Abstract
Purpose:
Microsurgical intussusception vasoepididymostomy (VE) is well-established treatment option for obstructive azoospermia due to epididymal obstruction. In this study, we evaluated patency rates and complications of our modified longitudinal intussusception technique of microsurgical VE. We have modified the intussusception technique by taking only adventitia of epididymal tubule.
Methods:
This was a prospective, single-center (tertiary care center) study conducted from February 2008 to January 2016. Study patients were men aged more than 18 years with infertility due to azoospermia. All participants underwent microscopic VE with our modified intussusception technique. Patency rates, complications, and improvement in semen quality were assessed.
Results:
A total of 42 patients were included in the study and underwent unilateral VE using longitudinal intussusceptions technique. The mean age of the patients was 30.21 years. Of these 42 patients, 40 patients had congenital obstruction. Average operative time was 130.42 min. A total of 36 (85.7%) patients had motile sperms in the epididymal fluid. Patency at 3 months was observed in 25 (62.5%) patients with an average sperm count of 17.1 million/mL. Only two patients (5%) had hemotoma at the site of surgery.
Conclusion:
Our modified technique of microsurgical longitudinal intussusception VE using epididymal adventitial stitch showed a reasonable patency rate after surgery.
Keywords: Azoospermia, infertility, intussusception, microsurgical vasoepididymostomy
INTRODUCTION
Infertility is defined as the inability of the sexually active, noncontracepting couple to achieve pregnancy within 1 year. The disease of infertility affects approximately 15% of couples, resulting in nearly one of six childless.[1] As per WHO in 2010, an estimated 48.5 million couple worldwide were infertile.[2] Azoospermia is the absence of spermatozoa or spermatogenic cells in at least two samples of semen. The European Association of Urology guidelines on male infertility defined obstructive azoospermia as “absence of both spermatozoa and spermatogenic cells in semen and postejaculate urine due to bilateral obstruction of the seminal ducts.”[3] Male infertility best practice policy committee of the American Urology Association reported that around 15% of infertile men present with azoospermia and 40% of these patients have ductal obstruction.[4] Although the site of obstruction can be anywhere along the path of sperm through a male reproductive system, the obstruction at epididymal level is among the most common causes of obstructive azoospermia.
Sperm retrieval for in vitro fertilization (IVF) and vaso-epididymal anastomosis (VEA, microsurgical reconstruction by vasoepididymostomy [VE]) are the two treatment options available for obstructive azoospermia due to epididymal tubular obstruction. Outcomes of microsurgery for obstructive azoospermia also depend on the technical expertise and experience of the surgeon. Microsurgery for obstructive azoospermia is technically demanding procedure, and the surgical expertise of the surgeon plays a crucial role in the determining the outcome of the surgery. However, microsurgical VE is considered more cost-effective than sperm retrieval and IVF/intracytoplasmic sperm injection (ICSI). With advances in the sperm retrieval technique and the introduction of ICSI in the early 1990s,[5] the live delivery rate of assisted reproduction technique has improved significantly. However, assisted reproduction technique increases the risk of ovarian hyperstimulation, multiple gestations, prematurity, lower birth rates, and increased perinatal morbidity.
Over the period of time, the technique of VE has undergone tremendous improvement. Various anastomosis techniques such as end-to-end, end-to-side, and intussusceptions techniques are described by various researchers. The end-to-end technique of VE had the disadvantage of difficult hemostasis, difficulty in identifying proper tubule for anastomosis, and sacrifice of vasal blood supply of vas from the inferior epididymal artery. The end-to-side VE technique is less traumatic and relatively bloodless, but the disadvantage of this technique is that it is difficult to place a suture in collapsed tubules. Thus, intussusception technique of VE came up with better of anastomosis results and ease of performance. Use of microscope resulted in successful anastomosis with excellent precision but with long learning duration.
