSM. In an effort to reduce HIV transmission and acquisition, some MSM use “seroadaptive” behaviors, which consist of men adapting their sexual practices based on perceived HIV serostatus. This can be accomplished through “serosorting” (choosing partners based on similar HIV status) or, in the case of discordant couples, “seropositioning” (the HIV positive partner engages in the receptive role and the HIV negative partner engages in the insertive role).18-20 These behaviors have been depicted as a “double edged sword” in terms of their ability to reduce HIV transmission, but at the expense of potentially increasing the rates of other STIs, like syphilis. These behaviors are attributed to the perception that condomless anal sex may be made more safe based on seroadaptive behaviors.10,18-20 For example, in Surkan et al’s21 study, it was found that the odds of engaging in unprotected anal intercourse (UAI) were 6.62 times higher (95% CI 4.86-9.01) among those who reported using serosorting compared with those who did not.
From a social context, MSM appear to be more likely to engage in densely connected sexual networks.11 In Pathela et al’s22 study of New York City men, a community health survey was utilized (n = 11 217 men, ages 18 to 64) to access sexual behavioral trends. They found that MSM were more likely to report multiple sex partners compared with their heterosexual counterparts.22 It is important to note that beneath the surface of social-sexual networks, there are societal influences such as social sub cultures, social norms, cultural tendencies, and technological advances.10 For example, bath houses in San Francisco were an influential part of the gay culture in the 1970s to 1980s and also where the first cases of AIDS appeared in the United States, but evolving technologies have facilitated new ways of sexual networking.10
Online sex partners and dating apps
Advancements in technology have made it easier for people to meet anonymous sex partners online through various Internet chat rooms and more recently, through “dating apps,” otherwise known as Geospatial Networking Applications (ie, Tinder, Grindr, and Manhunt, among others).23,24 The increasing popularity of dating apps, especially among the younger MSM population, has created a virtual landscape of social-sexual networks that are not only more complex and challenging to identify, but potentially increase risky sexual behavior.10,23,25 For example, in Wong et al’s23 study, it was observed that MSM who used Internet services to meet sex partners were more likely (OR = 2.1, 95% CI 1.0-4.3) to have syphilis than those who did not. In addition, a study by Lehmiller and Ioerger26 showed MSM who are app users report having a significantly greater number of lifetime partners compared with non-app users (median of 30 lifetime partners, compared with 7 lifetime partners). Furthermore, a meta-analysis by Liau et al27 demonstrated that MSM who sought sex partners online were more likely to report UAI compared with those who sought partners offline (OR = 1.68, 95% CI 1.18-2.40).
HIV risk compensation
Advances in recent years in the development of effective drugs for both treatment and prevention of HIV has brought with it a growing concern that “risk compensation” is reducing the use of STI preventive behaviors, especially among the younger generation.28 In addition, the improved outcomes associated with combined antiretroviral therapy (cART) has potentially altered the perceived susceptibility and/or severity associated with HIV infection (ie, chronic vs fatal disease).12 Similar to cART, pre-exposure prophylaxis (PrEP)“may perpetuate unsafe sexual activity,” including a reduction of condom use.29-31 These combined observations provide suggested reasons for the increasing syphilis rates across the United States.28 Several studies provide evidence for the existence of risk compensation in the setting of HIV and highlight the potential impacts on other STI incidence rates.32,33
A longitudinal study (for the time period of 1999-2012) done by Surkan et al21 consisted of a cohort of 417 HIV positive MSM who were enrolled in the Multicenter Aids Cohort Study (MACS), an ongoing study across four major cities in the United States (Baltimore, Chicago, Los Angeles, and Pittsburgh). Study participants were limited to those who reported multiple sex partners and were enrolled prior to the cART era. The participants completed a “men’s attitude survey” (MAS) every 2 years related to perceived threat of HIV, safe sex fatigue, viral/load transmission beliefs, and sexual sensation seeking. This study found a strong correlation between the length of time an individual was on cART and high-risk sexual behaviors. Their results showed being on cART for a median of 5.3 years was associated with a 33% increase in unprotected insertive anal intercourse and a 47% increase in unprotected receptive anal intercourse (URAI). Those who reported a reduced HIV concern or “safe sex fatigue” while on cART had a significantly higher odds of reporting UAI (OR = 2.27, 95% CI 0.82-6.23, and OR 2.85, 95% CI 1.92-4.22, respectively).21 In fact, according to the National HIV Behavioral Surveillance System (NHBS), condom usage among MSM has decreased by 20% from 2005 to 2011.34
HIV coinfection
HIV infection is considered a risk factor for syphilis,1 and syphilis infection has been associated with HIV acquisition. HIV incidence among individuals diagnosed with syphilis is high; almost half of the syphilis cases reported in 2016 among the MSM population were coinfected with HIV.1 Pathela et al’s22 study, a cohort (n = 2805) of P&S syphilis cases that were HIV negative at the start of the study, were followed for an average of 4.2 years (11 714 person-years). Within that time frame, 423 of those men were subsequently diagnosed with HIV (on average, 1.6 years after diagnosis of syphilis).22 Coinfection of HIV and syphilis has been illustrated in several different study cohorts. In New York city, of the 6503 men diagnosed with syphilis between 2000 and 2010, more than half (3081) also had a previous HIV diagnosis.11 In Wong et al’s23 study, it was found that patients who were HIV positive were more than 5 times (95% CI 2.9-9.6) more likely to have syphilis compared with those who were HIV negative. In Phipps et al20 study, having HIV was the single biggest risk factor observed for repeat syphilis infection (OR = 4.7, 95% CI 2.6-7). In the HIV outpatient cohort study from 1999 to 2015, 641 out of 6888 HIV-infected participants had a new diagnosis of syphilis at least once and the study reported an overall incidence rate of 1.8/100 person-years.35
