Abstract
Background
Immunosuppressive therapy for connective tissue diseases (CTDs) increases risk for opportunistic infections including Pneumocystis pneumonia (PCP). High mortality rates are reported in CTD patients with PCP, which suggests a potential need for prophylaxis, but indications remain poorly defined. Wide variations in the use of PCP prophylaxis among rheumatologists have been documented. This study evaluated PCP prophylaxis patterns for CTD patients among infectious disease (ID) physicians.
Methods
An electronic survey was emailed to 1264 adult ID physicians who are members of the Infectious Diseases Society of America Emerging Infections Network.
Results
Six hundred thirty-one physicians responded to the survey. Respondents to the survey were more likely to work in academics (P = .02) and be early (<5 years) or late (≥25 years) in their careers (P = .0002). Forty-three percent (n = 269) made no recommendations for PCP prophylaxis in non-HIV patients. Of the 362 respondents who did make such recommendations, the greatest consensus for disease-based prophylaxis was for granulomatosis with polyangiitis (53%). For therapy-based prophylaxis, corticosteroids ≥20 mg/d was the most frequently cited indication (87%). Surrogate laboratory markers to aid in decisions about prophylaxis were not routinely used (21%). Although the majority recommended discontinuation of PCP prophylaxis with tapering of corticosteroids (65%), there was variability in the specific dose. Eighty-nine percent of respondents felt that guidelines about PCP prophylaxis would be helpful.
Conclusions
There is little consensus about PCP prophylaxis in CTDs among ID physicians. Guidelines for PCP prophylaxis would be helpful when caring for these complex patients.
Keywords: connective tissue diseases, PCP, Pneumocystis pneumonia, prophylaxis, rheumatologic disorders
The management of connective tissue diseases (CTDs) continues to evolve rapidly with the ever-increasing number of biologic therapies, which can be used in conjunction with or in lieu of traditional nonbiologic immunosuppressants such as corticosteroids and methotrexate [1]. One consequence of these more “aggressive” treatment regimens is an increased risk of opportunistic infections (OIs) [2, 3]. Pneumocystis pneumonia (PCP) is a traditional OI that occurs at varying frequencies in non-HIV-infected immunocompromised patients [4, 5] and is particularly associated with significant mortality in patients with underlying CTDs [6]. Thus, identification of effective strategies to prevent OIs in these vulnerable patient populations is important. In the absence of randomized controlled clinical trials to inform decision-making, recommendations about the use of PCP prophylaxis in patients with CTDs are most frequently based upon expert opinion or personal experience and are varied as to if and when PCP prophylaxis is indicated. Confounding the debate is that not only do the medications used to treat CTD potentially increase the risk of PCP, but also the diseases themselves may predispose these patients to PCP independent of the treatment regimen [6]. Thus, there is the consideration for disease-based prophylaxis (prophylaxis based on underlying disease regardless of medications) vs therapy-based prophylaxis (prophylaxis based on therapy regimen regardless of underlying disease) and a combination of the 2. Previous surveys of practicing rheumatologists have shown limited consensus of when to use PCP prophylaxis not only in terms of therapeutic regimens but also in terms of underlying diseases [7, 8]. The purpose of this survey was to assess current clinical practice and recommendations for PCP prophylaxis from the perspective of the infectious disease (ID) consultant in non-HIV-infected immunocompromised patients with CTDs.
METHODS
An electronic 7-question survey of adult ID physician members of the Emerging Infections Network (EIN) was conducted from September 7, 2016, to October 3, 2016 (Figure 1). The EIN is a provider-based network comprised of Infectious Diseases Society of America (IDSA) members in the United States, Puerto Rico, and Canada. The EIN is funded through a collaboration between the Centers for Disease Control and Prevention and the IDSA [9]. The survey was developed primarily by R.M.W. and J.E.P. with input from the EIN program staff. The goal of the survey was to assess ID physician patterns on the use of prophylaxis for PCP in non-HIV-infected patients with various CTDs who were receiving various immunosuppressive therapies.
