Abstract
Purpose of review:
Initial and subsequent modality decisions are important, impacting both clinical outcomes and quality of life. Transition from chronic kidney disease to dialysis and between dialysis modalities are periods were patients may be especially vulnerable. Reviewing our current knowledge surrounding these critical periods and identifying areas for future research may allow us to develop dialysis strategies beneficial to patients.
Sources of information:
We searched the electronic database PubMed and queried Google Scholar for English peer-reviewed articles using appropriate keywords (non-exhaustive list): dialysis transitions, peritoneal dialysis, home hemodialysis, integrated care pathway, and health-related quality of life. Primary sources were accessed whenever possible.
Methods:
In this narrative review, we aim to expose the controversies surrounding home-dialysis first strategies and examine the evidence underpinning home-dialysis first strategies as well as home-to-home and home-to-in-center transitions.
Key findings:
Diverse factors must be taken into consideration when choosing initial and subsequent dialysis modalities. Given the limitations of available data (and lack of convincing benefit or detriment of one modality over the other), patient-centered considerations may prime over suspected mortality benefits of one modality or another.
Limitations:
Available data stem almost exclusively from retrospective and observational studies, often using large national and international databases, susceptible to bias. Furthermore, this is a narrative review which takes into account the views and opinions of the authors, especially as it pertains to optimal dialysis pathways.
Implications:
Emphasis must be placed on individual patient goals and preferences during modality selection while planning ahead to achieve timely and appropriate transitions limiting discomfort and anxiety for patients. Further research is required to ascertain specific interventions which may be beneficial to patients.
Keywords: dialysis transitions, controversies in dialysis, home-dialysis, integrated care pathway, peritoneal dialysis, home hemodialysis
Abrégé
Contexte motivant la revue:
Les décisions entourant le choix de la modalité de dialyse initiale et subséquente sont importantes puisqu’elles ont des répercussions sur les résultats cliniques et la qualité de vie du patient. La transition entre la période l’insuffisance rénale chronique et l’amorce de la dialyse, de même que les périodes de transition entre différentes modalités de dialyse sont des moments où les patients sont particulièrement vulnérables. L’évaluation des données probantes entourant ces périodes et la définition de futurs axes de recherche pourraient contribuer à l’optimisation des soins aux patients.
Sources:
Nous avons identifié dans PubMed et Google Scholar les articles révisés par les pairs et rédigés en anglais répondant aux mots-clés appropriés (liste non exhaustive): dialysis transitions (transitions en dialyse), peritoneal dialysis (dialyse péritonéale), home hemodialysis (hémodialyse à domicile), integrated care pathway (schéma de soins intégrés), et health-related quality of life (qualité de vie liée à l’état de santé). Dans la mesure du possible, les sources principales ont été consultées.
Méthodologie:
Dans notre revue narrative, nous souhaitons exposer les controverses entourant les stratégies initiales de dialyse à domicile et examiner les données probantes qui sous-tendent les stratégies de dialyse à domicile d’abord, mais également les transitions « de domicile à domicile » et « de domicile à centre ».
Principaux résultats:
Plusieurs facteurs sont à considérer au moment de choisir les modalités de dialyse initiale et subséquente. Compte tenu des limites imposées par le manque de données disponibles (et de l’absence d’arguments convaincants quant aux avantages ou désavantages d’une modalité par rapport à une autre), les facteurs axés sur les patients sont susceptibles de l’emporter sur les avantages présumés de l’une ou l’autre modalité sur le taux de mortalité.
Limites:
Les données disponibles proviennent presque exclusivement d’études rétrospectives et observationnelles, lesquelles ayant souvent eu recours aux vastes bases de données nationales et internationales, et sont donc sujettes à l’introduction de biais. En outre, il s’agit d’une revue narrative qui tient compte du point de vue des auteurs, particulièrement en regard des schémas de dialyse optimaux.
Conclusion:
Au moment de choisir la modalité de dialyse, il importe d’accorder une importance aux objectifs et préférences du patient, tout en planifiant la réalisation de transitions opportunes et appropriées, afin de limiter l’inconfort et l’anxiété du patient. D’autres études sont nécessaires pour définir les interventions susceptibles de bénéficier aux patients.
Why is this review important?
In an era where policymakers are promoting a home dialysis-first strategy to manage end-stage renal disease, reflecting upon the basis for such recommendations and subsequent impacts on patients is essential. This review examines the controversies and evidence underpinning home dialysis-first strategies, as well as home-to-home and home-to-in-center transitions, allowing us to propose optimal dialysis pathways for patients.
What are the key messages?
There is no unique ‘best’ dialysis modality for a patient but rather a combination of different modalities over time creating an optimal dialysis pathway. Though we emphasize the importance of individualizing modality selection, we propose optimal dialysis pathways favouring home dialysis modalities whenever possible.
Introduction
Choice of the optimal dialysis modality is central to patients’ experience on renal replacement therapy (RRT). The best dialysis modality is influenced by various factors including health care system, dialysis center expertise, economic restrictions, patient demographics, comorbidities, frailty, and others. Often, there is no unique “best” dialysis modality for a patient but rather a combination of different modalities over time creating an optimal dialysis pathway. Patients’ needs, resources, and objectives may vary through time, influencing their current or future dialysis choices. Given that most patients will require several modality changes during their life on RRT,1 optimizing treatment pathways to offer the best RRT at the right time for the right patient is crucial. An individualized dialysis sequence may prove to be beneficial for both clinical and patient-centered outcomes, especially in an era where policymakers are promoting home-dialysis first policies.