Silber in 1978 described the technique of microscopic anastomosis of the inner lumen of the vas deferens directly to the epididymal tubule.[6] Berger described original intussusception technique using three double-arm sutures in triangular fashion.[7] In the year 2000, Marmar published a paper and suggested placing two needles simultaneously transversely in the epididymal tubule to avoid leakage of epididymal fluid and collapse of tubule.[8] Chan et al. described the technique of longitudinal intussusceptions in 2005.[9] Monosky developed a single-arm technique of VEA in 2007, which had similar efficacy to that of the double-arm technique.[10] The short-term outcomes of this microscopic intussusceptions' VE are superior with a patency rate of 78%–100%.[7,8,11]
We have modified the intussusception technique of microscopic VE by taking only adventitia of epididymal tubule. This technique has proposed advantages: (a) no leakage of epididymal fluid and resultant prevention of collapse of the tubule, (b) improved watertight anastomosis, and (c) improved patency results.
In this paper, we are reporting outcomes of our series in the form of patency rates and complications of our modified longitudinal intussusception technique of microsurgical VE. We also studied the improvement in semen quality (sperm count, progressive motility, and sperm morphology).
METHODS
Study design
This was a prospective, single-center (tertiary care center) study conducted from February 2008 to January 2016. Study patients were men aged more than 18 years with infertility due to azoospermia. The inclusion criteria for VE were documented azoospermia on at least 3 consecutive semen sample collected 6 weeks apart with normal volume, pH and presence of fructose, normal size testes, normal or marginally raised follicle-stimulating hormone (FSH), no history of paternity, at least one palpable vas deference, normal transrectal ultrasonography, and stable female partner.
The study protocol was reviewed and approved by the institutional ethics committee and scientific committee of our institute. The study was conducted in accordance with International Conference on Harmonization Good Clinical Practice and ethical principles that have their origin in Declaration of Helsinki. Each study participant provided written informed consent before any study-related procedure.
In this study, all microscopic VE were performed unilaterally by one surgeon with our modification of intussusception technique. We decided to operate only on one side because in case if we fail with our approach, we would like other center to take over the case for the opposite side procedure. All patients stayed overnight in our hospital as they underwent spinal anesthesia. They were discharged on the first postoperative day. Patients were advised to avoid ejaculation for the next 6 weeks postoperatively. Semen analysis was done at 3-month, 6-month, and 1-year visit. Patency was defined by the presence of sperm on semen examination. Semen analysis parameter such as sperm count, progressive motility, and sperm morphology were noted.
Surgical technique
Testicular aspiration biopsy was done on the operative side before the surgery to confirm normal spermatogenesis. No genetic studies were conducted for karyotyping and identification of Y chromosomal microdeletions.
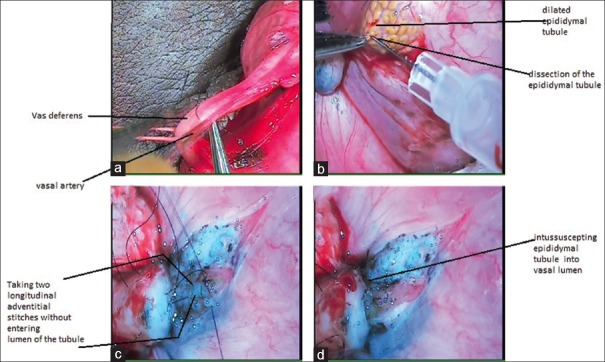
After obtaining written informed consent, scrotal exploration was performed under regional anesthesia. Testis and epididymis were exposed, and vas deferens was identified. Vasal artery was ligated with 8-0 Ethilon [Figure 1a]. As we are disconnecting vas deferens totally, bleeding from the vassal artery can obscure vision. Cauterizing this artery can damage vas deferens in vicinity. Hemisection of vas was done near epididymis. Patency of vas was confirmed with instillation of normal saline through vassal lumen. If this went smoothly, vasography was not performed. In some cases where resistance was found while instilling normal saline, vasography was done with diluted radiopaque contrast to confirm vassal patency. After confirming the patency of vas, it was transacted completely. Methylene blue was instilled on the cut end of vas deferens to delineate mucosa from muscularis.