Figure 1.
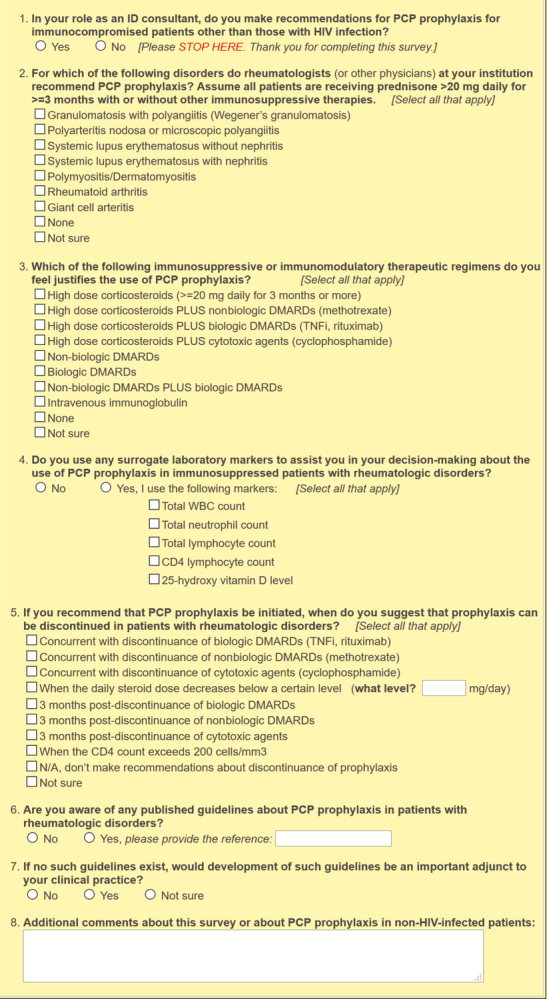
EIN survey on PCP prophylaxis for patients with connective tissue diseases.
The survey was distributed by email, with 2 reminders at weekly intervals for nonrespondents. An opt-out option was provided for ID physicians who indicated that they did not provide recommendations for PCP prophylaxis in non-HIV-infected patients. Respondents were not required to answer all questions, so total responses for individual questions could vary. The survey was designed to provide respondents with the option of recommending PCP prophylaxis based on either the underlying CTD (disease-based prophylaxis) or the immunosuppressive treatment regimen (therapy-based prophylaxis) or both. For the underlying CTD, the qualifying caveat was included that all patients had to be receiving ≥20 mg of prednisone daily with or without other immunosuppressive therapies. For most questions, the option of selecting multiple answers was available. Categorical variables were compared using a χ 2 test or Fisher exact test with SAS, version 9.3 (Cary, NC). P values <.05 were considered significant.
RESULTS
Of the 1264 ID physicians receiving the survey, 631 (50%) responded, but only 362 (29% of the total survey recipients, 57% of respondents) reported that they made recommendations for PCP prophylaxis for non-HIV-infected patients. Two hundred sixty-nine respondents (43%) indicated that they did not make recommendations and, subsequently, did not complete the remainder of the survey. The demographics and practice attributes of the nonrespondents (n = 633), total respondents (n = 631), and respondents who made recommendations about PCP prophylaxis (n = 362) are summarized in Table 1. Total respondents were significantly more likely to work in an academic/university system (P = .02) and either be early (<5 years) or late (≥25 years) in their career (P = .0002). Respondents making recommendations about PCP prophylaxis were more likely to work in a nonuniversity teaching hospital and less likely to work in a city/county/public hospital (P = .0455). There was no statistical difference in years of ID experience between respondents who did vs did not offer PCP prophylaxis recommendations.
Table 1.