Overall, international registry data show that though mortality rates on dialysis have been decreasing over the last decade,1,2 they remain quite high at more than 10-fold that of the general population.3 Attempts to identify periods of increased vulnerability have shown that transitions from chronic kidney disease (CKD) to RRT and between RRTs are especially challenging. Through this review, we will critically discuss the current data and potential controversies surrounding home dialysis modality transitions.
Transition From Chronic Kidney Disease to Renal Replacement Therapy: Home-Dialysis First for All?
The period surrounding dialysis initiation is known to be associated with the highest mortality. Using data from the Dialysis Outcomes and Practice Patterns Study (DOPPS), Bradbury et al4 showed that mortality was highest in the first 120 days following initiation of dialysis. Early mortality was associated with older age, use of a catheter, hypoalbuminemia, hypophosphatemia, cancer, and congestive heart failure. Conversely, the authors showed a 50% decrease in mortality associated with pre-dialysis nephrology care. Two other studies5,6 found very similar associations, with mortality risk at its highest in the first two months and falling to prevalent levels at approximately 6 months. It appears that the period surrounding dialysis initiation is critical and a phase where timely interventions, such as home dialysis modality education, could improve outcomes.
Should Peritoneal Dialysis Always be the First Dialysis Modality?
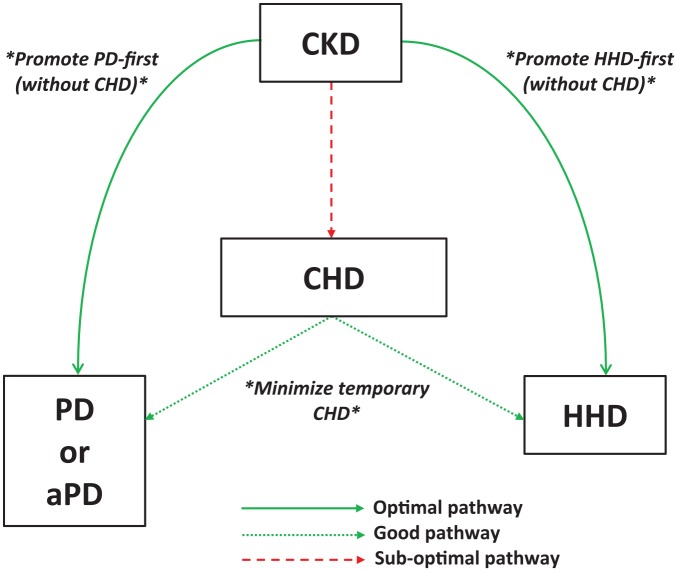
There are multiple theoretical advantages to initiating RRT with peritoneal dialysis (PD), including preservation of residual renal function7,8 and potential vascular accesses. Quality of life may also favor PD given its potential for preservation of lifestyle, independence (and hospital avoidance), travel ease, and flexible schedules. Despite these characteristics of PD, quality of life data have been inconsistent with studies showing superior,9 equal,10,11 or inferior12 health-related quality of life scores compared to conventional hemodialysis (CHD). More data are needed to assess the influence of dialysis modality on patient-centered outcomes such as quality of life.13 Incremental dialysis, with a progressive increase in PD dwell number (continuous ambulatory peritoneal dialysis) or day-dry abdomen (nocturnal intermittent peritoneal dialysis) and non-daily PD, is a promising avenue to improve dialysis patients’ quality of life. Conflicting data have been reported in studies comparing mortality in PD versus CHD cohorts. Several retrospective studies14-16 have shown a relative mortality benefit for patients starting dialysis on PD, especially in the first year and in patients requiring more than one modality over time. However, a recent study by Wong et al17 found a lack of the early PD survival advantage, and rather equal overall survival with PD and HD when comparing a subgroup of patients who were equally eligible for both CHD and PD at the start. The reduced survival of CHD patients in previous studies may have been driven by a selection bias with a greater proportion of more vulnerable patients in this group who were not eligible for PD. Similarly, it has previously been shown that the apparent survival advantage of PD is driven mostly by increased mortality in patients on CHD dialyzing with central venous catheters (CVC).18 As PD has advantages beyond putative improved survival, it remains a promising option for eligible patients (Figure 1).
Figure 1.
Proposed optimal pathway for initiating home dialysis.
Note. CKD = chronic kidney disease; CHD = conventional hemodialysis; HHD = home hemodialysis; PD = peritoneal dialysis; aPD = assisted peritoneal dialysis.
A PD-first strategy to dialysis initiation has been promoted by many regulatory bodies. A caveat to this approach is high attrition rates seen in the first year after initiation in some studies. In a study by Guo and Mujais19 which included >30 000 incident US PD patients, about 20% of PD patients were transferred to HD during the first year. A more recent US cohort study showed similar results, with 21% of PD patients switching to CHD within their first year of PD.20 In contrast, only 6.3% of French PD patients21 transitioned away from PD in the first 6 months. Often, early technique failure is driven by catheter-related complications while later technique failure may be related to infectious and psychosocial issues. Identifying patients at high-risk for early technique failure is imperative. A recent Australia and New Zealand (ANZDATA) registry study22 identified the following factors: age over 70, body mass index (BMI) less than 18.5, diabetes, ischemic heart disease, cerebrovascular disease, peripheral vascular disease, prior RRT, late referral to nephrology service, and being cared for in a smaller center. In the aforementioned French cohort,21 CHD prior to PD, allograft failure, and early peritonitis were associated with more technique failure whereas being treated by an experienced center (more than 20 new patients per year) was protective. Although this highlights the importance of pre-dialysis care and experience of the PD team, the other risk factors may be non-modifiable. In patients without a strong personal inclination toward PD as initial modality, accumulation of multiple risk factors for technique failure, especially if compounded by psychosocial issues, may make for a more challenging PD candidate. These patients should likely be supported by an experienced team and followed more closely if oriented to PD. In all cases, we should stay away from being dogmatic about a PD-first approach, but rather encourage PD as a first modality whenever possible for the patient and dialysis center dyad.