From a biological perspective, there are several implications for the apparent synergistic interaction between HIV and syphilis.12,28,36 The risk of transmitting HIV is increased fivefold if either partner has another STI, particularly an ulcerative STI like syphilis.12 The lesions associated with syphilis contain an abundance of lymphocytes, the cells that HIV target. This allows direct access for the infiltration of HIV into the host.2,12,16 In addition, the immunosuppression caused by HIV may aid in the ability of T pallidum to more easily evade the host defense mechanisms and lead to more advanced disease, such as neurosyphilis.20,37
Sexual networks
The ability of syphilis to disseminate in the population is partially reliant on the number of sexual partners an infected person has.36 The per-act and per-partnership estimates of syphilis transmission rate are very limited and vary widely. The general transmission rate of syphilis has been estimated to be about 20% to 30% per sexual act (based on contact with an infectious lesion), and increasing the number of partners an individual has will increase the likelihood of transmission and widespread dissemination across a population.12,28,38 In a study by Gray et al14 focusing on the MSM population, the authors estimated a syphilis transmission probability of 1.4% per penile-anal sexual act and 1.0% per penile-oral sexual act. MSM who reported 6 or more partners within the previous month were 3 times more likely to be diagnosed with syphilis compared with those reporting a single partner.23 In addition, several outbreaks in North Carolina that occurred in the heterosexual population were attributed to concurrency (overlap of sexual partners) and drug use (which often coincide).25,38 It is not uncommon for MSM with multiple partners to cluster in sexual networks that may facilitate repeated exposures to STIs.
The concept of “core groups” within sexual networks may have significant influence on disease persistence within a population. A core group is defined as clusters of individuals that have a greater likelihood of contracting an STI compared with other members of the general population (ie, illicit drug users, MSM).12 For example, in a study by Phipps et al20 during 2000 to 2001, the authors describe the number of repeat syphilis infections within core groups to be “integral to sustaining the current syphilis epidemic.” In their study, they observed 624 syphilis cases among San Francisco men and found that 47% of these individuals reported having 3 or more partners within the previous 3 months, and almost 7% developed a repeat infection within a year. Interestingly, HIV status was the only risk factor found to be associated with repeat infections, whereas the risk factors for initial infection were age, race, number of sex partners, and illicit drug use. This finding is important because it demonstrates the ability of T pallidum to persist in closely connected sexual core groups.20
Drug abuse
The rate of fatal drug overdoses has tripled during the years 1999 to 2014.39 In addition, the widespread misuse of opioids has created a public health epidemic, resulting in 30 000 deaths for 2015 alone.40 The illicit use of drugs (both injection and noninjection use), including trading sex for drugs, is another important risk factor for consideration in the resurgence of syphilis and has been consistently linked to outbreaks.10,12,20,23,29,38,41-43
Drug use can lead to altered judgment, decreased inhibition, an increase of impulsive behavior, enhanced sexual pleasure (especially among stimulants such as methamphetamine and cocaine); all of these factors are potentially associated with an increased number of partners and encounters, as well as other high-risk sexual behaviors (ie, condomless intercourse).10,29 In addition, stimulants have been found to delay ejaculation which may prolong sexual intercourse and increase the likelihood of damaging the epithelium.42 In Wong et al’s23 study of MSM who were seen in a San Francisco STD clinic during 2002 and 2003, men who reported using methamphetamines and sildenafil (Viagra) within the past 4 weeks were over 7 times more likely (95% CI 3.3-15.3) to have syphilis when compared with men who did not report methamphetamine usage. They also determined men who used methamphetamine in the absence of Viagra, were more than 4 times (95% CI 2-9.6) as likely to have syphilis. They did not determine an increased risk of syphilis in those who used Viagra in the absence of methamphetamine, which may not be surprising given the fact that Viagra is often used to counteract the sexual side effects (erectile dysfunction) of illicit drugs.23 A prospective longitudinal study by McKetin et al44 compared sexual behavior during periods of methamphetamine use and periods of sobriety among heterosexuals users (n = 319). They found that while using methamphetamine, participants were 4.3 times (95% CI 2.6-7) more likely to have multiple sex partners and 5.1 times (95% CI 2.7-9.6) more likely to have unprotected “casual sex” compared with periods of sobriety.44
Recommendations
Preliminary data from 2017 show the highest number of primary and secondary (P&S) syphilis cases reported since the onset of the current resurgence.45 In fact, there has been a 76% increase in the number of reported cases between the years 2013 and 2017.45 As stated by the director of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP) during the 2018 National STD Prevention conference, “We are sliding backward. It is evident the systems that identify, treat, and ultimately prevent STDs are strained to near breaking point.”45 The continuous increase of cases is an indication that new strategies need to be used in the United States to combat syphilis resurgence.