Profile of Survey Respondents (n = 631) vs Nonrespondents (n = 633)
| Respondents (n = 631) | Nonrespondents (n = 633) | Respondents who Provide PCP Prophylaxis Recommendations (n = 362) | |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Region | |||
| Northeast | 139 (22) | 133 (21) | 71 (20) |
| South | 183 (29) | 179 (28.3) | 103 (28) |
| Midwest | 172 (27.3) | 144 (22.8) | 104 (29) |
| West | 133 (21.1) | 164 (25.9) | 82 (23) |
| Canada and Puerto Rico | 4 (0.6) | 13 (2) | 2 (0.6) |
| Years of experience since completion of ID fellowship | |||
| <5 | 121 (19.2)* | 100 (15.8) | 77 (21) |
| 5–14 | 202 (32) | 252 (39.8) | 122 (34) |
| 15–24 | 111 (17.6) | 140 (22.1) | 54 (15) |
| ≥25 | 197 (31.2)* | 141 (22.3) | 109 (30) |
| Employment | |||
| Hospital/clinic | 186 (29.5) | 207 (32.7) | 104 (29) |
| Private/group practice | 170 (26.9) | 193 (30.5) | 105 (29) |
| University/medical school | 231 (36.6)* | 199 (31.4) | 131 (36) |
| VA and military | 40 (6.3) | 33 (5.2) | 21 (6) |
| State government | 4 (0.6)* | 2 (0.3) | 1 (0.3) |
| Primary hospital type | |||
| Community | 173 (27.4) | 213 (32.7) | 91 (25) |
| Nonuniversity teaching | 150 (23.8) | 164 (25.9) | 91 (25) |
| University | 235 (37.2) | 194 (30.7) | 138 (38) |
| VA hospital or DOD | 45 (7.1) | 34 (5.4) | 24 (7) |
| City/county | 28 (4.4) | 28 (4.4) | 11 (3) |
Abbreviations: DOD, Department of Defense; ID, infectious diseases; PCP, Pneumocystis pneumonia; VA, Veterans’ Administration.
*P value <.05 when comparing respondents with nonrespondents.
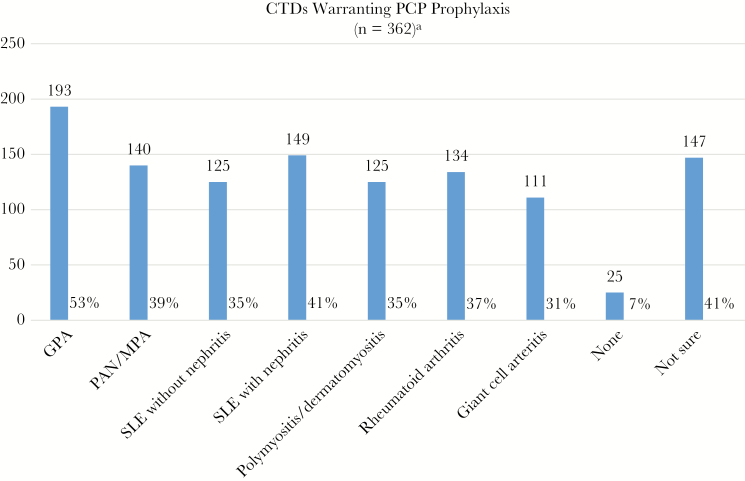
When considering the underlying CTD (disease-based prophylaxis), 53% of 362 respondents indicated that PCP prophylaxis would be recommended for patients diagnosed as having granulomatosis with polyangiitis (GPA), whereas 41% would recommend PCP prophylaxis for SLE with nephritis (Figure 2). Only 7% of respondents (n = 25) reported that PCP prophylaxis would not be recommended for any of the listed CTDs, whereas 93 (26%) selected all 7 of the listed CTDs. The single most frequent response was “not sure,” which was chosen by 127 (35%) respondents, although a total of 147 (41%) selected this answer choice with other options.
Figure 2. .
aRespondents were instructed to assume all patients were receiving prednisone >20 mg daily for a minimum of 3 months with or without other immunosuppressive therapies. Additionally, respondents could select all options that apply, so totals may exceed 100%. Abbreviations: CTDs, connective tissue diseases; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; PAN, polyarteritis nodosa; PCP, Pneumocystis pneumonia; SLE, systemic lupus erythematosus.