Is Peritoneal Dialysis Only for Independent Patients?
Assisted PD (aPD) is an emerging modality where (mostly older or comorbid) patients who wish to do PD but cannot because of physical, social, or cognitive limitations can do so with help from PD-trained home nursing staff. Models for its implementation vary from country to country, with Canada and many European countries favoring an approach using aPD with once to twice daily nursing visits for cycler setup and connection at night (with or without independent disconnection in the morning), and France favoring CAPD with 3 to 4 nursing visits per day.23 Observational data have shown that both quality of life10 and hospitalization rates24 for aPD patients are comparable to CHD patients, all the while exhibiting superior treatment satisfaction.10 Furthermore, caregiver burden of aPD as compared to self-care PD does not seem to be increased.25
Not only is aPD well-tolerated by patients and their caregivers, but clinical outcomes are very reassuring as well. This is true for destination-aPD, where patients require assistance for long-term treatment, and respite-aPD, where patients transition to aPD from self-care PD for a short period of time only (ie, in the context of an acute illness) or receive support through aPD at time of PD initiation while gaining experience and confidence toward their ability to perform independent PD. In an aPD study from British Columbia,26 both mortality and technique survival was comparable between PD modalities. As an added benefit, though aPD costs approximately 15 000 CAD dollars more than self-care PD per year, it remained cost-effective when compared to switching these patients to CHD.
Home Hemodialysis as the First Dialysis Modality
A minority of patients initiate home hemodialysis (HHD) immediately at start of RRT and, in many programs, patients will transition through CHD first. HHD requires investing more time and resources early on, for both the treating center and the patient. This is highlighted by the fact that cost-neutrality with respect to CHD is only achieved after 12.6 months on HHD.27 Patient preferences are likely to dominate the choice to initiate dialysis with HHD. HHD has generally been associated with improved quality of life in kidney-related domains compared to CHD, although study results were not always significant.28-32 Of note, the frequent hemodialysis network (FHN) trial,29 a randomized controlled trial comparing nocturnal HHD and CHD, did not find a statistically significant difference in quality of life between the conventional and nocturnal HHD groups. Nonetheless, both groups improved their quality of life during follow-up and a large proportion of the conventional HD patients received their dialysis treatments at home. Technique failure can come at great cost, and identifying at-risk patients while improving delivery of HHD by the treating center is important. Recently, Perl and colleagues33 showed that HHD technique failure in Canada has been increasing in the most recent era, most likely due to expansion of recruitment criteria and an influx of older and more vulnerable patients as experience with the technique grew. Data from the Northern Albertan Renal program34 showed that patients who failed HHD consumed more health-care resources in the last 6 months of HHD and ultimately had higher mortality after switching to another modality. In a recent Canadian multicenter study, Pauly and colleagues35 found that home HD center was an independent predictor of technique failure. It seems clear, then, that processes of care are important and should be the subject of further research.
Initiating RRT with HHD influences hospitalization rate. Suri and colleagues,36 using a prevalent dialysis cohort from the United States Renal Data System (USRDS), showed that though there was no difference in hospitalization rate between HHD and CHD, cardiovascular hospitalizations were lower with HHD. Conversely, infectious and access hospitalizations were higher for the HHD cohort.
What is the best first home dialysis modality?
One of the most common clinical questions about home dialysis modality is the debate about the benefit (or not) of HHD over PD. Specific data on this subject are scarce and remain susceptible to residual confounding. Two large database studies from the United States37 and Australia/New Zealand38 have compared mortality and technique failure on PD and HDD. In the ANZDATA study, which included 10 710 incident PD and 706 incident HHD patients, HHD was associated with lower mortality (hazard ratio [HR], 0.47; 95% confidence interval [CI], 0.38-0.59) and technique failure (HR, 0.34; 95% CI, 0.29-0.40).38 Results were consistent in different subgroups, including age, race, and diabetic status. Patients in this study were started on home dialysis less than 90 days after initiation of RRT. Ultimately, however, results were limited by lack of adjustment for psychosocial and economic factors. Furthermore, inclusion of patients initiated on home dialysis very early after RRT start may have selected a subgroup of highly motivated HHD patients and the study results may not apply to all HHD cohorts. The study from Weinhandl et al37 also showed lower risk of mortality and technique failure with short-daily HD using NxStage compared to PD (HR, 0.80 and 0.63, respectively) in a US cohort. However, in this study, the mean duration ESRD (end-stage renal disease) before home dialysis initiation was approximately 44 months in both groups, which likely represents a subgroup of “dialysis survivors” and may have influenced outcomes knowing that mortality risk is highest early after starting RRT. In the subgroup of patients who initiated either modality within the first six months of ESRD, mortality risk was similar with PD and HHD, and the HHD technique failure benefit was attenuated. Recently, a registry study of dialysis patients from Sweden39 also showed higher survival with HHD compared to PD and CHD, although the HHD cohort included in this study was very small, limiting the generalizability of its findings. Furthermore, subsequent graft survival was not influenced by dialysis modality. In addition, these three studies did not include data on vascular access and residual renal function, which may have influenced outcomes. Overall, current data suggest a potential mortality benefit from initiation of dialysis with HHD and likely a technique survival benefit, although these studies held limitations as highlighted above.