As illustrated throughout this article, the risk factors for syphilis are complex, with many social and behavioral factors at play. As such, interventions to address the current resurgence will need to take many forms from a multitude of sectors including the medical and public health fields and biomedical researchers.
Medical provider’s role
Risk assessment
The importance of continued education about syphilis, as well as the ability to take effective sexual histories of patients, needs to be a vital component of STI interventions. Focusing on “sexual health” as opposed to “sexual disease” is of importance because of the negative connotation often associated to the latter; this can help a patient feel more comfortable talking about sex with their provider.12 The CDC recommends that sexual histories include a behavioral risk assessment followed by counseling risk reduction strategies. The “Five P’s” strategy can be used as a guideline for comprehensive sexual histories (partners, practices, pregnancy prevention, protection from STIs, and past history of STDs.)46 The use of plain language and open-ended questions that allow the patient to do most of the talking, and delivery of questions and information in a nonjudgmental and culturally appropriate way are recommended.46 In addition, it is important for providers to council on the limitations of seroadaptive behaviors in MSM, proper condom usage (and their shortcomings for protection), and the associations between HIV and syphilis. It is important to note that condoms are not as effective at preventing infections that are transmitted through lesions, such as syphilis (as well as chancroid and potentially herpes simplex virus and human papillomavirus) if the lesion is not covered by the condom.46,47 Finally, handouts with social media links and Internet references that reinforce in-office discussions can be useful in the clinical setting.48
Screening
Provider adherence to screening recommendations (Table 1) put out by the CDC, the US Preventive Services Task Force (USPSTF), and the American College of Obstetricians and Gynecologists (ACOG) is crucial to the success of syphilis intervention strategies. Several studies suggest less than adequate provider adherence to STI screening practices; this includes low syphilis testing rates among positive HIV individuals,50,51 missed opportunities for preventing congenital syphilis cases,52 and low syphilis testing for females who gave birth to stillborns.53 Health care systems should look to implement quality control initiatives that monitor provider adherence to syphilis screening, and third party payers should consider implementing reimbursement incentives that reward health systems for better screening practices.
Table 1.
Guidelines for Syphilis Screening in the United States.
| Pregnant women: | 1st prenatal visit (all pregnant women) Early in 3rd trimester and delivery (high-risk pregnant women) |
| Men who have sex with men (MSM): | Annually (all sexually active MSM) Every 3 to 6 months (high-risk MSM) |
| HIV positive individuals: | 1st HIV evaluation and at least annually
thereafter More frequent screening may be appropriate based on behavioral risk factors and local epidemiology |
Given the shortcomings of provider adherence (as discussed above), it should be noted the importance of syphilis screening as an important preventive approach for several reasons. Given the often asymptomatic nature of many STIs, individuals can be unaware they have an infection. The CDC recommends screening all pregnant women at the first prenatal visit, at a minimum, and again in the third trimester, and at delivery if she is considered at high risk (ie, multiple partners, previous STI diagnosis).46 MSM should be screened at least annually, but more frequently (every 3-6 months) is recommended if high-risk behaviors (illicit drug use, multiple partners, UAI) are present and/or the patient is HIV positive.9,46,54 Posttreatment serologic tests are recommended at 6 and 12 months to ensure a declining nontreponemal titer (indicative of treatment success). 46 For women treated during pregnancy, the CDC recommends monthly monitoring of titer levels, if feasible.46 In a dynamic model of syphilis transmission by Tuite et al,55 screening high-risk MSM for syphilis more frequently (every 3-6 months) had the greatest impact on reducing incidence; in fact, according to their model, concentrating efforts on continual screening of individuals already deemed as high risk worked better than efforts to expand screening outreach to those not already in care.
Public health’s role
The role of public health (including local, state, and federal health departments) is important for successful disease interventions across multiple levels, from the individual level to the public policy level. Public health can influence policy makers to ensure financial stability of STI clinics and put into place regulations that reduce barriers for individuals who need medical preventive services. In addition, public health can work to ensure proper education and training is provided to medical professionals about syphilis in regard to prevention, interpretation of diagnostic tests, treatment guidelines, and updated disease incidence surveillance. Public health can also work toward reaching out to organizations that provide services to individuals at high risk for acquiring syphilis (drug treatment facilities, jails/prisons, local MSM community health organizations, maternal-child health programs, etc).9,23
One area public health can improve on is to increase communication and collaborate goals between different program areas (such as maternal-child health, HIV/STI and family planning, disease surveillance, and field services); in doing so, this will help to bridge gaps in information and make interventions more successful in the long term.56 Also, utilizing local epidemiological data to create guidelines for specific population demographics (age, race, gender, etc) may help to ensure public health interventions and resources are used most efficiently.56 It is also vital that public health agencies are using all approaches to reaching patients and partners including online services.56
Despite the impact public health has on disease prevention, budget cuts over the last several years have hindered these efforts and may be leading to a gap in important STI preventive measures.57-59 In a study by Leichliter et al,58 over 61% of local health departments they surveyed reported budget cuts that often led to a decrease in STI screening and partner services.