When focusing on the immunosuppressive regimen itself (therapy-based prophylaxis), 87% of respondents (n = 316) would utilize prophylaxis for patients receiving ≥20 mg of prednisone daily for ≥3 months (Table 2). Interestingly, those figures declined to 77% and 78% if high-dose corticosteroids were given in conjunction with biologic disease-modifying antirheumatic drugs (DMARDs) such as tumor necrosis factor inhibitors (TNFi) or rituximab, or with cytotoxic agents such as cyclophosphamide. Only 53 respondents (15%) indicated that they would consider PCP prophylaxis for patients receiving biologic DMARDs alone. One percent of respondents felt that PCP prophylaxis was not recommended for any of the listed immunosuppressive regimens. The single most frequent response to Question 3 was the selection of all immunosuppressive regimens that included high-dose corticosteroids, which was the choice of 49% of respondents (n = 179).
Table 2.
Immunosuppressive or Immunomodulatory Therapeutic Regimens Justifying PCP Prophylaxis (n = 362)
| Therapeutic Regimen | No. of Respondents (%)a |
|---|---|
| High-dose corticosteroids (≥20 mg daily for ≥3 mo) | 316 (87) |
| High-dose corticosteroids PLUS nonbiologic DMARDs (methotrexate) | 249 (69) |
| High-dose corticosteroids PLUS biologic DMARDs (TNFi, rituximab) | 280 (77) |
| High-dose corticosteroids PLUS cytotoxic agents (cyclophosphamide) | 281 (78) |
| Nonbiologic DMARDs | 8 (2) |
| Biologic DMARDs | 53 (15) |
| Nonbiologic DMARDs PLUS biologic DMARDs | 43 (12) |
| Intravenous immunoglobulin | 0 |
| None | 4 (1) |
| Not sure | 18 (5) |
Abbreviations: DMARDs, disease-modifying antirheumatic drugs; PCP, Pneumocystis pneumonia; TNFi, tumor necrosis factor inhibitor.
aRespondents could select all that apply so totals may exceed 100%.
Surrogate laboratory markers such as absolute lymphocyte counts and CD4 (helper) lymphocyte counts were not felt to provide useful information in determining the need for PCP prophylaxis in immunosuppressed patients with rheumatologic disorders by 79% of respondents (Question 4). Of the 76 physicians who felt that surrogate laboratory markers were aids in deciding about PCP prophylaxis, the CD4 lymphocyte count was most often the test of choice, as it was selected by 48 of the 76 physicians (63%).
Sixty-five percent of respondents indicated that they would utilize the corticosteroid dose as the primary determinant for stopping PCP prophylaxis, but there was no uniform consensus about discrete end points for this discontinuance (Table 3). Among the offered options as to the daily dose of corticosteroids below which it was safe to discontinue therapy, 52% cited 16–20 mg, whereas 30% chose ≤10 mg. Nineteen percent of respondents chose a CD4 count >200 cells/mm3 as the criterion utilized to stop corticosteroids. “Not sure” was the response selected by 12%.
Table 3.
Criteria for Discontinuance of PCP Prophylaxis in Patients With Rheumatologic Disorders (n = 362)
| Criterion for Discontinuing PCP Prophylaxis | No. of Respondents (%)a |
|---|---|
| Concurrent with discontinuance of biologic DMARDs (TNFi, rituximab) | 50 (14) |
| Concurrent with discontinuance of nonbiologic DMARDs (methotrexate) | 26 (7) |
| Concurrent with discontinuance of cytotoxic agents (cyclophosphamide) | 45 (12) |
| When the daily steroid dose decreases below a certain level | 235 (65) |
| 0.5–10 mg | 70 (30) |
| 11–15 mg | 37 (16) |
| 16–20 mg | 123 (52) |
| 30 mg | 4 (2) |
| 40 mg | 2 (1) |
| 3 mo post-discontinuance of biologic DMARDs (TNFi, rituximab) | 64 (18) |
| 3 mo post-discontinuance of nonbiologic DMARDs (methotrexate) | 25 (7) |
| 3 mo post-discontinuance of cytotoxic agents (cyclophosphamide) | 54 (15) |
| When the CD4 count exceeds 200 cells/mm3 | 70 (19) |
| N/A, don’t make recommendations about discontinuance of prophylaxis | 29 (8) |
| Not sure | 44 (12) |
Abbreviations: DMARDs, disease-modifying antirheumatic drugs; PCP, Pneumocystis pneumonia; TNFi, tumor necrosis factor inhibitor.