The optimal initial dialysis modality for any given patient goes beyond considerations of mortality and technique survival, especially in light of the quality of available evidence. Patient preferences and quality of life should weigh heavily in the initial and subsequent modality choice.
Home-to-home transition; when PD ending is not a “failure”
The transition from PD to HHD is intuitively the most desirable. It allows patients already accustomed to a home modality to remain independent while taking advantage of long-term HHD benefits such as increased solute clearance and optimal volume control. Unfortunately, PD to HHD transitions are relatively infrequent, accounting for only 5.4% of incident PD patient with technique failure in an ANZDATA study.40 In a single-center Canadian report, 16% (12/75) of all PD failures were eventually transferred to HHD.41 More recently, the Ontario Renal Network Home Dialysis Attrition Task Force published data regarding their experience with PD to HHD transitions between 2010 and 2016.42 Province-wide, 14% of patients with PD technique failure transitioned to HHD. Of note, Ontario has previously implemented a Home First Strategy, where the home-to-home strategy is preferred if a kidney transplant is unavailable, though this is no longer the case.
A recurrent clinical question for patients and nephrologists is whether PD followed by HHD is equivalent to a HHD first strategy. This dialysis pathway has been referred to as the Integrated Home Dialysis Model. The largest available study looking to answer this question comes from the Australia and New Zealand Dialysis and Transplant Registry.43 In this cohort, 156 of 10 710 patients on incident PD transitioned to HHD within less than 180 days of PD ending. Men, obese patients, and patients with longer PD vintage were more likely to transition to HHD compared to facility-HD. Mortality risk and home dialysis technique failure were similar for patients who transitioned from PD to HHD and those treated directly with HHD at dialysis initiation.
These results were consistent to those of a smaller single-center Ontario study where HHD patients had similar patient and technique survival with and without previous exposure to PD.44 Recently, a US study identified that 3.6% of all new HHD patients using NxStage transitioned from PD to HHD. Patients who transferred to HHD had a lower mortality risk than a matched cohort transferred to in-center HD.45
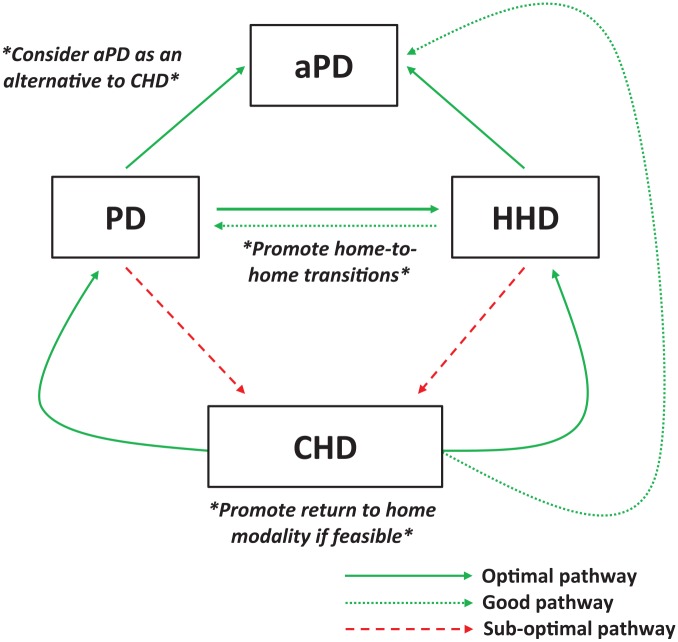
These results favor broader use of the home-to-home transition for patients failing PD (Figure 2). Nonetheless, unanswered questions include the optimal timing for such transition and the need to adequately plan those transfers whenever possible. Of note, patients in the ANZDATA study had a median time on PD of 2.3 years before their transfer to HHD while ESRD duration was 4.3 years before HHD start in the US study.43
Figure 2.
Proposed optimal pathway for home dialysis ending.
Note. CKD = chronic kidney disease; CHD = conventional hemodialysis; HHD = home hemodialysis; PD = peritoneal dialysis; aPD = assisted peritoneal dialysis.
Is it ever too late to start home dialysis?
Peritoneal dialysis
It is well recognized that a small but significant number of patients who ultimately start PD will transition through CHD first. Reasons for this delay include patient preference, modality indecision, logistical limitations (e.g. PD catheter placement), unplanned dialysis initiation (“crash-starts”), and lack of pre-dialysis nephrology follow-up. Canadian registry data have shown that, compared to PD-first patients, patients transferred from CHD had higher mortality46 and experience more technique failure46 and peritonitis.47 Mortality risk and technique failure was mostly increased in the first year after the switch.
Patients who fail CHD due to vascular access issues or cardiovascular disease may represent a subgroup more likely to do well after transitioning to PD. In a Chinese study48 specifically assessing this group, there was no statistically significant differences in technique failure or mortality between patients transferred to PD and a matched cohort initiated directly on PD. Overall, patients transferred to PD due to “CHD technique failure” will likely have different (poorer) outcomes than those transferred due to personal choices and should be followed accordingly.