Partner notification
Partner notification is vital to reducing the chain of transmission because it can facilitate testing and treatment of individuals who otherwise would not be aware of their exposure, thus reducing the likelihood of further transmission in a population. Patients can elect to notify partners themselves or, if they prefer to remain anonymous, authorize their providers or a public health professional to notify them. In addition, many health departments offer partner notification services via the Internet that allows a person to anonymously notify potentially infected partners through a text message or email.46 Increasing partner notification should be a priority, especially for individuals with early syphilis.56 Future partner notification capabilities could include a built-in feature in all dating apps that is mandated by the federal government; this would provide a quick and easy way for those infected with STIs to inform their partners.
Network interventions using venue analysis and social network theory
Given the often limited resources available for public health agencies to implement STI interventions, it is important to ensure efforts are as efficient as possible. A more focused or targeted control method that utilizes “venue analysis” to locate key social venues (bars, clubs, etc) where high-risk sexual behavior is prominent could be an effective intervention tool for public health; both for inducing and sustaining behavioral change as well as opportunities for testing and treating STIs.60,61 Identifying locations such as these is important, as “place” is closely intertwined with social/sexual networks and ultimately impacts human behavior.61 In a study by Jennings et al,61 it was found that drug and sex market venues were more likely to be visited by core transmitters than other social venues. In addition, in a study by Fujimoto et al,62 a similar connection was made among young, black MSM; syphilis cases were associated with a higher number of social venues attended, while HIV-syphilis coinfected cases were more likely to be associated with social network. Finally, in Behler et al’s63 uConnect study, it was found that social venues impact the prevention and treatment services a person connects to. Public health interventions to reduce syphilis (and HIV) incidence should take into consideration the impact of social venues, not just sexual networks, on infectious disease transmission.62,63
The Social Network Theory is a useful tool for understanding the role social peers (and venues) play in influencing human behavior, which has a large role in syphilis transmission.64 Valente and Pitts64 propose that using this analytical tool may enable public health interventions to sustain change even after funding and apparent interest has reached a plateau or has been lost (an issue that often emerges after a successful intervention is implemented).
Integration of public health & medical field
Historically, the public health and medical fields have operated as parallel entities with often overlapping goals. Efforts to integrate these two fields through shared resources will enhance STI surveillance, preventive approaches, and improve treatment outcomes. This linkage has the potential to increase the efficiency of services to individuals while promoting sexual health at the population level. Aligning electronic medical records across US health care systems to include complete and accurate sexual histories, STI data, and laboratory data can facilitate a more efficient response to the growing incidence of STIs. In addition, this information could be included when reporting case counts to the federal government to avoid potential gaps in STI data. As an important pillar in promoting overall health and well-being, these two entities can work jointly to reduce the stigma associated with STIs and to work toward normalizing sexual health in the United States. Together these two entities can address health disparities that often create barriers to care: such as poverty, homophobia, educational status, racism, and sexism.9,65,66
Biomedical research
The field of biomedical research can offer important solutions to aiding in the resurgence of syphilis, especially given that historically, behavioral strategies have failed to offer long-term reductions in incidence. More efficient diagnostic tests, additional treatment options, and effective vaccines are areas that need more research, but may ultimately contribute to a significant reduction in syphilis incidence.
Better diagnostic tests
There is a need for single, reliable, simple, and accurate tests with a short turnaround time to reduce loss to follow-up. The current diagnostic tests used in clinical settings are presumptive, cumbersome (require 2 tests), and can be complicated to decipher (difficult to ascertain an active infection, false positives and negatives occur depending on the stage of infection, etc). The current serological tests include nontreponemal and treponemal tests, which are used in combination to increase the sensitivity and specificity of the results.46 Traditionally, the nontreponemal test is used first, followed by a treponemal test to confirm a positive result, although some clinicians are now using a reverse algorithm to improve sensitivity.46 The nontreponemal test is not specific to T pallidum (it is used to detect antibodies to cardiolipin, a phospholipid, that is released from infected cells) and is both qualitative (reactive or nonreactive) and quantitative (given as a titer).67 Disadvantages of this test include potential false positives (due to autoimmune disorders, pregnancy, malaria, cardiovascular diseases, etc), low sensitivity in the primary and late latent stages, labor intensive, and a slow turnaround time for results (up to 7 days).68,69 In contrast, the treponemal test is specific to T pallidum antibodies and is qualitative only.67,68 Once a person has been infected with T pallidum, the treponemal test will remain positive indefinitely, making it difficult to distinguish between current and past infections.2,69
In addition, point-of-care tests (POCT) can be used in the diagnosis of syphilis (and HIV).67,70 These tests are more commonly used in developing countries than developed countries, with only one test being FDA-approved in the United States.67 POCT are advantageous because they provide almost immediate results and are cost effective in areas with limited resources.67,70
Direct diagnosis of syphilis is made by darkfield microscopy or PCR.2 However, these tests are generally only used in the research setting and are not readily available for clinical use.2,69 Darkfield microscopy is prone to human error and it is becoming more difficult to find individuals with knowledge of this technique; PCR in the clinical setting would offer a method that is less prone to human error.71