aRespondents could select all that apply so totals may exceed 100%.
Ninety-six percent of respondents indicated that they were unaware of any preexisting guidelines for PCP prophylaxis in non-HIV-infected patients with rheumatologic disorders. Eighty-nine percent felt that the development of such guidelines would be a useful adjunct to their clinical practices, though some respondents expressed concern and skepticism about guidelines based on expert opinion rather than clinical trial data.
DISCUSSION
Although trends in practice patterns in PCP prophylaxis in CTDs can be mined from this survey, overall, there is minimal consensus on if and when prophylaxis should be recommended and what factors (disease, treatment regimen, laboratory values) should influence that decision. Clear evidence-based guidelines exist for other disease entities with endogenously or iatrogenically suppressed T-cell immunity such as HIV, hematologic malignancies, and transplant patients [10–14], but no such guidelines exist for CTDs. Patients with CTDs, who are often on treatments that induce that same profound suppression of T-cell-mediated immunity, are an ever-growing group of patients who are considered to be at risk for PCP [6, 11, 12]. However, the exact frequency of occurrence of PCP among patients with various rheumatologic disorders is difficult to define given the heterogeneity of that population. It has been estimated that persons with rheumatologic and other autoimmune disorders comprise 13%–36% of cases of PCP in non-HIV-infected patients [15]. Ognibene et al. [16] found an overall incidence of PCP of 6% in patients with GPA, whereas Chew and colleagues [17] documented an incidence of 75 cases per 100 000 patients per year in a general hospital population of patients with autoimmune disorders (defined by discharge diagnoses). Yet these estimated incidence numbers are confounded by the rarity of both the rheumatologic disease and the low absolute number of PCP cases in each study group. Additionally, given the retrospective nature of these studies, confirmation of the underlying connective tissue disease and the diagnosis of PCP can be quite challenging and fraught with inher error. Mortality rates for PCP in CTDs are noted to be high, but these numbers are subject to the same shortcomings as data on the overall prevalence of PCP. These shortcomings likely explain the diverse range of reported mortality for PCP in this patient population, which extends from 9% to 85% [6].
Regardless of the shortcomings of the available data, PCP does occur in CTDs and can be severe, if not fatal. In the absence of consensus evidence-based recommendations for the use of prophylaxis in immunosuppressed patients with rheumatologic disorders, recommendations based on observational data and personal experience guide practice patterns. Prior surveys on PCP prophylaxis targeting rheumatologists [7, 8] have revealed a great deal of variability in clinical practice. The 2008 survey by Gupta and colleagues [7] focused only on patients with SLE who were receiving cyclophosphamide. In that survey, 50% of rheumatologists reported using prophylaxis for PCP in that specific patient population. The authors concluded that the low rate of PCP in the population, estimated to be only 0.16%, did not justify the routine use of PCP prophylaxis. Cettomai and colleagues surveyed rheumatologists focusing on the use of PCP prophylaxis in their patients with any rheumatic disease or therapy [8]. Prophylaxis was prescribed by 69.5% of respondents, with the most important determinant for utilizing prophylaxis being the treatment regimen rather than the underlying rheumatologic disease or the specific medication dosage [8]. This observation stands in contrast to evidence that suggests that the underlying disease itself plays a significant role in development of PCP, rather than therapeutic regimen alone [6].