Time on CHD has been associated with the loss of residual kidney function, which, in turn, is known to influence survival on PD.49,50 While it may never be too late to transfer a patient to PD for lifestyle and quality-of-life considerations, efforts should be made to promptly identify PD candidates in order to minimize unnecessary time on HD and thus preserve residual kidney function.
Transitioning anuric patients to PD poses a particular dilemma, as it is well recognized that residual kidney function is linked to survival on PD.49,51-54 Unfortunately, there is a paucity of data regarding transition to PD in this vulnerable patient population. The EAPOS (European Automated Peritoneal Dialysis Outcomes Study) prospective multicenter study55 demonstrated the feasibility of performing APD in anuric patients. At 2 years, patient survival was 78% and technique survival was 62%. Baseline ultrafiltration <750 mL per day predicted poor survival, and daily ultrafiltration positively correlated with survival in this population. This correlation was reaffirmed in the NECOSAD (Netherlands Cooperative Study on the Adequacy of Dialysis) study56 and in a more recent retrospective Chinese cohort.57 Of note, baseline transport status and creatinine clearance on PD did not correlate with survival in the EAPOS study.55
Home hemodialysis
There is little data regarding the timing of transition to HHD and subsequent outcomes. Generally, most HHD patients have a significant CHD vintage before transferring to HHD.58,59 In a systematic review of daily hemodialysis (including HHD) by Suri et al,60 mean time on CHD before transition to daily HHD ranged from 2 to 11 years, and there was no signal toward negative clinical outcomes with longer CHD vintage. Studies including prevalent cohorts should, however, be interpreted with caution because these patients may be considered “survivors” and prone to the Neyman (selective survival) bias.61 HHD candidates should also be oriented to the HHD training unit as soon as possible since it is common belief that interest toward any home modality may attenuate as patients get used to their current therapy.
Peritoneal Dialysis to Conventional Hemodialysis—A Frequent and Hazardous Transition
The switch from PD to CHD is the most frequent transition in dialysis, notwithstanding transplantation. Most patients who switch from PD to CHD do so permanently. In an ANZDATA study, 24% of patients returned to PD after 30 days on CHD, while only 3% did so after 180 days on CHD.62 Exploring clinical outcomes for this dialysis pathway is essential. A prospective cohort study of American PD patients63 showed that patients switching from PD to CHD had a similar mortality risk than those who stayed on PD. Mortality risk after transfer to HD may be influenced by the cause of PD technique failure. In an ANZDATA study,64 patients transferred to CHD due to inadequate dialysis or mechanical complications had lower mortality risk after transition to CHD than those with infectious causes of PD technique failure. In contrast, transfer to CHD due to social reasons was associated with an increased mortality risk once transferred. Globally, mortality after transition to CHD can be as high as 25% if the transition is unplanned.65
This is mirrored in international data from the INTEGRATED group which showed that mortality is highest during the first month after the switch to CHD with a subsequent decline and plateau after 3 to 4 months.66,67 This data may help us identify those patients who are likely to survive beyond the first months of CHD after transition, and pay particular attention to interventions which may improve their outcomes.
Planning arteriovenous fistula (AVF) or graft creation in patients failing PD may allow them to start hemodialysis with a functioning vascular access. As in the general CHD population, PD patients transferred to CHD and dialyzed with a CVC are considered at higher risk of morbidity and mortality as compared to patients who either remain on PD or CHD patients dialyzed via an AVF.68 Recently, a case-control study attempted to identify predictors of negative outcomes in PD patients and proposed a risk score to guide placement of a vascular access. In this small cohort, Kt/V < 1.7, low albumin, a peritonitis episode, and PD-related hospitalizations were associated with greater risk of transfer to CHD, which appears consistent with previous literature.69 The authors suggested that combination of two risk factors, ≥4 hospitalizations, and exhaustion or loss of autonomy should warrant AVF creation. Of note, in this study, placement of AVF during PD was not associated with PD failure, which may be related to practice patterns in this center or indicate that, unfortunately, clinicians are not good at predicting PD failure. Generally, creation of a permanent vascular access at the start of PD as a “back-up” plan is not advised. A small report of 24 patients in whom an AVF was created at time of PD catheter insertion found that only 3 patients (12.5%) were started on CHD using the AVF.70 This is similar to older data from the United Kingdom where 9% of PD patients with an AVF used this access to start CHD.71 Overall, identifying the optimal time for access creation and transition to CHD where time on PD is maximized while complications and crash-transitions are minimized remains key and should be explored in future research.
Conclusion
Overall, diverse factors must be taken into consideration when choosing initial and subsequent dialysis modalities. Given the limitations of available data (and lack of convincing benefit or detriment of one modality over the other), patient-centered and health system–level considerations may prime over suspected mortality benefits of one modality or another. Emphasis must be placed on individual patient goals and preferences while planning ahead to achieve timely and appropriate transitions limiting discomfort and anxiety for patients. The proposed integrated care pathway where PD is initiated first with timely transition to HHD should likely be suggested to patients if their goals and preferences align, acknowledging the limitations of the current data.
Footnotes
Ethics Approval and Consent to Participate: No patient consent or ethics approval was required for this narrative review.
Consent for Publication: The authors have consented publication of this article.