The absence of a highly sensitive diagnostic test for early infections (when syphilis is most likely to be spread to a partner and when the risk of HIV coinfection is the highest) as well as the challenges of diagnosing a repeat infection shed light on the salience of a vaccine.72
Additional treatment options
Additional treatment options are another realm of biomedical research that is in need. While there has not been any known resistance to penicillin by T pallidum, there has not been any treatment advances in 75 years for syphilis.9 According to the CDC, penicillin is the only available treatment for pregnant women that is known to prevent congenital syphilis and is complicated when penicillin allergies are present.9,46 However, the World Health Organization (WHO) lists ceftriaxone as an alternative with “very low quality evidence” in cases where penicillin is either unavailable or desensitization is not an option for pregnant women.73
Post exposure prophylaxis (PEP) is another possible biomedical intervention for syphilis, especially since penicillin resistance has not been observed in T pallidum to date.4 In an open-label randomized trial, Molina et al74 compared a group of high-risk MSM given doxycycline PEP 24 hours after sex to a control group that did not receive doxycycline; they observed a relative risk reduction of 47% of a new bacterial STI in the treatment group and a 73% relative reduction of syphilis alone. Using doxycycline for PEP may be a useful, but a temporary tool with a few limitations for prolonged use, including the potential for antibiotic resistance (which would be difficult to closely monitor given the inability to culture T pallidum) and the known resistance to doxycycline among gonococcal infections.75
Immunization
Elimination of syphilis is theoretically possible because it has no known animal or environmental reservoirs.28 In addition, there is a strong possibility of reducing HIV incidence through syphilis elimination, given the synergistic role between these two infections (as previously discussed).76 Given the recent resurgence, it is evident that treatment and prevention measures have not been enough to halt the spread of syphilis; thus, an effective vaccine may be the only feasible method for elimination.76
However, more research is needed in the realm of vaccine development; lack of research interest and funding have been impediments to this goal.76,77 Other STIs, such as gonorrhea, have had a higher precedence for vaccine research in light of the growing concerns of multidrug resistant infections.77 In addition, there are inherent hindrances to working with T pallidum in the lab setting, including the inability to culture and/or genetically manipulate this bacterium.72,76 Also, development of an effective vaccine is likely to prove difficult given that past infection is not protective against future infection. Because of these difficulties, there are still a lot of unknowns in regard to the host response mechanisms and the capability of T pallidum to establish latency in the human host.76,77
The World Health Organization and the National Institutes of Health established a “roadmap plan” for the research and development of vaccines against several STIs.77 However, syphilis vaccination studies are still in the basic research/preclinical stage.77 Although protective immunity in rabbits has been shown as possible through a vaccine developed by Miller et al in 1973,76,78 the vaccine regimen consisted of 60 IV injections given over the course of 37 weeks, which would not be cost effective or feasible in humans. Recently, there has been some encouraging progress made on identifying and characterizing outer membrane proteins (OMPs) on T pallidum.79
Conclusion
Sexual behavioral trends are key driving factors in the current resurgence of syphilis in the United States. MSM, HIV risk compensation, HIV coinfection, having multiple sex partners, practicing unsafe sex, and illicit drug use are all overlapping contributing factors to the marked increase in the incidence of syphilis in the United States. Finally, successful, permanent incidence reduction (or elimination) of syphilis may only be possible through biomedical advancements such as an effective vaccine. Developing effective prevention strategies will take joint efforts from both the medical and public health fields along with community members. Utilizing a “diversified prevention” approach that combines consistent practice of well-established public health interventions along with new interventions such as social venue analysis and biomedical advancements in treatment and prevention is likely required to turn the tide on this resurging epidemic.75
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Literature collection and review was done by RS and PJC. All authors contributed to drafting the manuscript for submission, followed by editing and modifying the manuscript based on the recommendations of the journal’s reviewers. All authors approved the final manuscript prior to publication.
ORCID iDs: Rebecca Schmidt  https://orcid.org/0000-0003-4482-2113
https://orcid.org/0000-0003-4482-2113
Rick J Jansen  https://orcid.org/0000-0001-5683-2584
https://orcid.org/0000-0001-5683-2584
References
- 1. Centers for Disease Control and Prevention (CDC). Syphilis – 2016 STD Surveillance Report. Atlanta; 2017. https://www.cdc.gov/std/stats16/Syphilis.htm. Accessed April 2, 2018. [Google Scholar]
- 2. Hook EW. Seminar syphilis. Lancet. 2017;389:1550-1557. [DOI] [PubMed] [Google Scholar]
- 3. Peterman TA, Kidd SE. Trends in deaths due to syphilis, United States, 1968-2015. Sex Transm Dis. 2019;46:37-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stamm LV. Syphilis: re-emergence of an old foe. Microb Cell. 2016;3:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Su JR, Brooks LC, Davis DW, Torrone EA, Weinstock HS, Kamb ML. Congenital syphilis: trends in mortality and morbidity in the United States, 1999 through 2013. Am J Obstet Gynecol. 2016;214:381.e1-381.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Owusu-Edusei K, Jr, Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis. 2013;40:197-201. [DOI] [PubMed] [Google Scholar]
- 7. United States Department of Health and Human Services (HHS), Centers for Disease Control and Prevention (CDC), Office of Infectious Diseases (OID), National Center for HIV, Viral Hepatitis, STD & TB Prevention (NCHHSTP), Division of STD Prevention (DSTDP). Sexually transmitted disease surveillance 2017. https://www.cdc.gov/std/stats17/2017-STD-Surveillance-Report_CDC-clearance-9.10.18.pdf. Published 2017. Accessed October 5, 2018.