As judged by the results of this survey, ID clinicians, like their rheumatology colleagues, also appear to have a great deal of uncertainty about if and when to use PCP prophylaxis in patients with CTDs and other rheumatologic disorders. Nearly 50% of respondents indicated that they did not prescribe PCP prophylaxis for non-HIV-infected patients. Whether that position was due to lack of involvement in decision-making about prophylaxis for PCP in non-HIV-related immunodeficiency disorders, a perception that the decision is the purview of others, or a general lack of interest in that issue cannot be determined based on the survey results. Among candidate rheumatologic disorders for disease-based prophylaxis, a slight majority of respondents (53%) selected GPA as a disease for which they would prescribe prophylaxis; no other rheumatologic disorder garnered a majority consensus for prophylaxis. In contrast, 87% of respondents indicated that therapy-based prophylaxis would be recommended for patients receiving high-dose corticosteroids (≥20 mg daily of prednisone equivalent for ≥3 months), whether other agents were used or not. That finding is consistent with the results from Cettomai et al. [8], where surveyed rheumatologists were more inclined to use the treatment regimen rather than the underlying rheumatologic disorder as the determinant for choosing prophylaxis.
Interestingly, 63% of ID clinicians who utilized lab studies to assist with decision-making about prophylaxis felt that the CD4 count was a useful surrogate marker, whereas another 21% indicated that the CD4 count plus the absolute lymphocyte count was an aid in decision-making. Even though several reviews and observational studies have proposed using the CD4 count to guide decision-making about PCP prophylaxis [12, 15, 18], a recent report by Baulier and colleagues [19] questioned the accuracy of that criterion in patients with rheumatologic and autoimmune disorders. Thus, evidence to support the use of the CD4 count in patients with CTDs is presently lacking, and additional studies are needed before widespread adoption of that tool can be justified [6].
Discontinuance of PCP prophylaxis, if initiated, was also associated with a great deal of indecision. Sixty-five percent of respondents indicated that a decrease of the prednisone dose below a predetermined level would be the primary end point utilized to stop PCP prophylaxis; that choice was the most frequently selected option. The most commonly identified prednisone dosage was 16–20 mg daily, which was chosen by 52% of those respondents who indicated that they would use the corticosteroid dose as the criterion for discontinuing prophylaxis. Cessation of prophylaxis in association with discontinuance of biologic or nonbiologic DMARDs was chosen as a logical end point by 14% and 7% of respondents, respectively. Interestingly, most of the studies addressing the use of PCP prophylaxis in patients with CTDs generally do not propose criteria for cessation of prophylaxis. However, Vernovsky and Dallaripa [18] set forth the recommendation that prophylaxis could be discontinued when corticosteroids were switched from daily to alternate-day dosing or when the daily corticosteroid dose decreased below 20 mg of prednisone equivalent.
Despite the uncertainties among clinicians and the dearth of randomized controlled clinical trial data addressing PCP prophylaxis in patients with rheumatologic disorders, observational data and cohort studies are appearing and perhaps gaining clinical traction [15, 20]. In one of the most thorough reviews of this issue to date, Park et al. retrospectively evaluated 1522 treatment episodes with prolonged (≥4 weeks) high-dose prednisone use (≥30 mg/d) in the setting of rheumatic disease and assessed the use of PCP prophylaxis [20]. Of the 1522 qualifying events, 262 cases received trimethoprim-sulfamethoxazole prophylaxis; the remaining cases did not receive prophylaxis and served as the control group. Overall, 30 cases of PCP were diagnosed (via PCR), with only 1 case being in the prophylaxis arm. Collectively, for all rheumatic diseases, the number needed to treat (n = 52) was lower than the number needed to harm (n = 131) given a low rate of adverse events. These results must be interpreted with caution for several reasons. First, the potential severity of adverse events from trimethoprim-sulfamethoxazole cannot be ignored, especially as higher adverse event rates have been reported in specific CTDs such as lupus [6]. Additionally, all rheumatic diseases were evaluated together, and consideration of treatment regimens that may have been concurrently administered was not evaluated. In an accompanying editorial to this article, PCP prophylaxis in CTDs was recommended for prednisone doses >30 mg when planned for more than 4 weeks until the dose was tapered below 15 mg daily [21]. The dose for tapering was based on the Park et al. data given the low risk of PCP after this dose. However, the caveat was given that if other PCP risk factors were present, consideration to continue prophylaxis until the prednisone dose was even lower would be warranted.