Availability of Data and Materials: No additional data or materials are available for this review. Please contact corresponding author with any requests.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Annie-Claire Nadeau-Fredette received salary support from Fonds de la recherche du Quebec - Santé and CEC peritoneal dialysis grant by Baxter Healthcare.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Benoit Imbeault  https://orcid.org/0000-0001-8614-8126
https://orcid.org/0000-0001-8614-8126
References
- 1. Marshall MR, Polkinghorne KR, Kerr PG, Agar JW, Hawley CM, McDonald SP. Temporal changes in mortality risk by dialysis modality in the Australian and New Zealand dialysis population. Am J Kidney Dis. 2015;66:489-498. [DOI] [PubMed] [Google Scholar]
- 2. Wakasugi M, Kazama JJ, Narita I. Mortality trends among Japanese dialysis patients, 1988-2013: a joinpoint regression analysis. Nephrol Dial Transplant. 2016;31:1501-1507. [DOI] [PubMed] [Google Scholar]
- 3. Kalantar-Zadeh K, Abbott KC, Kronenberg F, Anker SD, Horwich TB, Fonarow GC. Epidemiology of dialysis patients and heart failure patients. Semin Nephrol. 2006;26:118-133. [DOI] [PubMed] [Google Scholar]
- 4. Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2007;2:89-99. [DOI] [PubMed] [Google Scholar]
- 5. Lukowsky LR, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol. 2012;35:548-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Foley RN, Chen SC, Solid CA, Gilbertson DT, Collins AJ. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014;86:392-398. [DOI] [PubMed] [Google Scholar]
- 7. Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT; NECOSAD Study Group. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046-1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 8. Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556-564. [DOI] [PubMed] [Google Scholar]
- 9. Kutner NG, Zhang R, Barnhart H, Collins AJ. Health status and quality of life reported by incident patients after 1 year on haemodialysis or peritoneal dialysis. Nephrol Dial Transpl. 2005;20:2159-2167. [DOI] [PubMed] [Google Scholar]
- 10. Iyasere OU, Brown EA, Johansson L, et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clin J Am Soc Nephrol. 2016;11:423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manns B, Johnson JA, Taub K, Mortis G, Ghali WA, Donaldson C. Quality of life in patients treated with hemodialysis or peritoneal dialysis: what are the important determinants? Clin Nephrol. 2003;60:341-351. [DOI] [PubMed] [Google Scholar]
- 12. Kang SH, Do JY, Lee SY, Kim JC. Effect of dialysis modality on frailty phenotype, disability, and health-related quality of life in maintenance dialysis patients. PLoS ONE. 2017;12:e0176814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manera KE, Johnson DW, Craig JC, et al. Patient and caregiver priorities for outcomes in peritoneal dialysis: multinational nominal group technique study. Clin J Am Soc Nephrol. 2019;14:74-83. doi: 10.2215/CJN.05380518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant. 2012;27:3568-3575. doi: 10.1093/ndt/gfr674. [DOI] [PubMed] [Google Scholar]
- 15. Lukowsky LR, Mehrotra R, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: a marginal structural model analysis. Clin J Am Soc Nephrol. 2013;8:619-628. doi: 10.2215/CJN.04810512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol. 2010;21:499-505. doi: 10.1681/ASN.2009060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong B, Ravani P, Oliver MJ, Holroyd-Leduc J, Venturato L, Garg AX, Quinn RR. Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis. 2018;71:344-351. doi: 10.1053/j.ajkd.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 18. Perl J, Wald R, McFarlane P, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol. 2011;22:1113-1121. doi: 10.1681/ASN.2010111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo A, Mujais S. Patient and technique survival on peritoneal dialysis in the United States: evaluation in large incident cohorts. Kidney Int Suppl. 2003;88:S3-S12. doi: 10.1046/j.1523-1755.2003.08801.x. [DOI] [PubMed] [Google Scholar]
- 20. Chen JLT, Mehrotra R, Kalantar-Zadeh K. Surviving the first year of peritoneal dialysis: enduring hard times. Am J Kidney Dis. 2014;64:673-676. doi: 10.1053/j.ajkd.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 21. Bechade C, Guittet L, Evans D, Verger C, Ryckelynck JP, Lobbedez T. Early failure in patients starting peritoneal dialysis: a competing risks approach. Nephrol Dial Transplant. 2014;29:2127-2135. doi: 10.1093/ndt/gft055. [DOI] [PubMed] [Google Scholar]
- 22. See EJ, Johnson DW, Hawley CM, et al. Risk predictors and causes of technique failure within the first year of peritoneal dialysis: an Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) study. Am J Kidney Dis. 2018;72:188-197. doi: 10.1053/j.ajkd.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 23. Brown EA, Wilkie M. Assisted peritoneal dialysis as an alternative to in-center hemodialysis. Clin J Am Soc Nephrol. 2016;11:1522-1524. doi: 10.2215/cjn.07040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oliver MJ, Al-Jaishi AA, Dixon SN, et al. Hospitalization rates for patients on assisted peritoneal dialysis compared with in-center hemodialysis. Clin J Am Soc Nephrol. 2016;11:1606-1614. doi: 10.2215/CJN.10130915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Griva K, Goh CS, Kang WCA, et al. Quality of life and emotional distress in patients and burden in caregivers: a comparison between assisted peritoneal dialysis and self-care peritoneal dialysis. Qual Life Res. 2016;25:373-384. doi: 10.1007/s11136-015-1074-8. [DOI] [PubMed] [Google Scholar]