- 8. Grey JA, Bernstein KT, Sullivan PS, et al. Rates of primary and secondary syphilis among white and black non-Hispanic men who have sex with men, United States, 2014. J Acquir Immune Defic Syndr. 2017;76:e65-e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Division of STD Prevention (DSTDP), Centers for Disease Control and Prevention (CDC). CDC Call to Action: let’s work together to stem the tide of rising syphilis in the United States. https://www.cdc.gov/std/syphilis/syphiliscalltoactionapril2017.pdf. Published 2017. Accessed January 22, 2018.
- 10. Solomon MM, Mayer KH. Evolution of the syphilis epidemic among men who have sex with men. Sex Health. 2015;12:96-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pathela P, Braunstein SL, Schillinger JA, Shepard C, Sweeney M, Blank S. Men who have sex with men have a 140-fold higher risk for newly diagnosed HIV and syphilis compared with heterosexual men in New York City. J Acquir Immune Defic Syndr. 2011;58:408-416. [DOI] [PubMed] [Google Scholar]
- 12. Nelson K, Williams C. Infectious Disease Epidemiology. 3rd ed. Burlington, VT: Jones & Bartlett Learning; 2014. [Google Scholar]
- 13. Von Werssowetz AJ. The incidence of infection in contacts of early syphilis. J Vener Dis Inf. 1948;29:132-137. [PubMed] [Google Scholar]
- 14. Gray RT, Hoare A, Prestage GP, Donovan B, Kaldor JM, Wilson DP. Frequent testing of highly sexually active gay men is required to control syphilis. Sex Transm Dis. 2010;37:298-305. [DOI] [PubMed] [Google Scholar]
- 15. Gray RT, Hoare A, McCann PD, et al. Will changes in gay menʼs sexual behavior reduce syphilis rates? Sex Transm Dis. 2011;38:1151-1158. [DOI] [PubMed] [Google Scholar]
- 16. Norkin L. Virology Molecular Biology and Parthenogenesis. Amherst, MA: ASM Press; 2010. [Google Scholar]
- 17. Kelley CF, Kraft CS, de Man TJ, et al. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: implications for HIV transmission and prevention. Mucosal Immunol. 2017;10:996-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hart GJ, Elford J. Sexual risk behaviour of men who have sex with men: emerging patterns and new challenges. Curr Opin Infect Dis. 2010;23:39-44. [DOI] [PubMed] [Google Scholar]
- 19. Ronn M, White PJ, Hughes G, Ward H. Developing a conceptual framework of seroadaptive behaviors in HIV-diagnosed men who have sex with men. J Infect Dis. 2014;210:S586-S593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phipps W, Kent CK, Kohn R, Klausner JD. Risk factors for repeat syphilis in men who have sex with men, San Francisco. Sex Transm Dis. 2009;36: 331-335. [DOI] [PubMed] [Google Scholar]
- 21. Surkan PJ, Li Y, Jacobson LP, et al. Unsafe sexual behavior among gay/bisexual men in the era of combination antiretroviral therapy (cART). AIDS Behav. 2017;21:2874-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pathela P, Braunstein SL, Blank S, Shepard C, Schillinger JA. The high risk of an HIV diagnosis following a diagnosis of syphilis: a population-level analysis of New York City men. Clin Infect Dis. 2015;61:281-287. [DOI] [PubMed] [Google Scholar]
- 23. Wong W, Chaw JK, Kent CK, Klausner JD. Risk factors for early syphilis among gay and bisexual men seen in an STD clinic: San Francisco, 2002-2003. Sex Transm Dis. 2005;32:458-463. [DOI] [PubMed] [Google Scholar]
- 24. Holloway IW, Pulsipher CA, Gibbs J, Barman-Adhikari A, Rice E. Network influences on the sexual risk behaviors of gay, bisexual and other men who have sex with men using geosocial networking applications. AIDS Behav. 2015;19:112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doherty IA, Padian NS, Marlow C, Aral SO. Determinants and consequences of sexual networks as they affect the spread of sexually transmitted infections. J Infect Dis. 2005;191:S42-S54. [DOI] [PubMed] [Google Scholar]
- 26. Lehmiller JJ, Ioerger M. Social networking smartphone applications and sexual health outcomes among men who have sex with men. PLoS ONE. 2014;9: e86603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liau A, Millett G, Marks G. Meta-analytic examination of online sex-seeking and sexual risk behavior among men who have sex with men. Sex Transm Dis. 2006;33:576-584. [DOI] [PubMed] [Google Scholar]