A recent review by 2 of the authors of this survey (R.M.W., J.E.P.) concluded that, among patients with CTDs, PCP prophylaxis could be recommended with confidence only for patients with GPA undergoing induction therapy [6]. That proposal was supported by updated recommendations from the European League Against Rheumatism, which added PCP prophylaxis to treatment guidelines of any ANCA-associated vasculitis during induction therapy [22]. The review article by Wolfe and Peacock also offered conditional recommendations for prophylaxis for patients with SLE, inflammatory myositis, PAN, and ANCA-associated vasculitis but not for rheumatoid arthritis, giant cell arteritis, scleroderma, or other CTDs. Other review articles also advocate an individual approach to PCP prophylaxis for these complex patients with CTDs [17].
Surveys such as this one, which target a selected group of clinicians and are used to characterize clinical practice patterns, have obvious limitations, and this study was subject to these weaknesses. Our survey relied upon self-reported data from voluntary respondents who may or may not have been representative of the larger group as a whole. Only 362 survey recipients, representing 29% of the total ID providers surveyed, indicated that they made recommendations for PCP prophylaxis in CTD patients. Additionally, questions that permit more than a single response add to the difficulties in interpreting those responses. Lastly, the grouping of immunosuppressive regimens in this survey may not encompass all potential combinations and may not conform to local practice patterns.
Notwithstanding those limitations, this survey did provide insightful information. On the basis of the survey responses, the following conclusions can be drawn. Among ID clinicians who make recommendations for PCP prophylaxis in at-risk rheumatologic patients, there is a great deal of uncertainty as to which patients or treatment regimens warrant prophylaxis. The immunosuppressive regimen being used to treat the rheumatologic disorder rather than the disorder itself appears to have the greatest impact on the decision to recommend prophylaxis. Discontinuance of PCP prophylaxis is usually based on the corticosteroid dosage, with cessation considered when the daily dose is decreased to <20 mg. Even in the absence of evidence-based data supporting its use, the majority of respondents who utilized lab results to aid in decision-making felt that the CD4 count was a useful surrogate marker to identify patients at risk who should be considered for prophylaxis. Guidelines for PCP prophylaxis would provide an important adjunct in caring for complex immunosuppressed patients with underlying rheumatologic disorders.
Acknowledgments
The authors (R.M.W., J.E.P.) would like to express their appreciation to their respective Section Chiefs, Dennis C. Ang, MD, MS, in Rheumatology and John W. Sanders, III, MD, MPH, in Infectious Diseases, for providing the required publication fee for this manuscript.
Financial support. This publication was supported by Cooperative Agreement Number 1 U50 CK000477, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Potential conflicts of interest. J.E.P. owns common stock in Pfizer, Inc. K.L.W. receives research funding from BMS and serves as a consultant for BMS, Pfizer, Abbvie, Lilly, UCB, Gilead, and Galapagos. All other authors report no conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Concept/design: Peacock, Wolfe. Data analysis/interpretation: Beekmann, Polgreen, Wolfe, Peacock. Drafting article: Peacock, Wolfe. Critical revision of article: Wolfe, Peacock, Beekmann, Polgreen, Winthrop. Approval of article: All authors. Statistics: Beekmann. Funding secured by: Polgreen. Data collection: Beekmann, Polgreen, EIN.
Prior presentation. The results of this survey were presented in part as an oral abstract at the annual American College of Rheumatology meeting in San Diego, California, in November 2017 by R.M.W.