- 26. Bevilacqua MU, Turnbull L, Saunders S, et al. Evaluation of a 12-month pilot of long-term and temporary assisted peritoneal dialysis. Perit Dial Int. 2017;37:307-313. doi: 10.3747/pdi.2016.00201. [DOI] [PubMed] [Google Scholar]
- 27. Beaudry A, Ferguson TW, Rigatto C, Tangri N, Dumanski S, Komenda P. Cost of dialysis therapy by modality in Manitoba. Clin J Am Soc Nephrol. 2018;13:1197-1203. doi: 10.2215/CJN.10180917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291-1299. [DOI] [PubMed] [Google Scholar]
- 29. Rocco MV, Lockridge RS, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the frequent hemodialysis network nocturnal trial. Kidney Int. 2011;80:1080-1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. VanEps CL, Jeffries JK, Johnson DW, et al. Quality of life and alternate nightly nocturnal home hemodialysis. Hemodial Int. 2010;14:29-38. [DOI] [PubMed] [Google Scholar]
- 31. Vos PF, Zilch O, Jennekens-Schinkel A, et al. Effect of short daily home haemodialysis on quality of life, cognitive functioning and the electroencephalogram. Nephrol Dial Transplant. 2006;21:2529-2535. [DOI] [PubMed] [Google Scholar]
- 32. Heidenheim AP, Muirhead N, Moist L, Lindsay RM. Patient quality of life on quotidian hemodialysis. Am J Kidney Dis. 2003;42:36-41. doi: 10.1016/s0272-6386(03)00536-5. [DOI] [PubMed] [Google Scholar]
- 33. Perl J, Na Y, Tennankore KK, Chan CT. Temporal trends and factors associated with home hemodialysis technique survival in Canada. Clin J Am Soc Nephrol. 2017;12:1248-1258. doi: 10.2215/CJN.13271216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah N, Reintjes F, Courtney M, et al. Quality assurance audit of technique failure and 90-day mortality after program discharge in a Canadian home hemodialysis program. Clin J Am Soc Nephrol. 2017;12:1259-1264. doi: 10.2215/CJN.00140117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pauly RP, Rosychuk RJ, Usman I, et al. Technique failure in a multicenter Canadian home hemodialysis cohort. Am J Kidney Dis. 2019;73:230-239. doi: 10.1053/j.ajkd.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 36. Suri RS, Li L, Nesrallah GE. The risk of hospitalization and modality failure with home dialysis. Kidney Int. 2015;88:360-388. doi: 10.1038/ki.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weinhandl ED, Gilbertson DT, Collins AJ. Mortality, hospitalization, and technique failure in daily home hemodialysis and matched peritoneal dialysis patients: a matched cohort study. Am J Kidney Dis. 2016;67:98-110. doi: 10.1053/j.ajkd.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 38. Nadeau-Fredette AC, Hawley CM, Pascoe EM, et al. An incident cohort study comparing survival on home hemodialysis and peritoneal dialysis (Australia and New Zealand dialysis and transplantation registry). Clin J Am Soc Nephrol. 2015;10:1397-1407. doi: 10.2215/CJN.00840115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rydell H. Improved long-term survival with home hemodialysis compared with institutional hemodialysis and peritoneal dialysis: a matched cohort study. BMC Nephrol. 2019;20:Article 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nadeau-Fredette AC, Hawley C, Pascoe E, et al. Predictors of transfer to home hemodialysis after peritoneal dialysis completion. Perit Dial Int. 2016;36:547-545. doi: 10.3747/pdi.2015.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cina DP, Dacouris N, Kashani M, et al. Use of home hemodialysis after peritoneal dialysis technique failure. Perit Dial Int. 2013;33:96-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCormick BB, Chan CT. Striving to achieve an integrated home dialysis system: a report from the Ontario renal network home dialysis attrition task force. Clin J Am Soc Nephrol. 2018;13:468-470. doi: 10.2215/CJN.06900617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nadeau-Fredette AC, Chan CT, Cho Y, et al. Outcomes of integrated home dialysis care: a multi-centre, multi-national registry study. Nephrol Dial Transplant. 2015;30:1897-1904. doi: 10.1093/ndt/gfv132. [DOI] [PubMed] [Google Scholar]
- 44. Nadeau-Fredette AC, Bargman JM, Chan CT. Clinical outcome of home hemodialysis in patients with previous peritoneal dialysis exposure: evaluation of the integrated home dialysis model. Perit Dial Int. 2015;35:316-323. doi: 10.3747/pdi.2013.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kansal SK, Morfin JA, Weinhandl ED. Survival and kidney transplant incidence on home versus in-center hemodialysis, following peritoneal dialysis technique failure. Perit Dial Int. 2019;39:25-34. doi: 10.3747/pdi.2017.00207. [DOI] [PubMed] [Google Scholar]
- 46. Nessim SJ, Bargman JM, Jassal SV, Oliver MJ, Na Y, Perl J. The impact of transfer from hemodialysis on peritoneal dialysis technique survival. Perit Dial Int. 2015;35:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large, prospective Canadian database. Clin J Am Soc Nephrol. 2009;4:1195-1200. doi: 10.2215/CJN.00910209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang L, Cao T, Li Z, et al. Clinical outcomes of peritoneal dialysis patients transferred from hemodialysis: a matched case-control study. Perit Dial Int. 2013;33:259-266. doi: 10.3747/pdi.2011.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bargman JM, Thorpe KE, Churchill DN; CANUSA Peritoneal Dialysis Study Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158-20162. [DOI] [PubMed] [Google Scholar]