- 28. Lafond RE, Lukehart SA. Biological basis for syphilis. Clin Microbiol Rev. 2006;19:29-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vosvick M, Sarah Fritz B, Doug Henry B, Victor Prybutok B, Shane Sheu B, Jonathon Poe B. Correlates and racial/ethnic differences in bareback sex among men who have sex with men with unknown or negative HIV serostatus. AIDS Behav. 2016;20:2798-2811. [DOI] [PubMed] [Google Scholar]
- 30. Volk JE, Marcus JL, Phengrasamy T, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61:1601-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kojima N, Davey DJ, Klausner JD. Pre-exposure prophylaxis for HIV infection and new sexually transmitted infections among men who have sex with men. AIDS. 2016;30:2251-2252. [DOI] [PubMed] [Google Scholar]
- 32. Kalichman SC, Price D, Eaton LA, et al. Diminishing perceived threat of AIDS and increasing sexual risks of HIV among men who have sex with men, 1997–2015. Arch Sex Behav. 2017;46:895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. MacKellar DA, Hou S-I, Whalen CC, et al. HIV/AIDS complacency and HIV infection among young men who have sex with men, and the race-specific influence of underlying HAART beliefs. Sex Transm Dis. 2011;38:755-763. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention (CDC). HIV testing and risk behaviors among gay, bisexual, and other men who have sex with men—United States. MMWR Morb Mortal Wkly Rep. 2013;62:958-962. [PMC free article] [PubMed] [Google Scholar]
- 35. Novak RM, Ghanem A, Hart R, Ward D, Armon C, Buchacz K. Risk factors and incidence of syphilis in human immunodeficiency virus (HIV)-infected persons: the HIV outpatient study, 1999–2015. Clin Infect Dis. 2018;67: 1750-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doherty IA, Serre ML, Gesink D, et al. Sexual networks, surveillance, and geographical space during syphilis outbreaks in rural North Carolina. Epidemiology. 2012;23:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Centers for Disease Control Prevention (CDC). The National Plan to Eliminate Syphilis From the United States. Atlanta, GA: CDC; 2006. https://www.cdc.gov/stopsyphilis/seeplan2006.pdf. [Google Scholar]
- 38. Sena AC, Muth SQ, Heffelfinger JD, O’Dowd JO, Foust E, Leone P. Factors and the sociosexual network associated with a syphilis outbreak in rural North Carolina. Sex Transm Dis. 2007;34:280-287. [DOI] [PubMed] [Google Scholar]
- 39. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016; 65:1445-1452. [DOI] [PubMed] [Google Scholar]
- 40. Vadivelu N, Kai AM, Kodumudi V, Sramcik J, Kaye AD. The opioid crisis: a comprehensive overview. Curr Pain Headache Rep. 2018;22:16. [DOI] [PubMed] [Google Scholar]
- 41. Outbreak of syphilis among men who have sex with men—Southern California, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:117-120. [PubMed] [Google Scholar]
- 42. Chesney MA, Barrett DC, Stall R. Histories of substance use and risk behavior: precursors to HIV seroconversion in homosexual men. Am J Public Health. 1998;88:113-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beymer MR, Weiss RE, Bolan RK, et al. Sex on demand: geosocial networking phone apps and risk of sexually transmitted infections among a cross-sectional sample of men who have sex with men in Los Angeles County. Sex Transm Infect. 2014;90:567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKetin R, Lubman DI, Baker A, et al. The relationship between methamphetamine use and heterosexual behaviour: evidence from a prospective longitudinal study. Addiction. 2018;113:1276-1285. [DOI] [PubMed] [Google Scholar]
- 45. Centers for Disease Control Prevention (CDC). New CDC analysis shows steep and sustained increases in STDs in recent years. https://www.cdc.gov/nchhstp/newsroom/2018/press-release-2018-std-prevention-conference.html. Published 2018. Accessed September 8, 2018.
- 46. Workowski K, Bolan G. Sexually transmitted diseases treatment guidelines 2015. MMWR Morb Mortal Wkly Rep. 2015;64:34-49. [Google Scholar]
- 47. United States Department of Health and Human Services (HHS), Centers for Disease Control Prevention (CDC), National Center for HIV, Viral Hepatitis, STD & TB Prevention (NCHHSTP). Condoms and STDs: fact sheet for public health personnel sexually transmitted diseases, including HIV infection. https://www.cdc.gov/condomeffectiveness/docs/Condoms_and_STDS.pdf. Accessed March 30, 2018.