References
- 1. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016; 68:1–25. [DOI] [PubMed] [Google Scholar]
- 2. Singh JA, Cameron C, Noorbaloochi S, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet 2015; 386:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Korbeti IS, Ziakis PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58:1649–57. [DOI] [PubMed] [Google Scholar]
- 4. Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc 2007; 82:1052–9. [DOI] [PubMed] [Google Scholar]
- 5. Stern A, Green H, Paul M, et al. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev 2014; (10):CD005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolfe RM, Peacock JE Jr. Pneumocystis pneumonia and the rheumatologist: which patients are at risk and how can PCP be prevented? Curr Rheumatol Rep 2017; 19:35. [DOI] [PubMed] [Google Scholar]
- 7. Gupta D, Zachariah A, Roppelt H, et al. Prophylactic antibiotic usage for Pneumocystis jirovecii pneumonia in patients with systemic lupus erythematosus on cyclophosphamide: a survey of US rheumatologists and the review of literature. J Clin Rheumatol 2008; 14:267–72. [DOI] [PubMed] [Google Scholar]
- 8. Cettomai D, Gelber AC, Christopher-Stine L. A survey of rheumatologists’ practice for prescribing Pneumocystis prophylaxis. J Rheumatol 2010; 37:792–9. [DOI] [PubMed] [Google Scholar]
- 9. Pillai SK, Beekmann SE, Santibanez S, Polgreen PM. The Infectious Diseases Society of America Emerging Infections Network: bridging the gap between clinical infectious diseases and public health. Clin Infect Dis 2014; 58:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004; 350:2487–98. [DOI] [PubMed] [Google Scholar]
- 11. Wickramasekaran RN, Jewell MP, Sorvillo F, et al. The changing trends and profile of pneumocystosis mortality in the United States, 1999–2014. Mycoses 2017; 60:607–15. [DOI] [PubMed] [Google Scholar]
- 12. LoPiccolo J, Mehta SA, Lipson EJ. Corticosteroid use and Pneumocystis pneumonia prophylaxis. A teachable moment. JAMA Intern Med 2018; 178:1106–7. [DOI] [PubMed] [Google Scholar]
- 13. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents Available at: https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-opportunistic-infection/392/whats-new. Accessed 22 December 2018.
- 14. Maertens J, Cesaro S, Maschmeyer G, et al. ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 2016; 71:2397–404. [DOI] [PubMed] [Google Scholar]
- 15. Sowden E, Carmichael AJ. Autoimmune inflammatory disorders, systemic corticosteroids and Pneumocystis pneumonia: a strategy for prevention. BMC Infect Dis 2004; 4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ognibene FP, Shelhamer JH, Hoffman GS, et al. Pneumocystis carinii pneumonia: a major complication of immunosuppressive therapy in patients with Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:795–9. [DOI] [PubMed] [Google Scholar]
- 17. Chew LC, Maceda-Galang LM, Tan YK, et al. Pneumocystis jirovecii pneumonia in patients with autoimmune disease on high-dose glucocorticoid. J Clin Rheumatol 2015; 21:72–5. [DOI] [PubMed] [Google Scholar]
- 18. Vernovsky I, Dellaripa PF. Pneumocystis carinii pneumonia prophylaxis in patients with rheumatic diseases undergoing immunosuppressive therapy: prevalence and associated features. J Clin Rheumatol 2000; 6:94–101. [DOI] [PubMed] [Google Scholar]
- 19. Baulier G, Issa N, Gabriel F, et al. Guidelines for prophylaxis of Pneumocystis pneumonia cannot rely solely on CD4-cell count in autoimmune and inflammatory diseases. Clin Exp Rheumatol 2018; 36:490–3. [PubMed] [Google Scholar]
- 20. Park JW, Curtis JR, Moon J, et al. Prophylactic effect of trimethoprim-sulfamethoxazole for Pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018; 77:644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winthrop KL, Baddley JW. Pneumocystis and glucocorticoid use: to prophylax or not to prophylax (and when?); that is the question. Ann Rheum Dis 2018; 77:631–3. [DOI] [PubMed] [Google Scholar]
- 22. Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 2016; 75:1583–94. [DOI] [PubMed] [Google Scholar]