- 50. Misra M, Vonesh E, VanStone JC, Moore HL, Prowant B, Nolph KD. Effect of cause and time of dropout on the residual GFR: a comparative analysis of the decline of GFR on dialysis. Kidney Int. 2001;59:741-763. doi: 10.1046/j.1523-1755.2001.059002754.x. [DOI] [PubMed] [Google Scholar]
- 51. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53:1068-1081. doi: 10.1053/j.ajkd.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 52. Termorshuizen F, Dekker FW, vanManen JG, Korevaar JC, Boeschoten EW, Krediet RT; NECOSAD Study Group. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands cooperative study on the adequacy of dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061-1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 53. Termorshuizen F, Korevaar JC, Dekker FW, vanManen JG, Boeschoten EW, Krediet RT. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis. 2003;41:1293-1302. doi: 10.1016/s0272-6386(03)00362-7. [DOI] [PubMed] [Google Scholar]
- 54. Chung SH, Heimburger O, Stenvinkel P, Qureshi AR, Lindholm B. Association between residual renal function, inflammation and patient survival in new peritoneal dialysis patients. Nephrol Dial Transplant. 2003;18:590-597. doi: 10.1093/ndt/18.3.590. [DOI] [PubMed] [Google Scholar]
- 55. Brown EA, Davies SJ, Rutherford P, et al. Survival of functionally anuric patients on automated peritoneal dialysis: the European APD outcome study. J Am Soc Nephrol. 2003;14:2948-2957. doi: 10.1097/01.asn.0000092146.67909.e2. [DOI] [PubMed] [Google Scholar]
- 56. Jansen MAM, Termorshuizen F, Korevaar JC, et al. Predictors of survival in anuric peritoneal dialysis patients. Kidney Int. 2005;68:1199-1205. doi: 10.1111/j.1523-1755.2005.00512.x. [DOI] [PubMed] [Google Scholar]
- 57. Lin X, Lin A, Ni Z, et al. Daily peritoneal ultrafiltration predicts patient and technique survival in anuric peritoneal dialysis patients. Nephrol Dial Transplant. 2010;25:2322-2327. doi: 10.1093/ndt/gfq001. [DOI] [PubMed] [Google Scholar]
- 58. Marshall MR, Hawley CM, Kerr PG, et al. Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis. 2011;58:782-793. doi: 10.1053/j.ajkd.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 59. Rioux JP, Bargman JM, Chan CT. Systematic differences among patients initiated on home haemodialysis and peritoneal dialysis: the fallacy of potential competition. Nephrol Dial Transplant. 2010;25:2364-2367. doi: 10.1093/ndt/gfq192. [DOI] [PubMed] [Google Scholar]
- 60. Suri RS, Nesrallah GE, Mainra R, et al. Daily hemodialysis: a systematic review. CJASN. 2006;1:33-42. doi: 10.2215/CJN.00340705. [DOI] [PubMed] [Google Scholar]
- 61. Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58:635-641. doi: 10.1136/jech.2003.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lan PG, Clayton PA, Johnson DW, et al. Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardized definition of technique failure. Perit Dial Int. 2016;36:623-630. doi: 10.3747/pdi.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jaar BG, Plantinga LC, Crews DC, et al. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: a prospective study. BMC Nephrol. 2009;10:Article 3. doi: 10.1186/1471-2369-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen JHC, Johnson DW, Hawley C, Boudville N, Lim WH. Association between causes of peritoneal dialysis technique failure and all-cause mortality. Sci Rep. 2018;8:Article 3980. doi: 10.1038/s41598-018-22335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boissinot L, Landru I, Cardineau E, Zagdoun E, Ryckelycnk JP, Lobbedez T. Is transition between peritoneal dialysis and hemodialysis really a gradual process? Perit Dial Int. 2013;34:391-397. doi: 10.3747/pdi.2011.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chan C, Combes G, Davies S, et al. Transition between different renal replacement modalities: gaps in knowledge and care-the integrated research initiative. Perit Dial Int. 2019;39:4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sukul N, Chan C, Davies S, et al. International trends in mortality soon after switch from peritoneal dialysis to in-center hemodialysis: results from the integrated study group. J Am Soc Nephrol. 2018;29. [Google Scholar]
- 68. Pajek J, Hutchison AJ, Bhutani S, et al. Outcomes of peritoneal dialysis patients and switching to hemodialysis: a competing risks analysis. Perit Dial Int. 2014;34:289-298. doi: 10.3747/pdi.2012.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ferreira H, Nunes A, Oliveira A, Beco A, Santos J, Pestana M. Planning vascular access in peritoneal dialysis-defining high-risk patients. Perit Dial Int. 2018;38:271-277. [DOI] [PubMed] [Google Scholar]
- 70. Nezakatgoo N, Ndzengue A, Ramaiah M, Gosmanova EO. Outcomes of simultaneous peritoneal dialysis and arteriovenous fistula placement in end-stage renal disease patients. Perit Dial Int. 2017;37:658-661. doi: 10.3747/pdi.2017.00072 [DOI] [PubMed] [Google Scholar]
- 71. Beckingham IJ, O’Rourke JS, Bishop MC, Blamey RW. Are backup arteriovenous fistulae necessary for patients on continuous ambulatory peritoneal dialysis? Lancet. 1993;341:1384-1386. doi: 10.1016/0140-6736(93)90951. [DOI] [PubMed] [Google Scholar]