- 48. Curry SJ, Krist AH, Owens DK, et al. Screening for syphilis infection in pregnant women. JAMA. 2018;320:911-917. [DOI] [PubMed] [Google Scholar]
- 49. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents. JAMA. 2016;315:2321-2327. [DOI] [PubMed] [Google Scholar]
- 50. Landovitz RJ, Gildner JL, Leibowitz AA. Sexually transmitted infection testing of HIV-positive Medicare and Medicaid enrollees falls short of guidelines. Sex Transm Dis. 2018;45:8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Flagg EW, Weinstock HS, Frazier EL, Valverde EE, Heffelfinger JD, Skarbinski J. Bacterial sexually transmitted infections among HIV-infected patients in the United States. Sex Transm Dis. 2015;42:171-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patel SJ, Klinger EJ, O’toole D, Schillinger JA. Missed opportunities for preventing congenital syphilis infection in New York City level of evidence: III. Obs Gynecol. 2012;120:882-890. [DOI] [PubMed] [Google Scholar]
- 53. Patel CG, Huppert JS, Tao G. Provider adherence to syphilis testing recommendations for women delivering a stillbirth. Sex Transm Dis. 2017;44:685-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Centers for Disease Control Prevention (CDC). Syphilis pocket guide for providers. https://www.cdc.gov/std/syphilis/Syphilis-Pocket-Guide-FINAL-508.pdf. Accessed January 22, 2018.
- 55. Tuite AR, Fisman DN, Mishra S. Screen more or screen more often? Using mathematical models to inform syphilis control strategies. BMC Public Health. 2013;13:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Golden MR, Dombrowski JC. Syphilis control in the post-elimination era: implications of a new syphilis control initiative for STD/HIV programs. Sex Transm Dis. 2017;45:S86-S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chesson H, Owusu-Edusei K., Jr. Examining the impact of federally-funded syphilis elimination activities in the USA. Soc Sci Med. 2008;67:2059-2062. [DOI] [PubMed] [Google Scholar]
- 58. Leichliter JS, Heyer K, Peterman TA, et al. US public sexually transmitted disease clinical services in an era of declining public health funding. Sex Transm Dis. 2017;44:505-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Himmelstein DU, Woolhandler S. Public health’s falling share of US health spending. Am J Public Health. 2016;106:56-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Valente TW. Network interventions. Science. 2012;337:49-53. [DOI] [PubMed] [Google Scholar]
- 61. Jennings JM, Polk S, Fichtenberg C, et al. Social place as a location of potential core transmitters—Implications for the targeted control of sexually transmitted disease transmission in urban areas. Ann Epidemiol. 2015;25:861-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fujimoto K, Flash CA, Kuhns LM, Kim J-Y, Schneider JA. Social networks as drivers of syphilis and HIV infection among young men who have sex with men. Sex Transm Infect. 2018;94:365-371. [DOI] [PubMed] [Google Scholar]
- 63. Behler RL, Cornwell B, Schneider J. Patterns of social affiliations and healthcare engagement among young, black, men who have sex with men. AIDS Behav. 2018;22:806-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Valente TW, Pitts SR. An appraisal of social network theory and analysis as applied to public health: challenges and opportunities. Annu Rev Public Health. 2017;38:103-118. [DOI] [PubMed] [Google Scholar]
- 65. Ford JV, Ivankovich MB, Douglas JM, Jr, et al. The need to promote sexual health in America: a new vision for public health action. Sex Transm Dis. 2017;44:579-585. [DOI] [PubMed] [Google Scholar]
- 66. A practical look at using integration to better prevent and treat sexually transmitted diseases integration of public health and primary care. www.astho.org. Accessed March 28, 2018.
- 67. Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol. 2015;22:137-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hahn A, Barbee L. Syphilis. https://www.std.uw.edu/go/pathogen-based/syphilis/core-concept/all#clinical-manifestations. Published 2017. Accessed February 3, 2018.
- 69. Mattei PL, Beachkofsky TM, Gilson RT. Syphilis: a reemerging infection. Am Fam Physician. 2012;86:433-440. [PubMed] [Google Scholar]
- 70. Marks M, Mabey DC. The introduction of syphilis point of care tests in resource limited settings. Expert Rev Mol Diagn. 2017;17:321-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gayet-Ageron A, Sednaoui P, Lautenschlager S, et al. Use of Treponema pallidum PCR in testing of ulcers for diagnosis of primary syphilis. Emerg Infect Dis. 2015;21:127-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lithgow KV, Cameron CE. Vaccine development for syphilis. Expert Rev Vaccines. 2017;16:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. WHO. Syphilis Screening and Treatment for Pregnant Women. Geneva, Switzerland: WHO; 2017. [PubMed] [Google Scholar]
- 74. Molina J-M, Charreau I, Chidiac C, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis. 2018;18:308-317. [DOI] [PubMed] [Google Scholar]
- 75. Siguier M, Molina J-M. Doxycycline prophylaxis for bacterial sexually transmitted infections: promises and perils. ACS Infect Dis. 2018;4:660-663. [DOI] [PubMed] [Google Scholar]
- 76. Cameron CE, Lukehart SA. Current status of syphilis vaccine development: need, challenges, prospects. Vaccine. 2014;32:1602-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gottlieb SL, Deal CD, Giersing B, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: update and next steps. Vaccine. 2016;34:2939-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Miller JN. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973;110:1206–1215. [PubMed] [Google Scholar]
- 79. Kumar S, Caimano MJ, Anand A, et al. Sequence variation of rare outer membrane protein β-barrel domains in clinical strains provides insights into the evolution of Treponema pallidum subsp. pallidum, the Syphilis Spirochete. MBio. 2018;9:e01006-e01018. [DOI] [PMC free article] [PubMed] [Google Scholar]