Figure 1.
Surgical technique, (a) ligation of vasal artery with Ethilon 8-0, (b) incision over tunica over epididymis to expose underlying tubule, (c) two longitudinal stitches were taken through adventitia of epididymal tubule, which were later passed through vasal lumen, (d) intussuscepted epididymal tubule into distal vasal lumen
Tunica albuginea was incised over epididymis to expose underlying dilated tubules [Figure 1b]. Single dilated tubule was dissected. Two sutures with 10-0 Ethilon (double arm; 5 cm microtip needle) were taken longitudinally through the adventitia of tubule without entering its lumen [Figure 1c]. Tubule was punctured longitudinally in-between two sutures. Escaping tubular fluid was examined under 40× magnification. The presence of sperms, their number, and motility were noted. If sperms were present, then epididymal tubule was intussuscepted into vasal lumen with two sutures which are used to invaginate the tubule in the vas [Figure 1d]. Reinforcing sutures were taken between adventitia of vas and tunica of the epididymis. The tunical sac was closed with 4-0 Vicryl suture material, and the testis was reposited back; the scrotal incision was closed in layers.
RESULTS
A total of 54 patients with infertility due to azoospermia were screened, of which 42 patients had obstructive azoospermia due to obstruction at the level of the epididymis and were included in the study. Twelve patients had absent sperm in the epididymal fluid on scrotal exploration and were excluded. On the evaluation of etiology, of 42 patients, 40 had a congenital obstruction and 2 had the inflammatory obstruction [Table 1]. Obstruction was considered congenital by ruling out history of tuberculosis or any kind of genitourinaray infection in the past.
Table 1.
Baseline demographics and clinical characteristics (n=40)
| Parameter | n |
|---|---|
| Age (years), mean | 30.21 |
| Etiology, n (%) | |
| Congenital obstruction | 40 |
| FSH value (IU/L), mean | 5.48 |
| Operative time (min), mean | 130.42 |
| Anastomosis, n (%) | |
| Right side | 23 (57.5) |
| Left side | 17 (42.5) |
| Site of vaso-epididymal anastomosis, n (%) | |
| Head | 5 (12.5) |
| Body | 29 (72.5) |
| Tail | 6 (15) |
FSH: Follicle-stimulating hormone
We had considered patient with congenital obstruction only for further discussion. The mean age of the patients was 30.21 years (range: 24–37 years). On examination, 30 patients had turgid epididymis and 10 patients had normal sized epididymis. Vas was palpable bilaterally in all 40 patients. All 40 patients underwent unilateral VE using our modified longitudinal intussusceptions technique.
The mean FSH value was 5.48 IU/L with a range of 2.1–7.6 IU/L. On semen examination, the mean semen volume was 2.15 ml with a minimum volume of 1.5 ml and maximum of 3 ml. Fructose was present in semen of all patients, and spermatozoa was absent in the semen of all patients before surgery. All patients had normal testis size, volume and normal seminal vesicles and ejaculatory duct on transrectal ultrasonography.
The two-stitch intussusception VE technique was used with our modification of taking only adventitia of epididymal tubule in all patients. Average operative time was 130.42 min (range: 100–160 min). Anastomosis was done on the right side in 23 patients, and 17 patients were operated on the left side. On scrotal exploration, 34 (85%) patients had motile sperms in the epididymal fluid on microscopic examination while 6 patients showed nonmotile sperm. The site of VEA was head in five (12.5%), body in 29 (72.5%), and tail in six (15%) patients.
Out of 40 operated patients, 25 (62.5%) patients had patency on follow-up at 3 months with an average sperm count of 1761 million/ml with minimum sperm count – 10 million and the maximum – 24 million/mL. Among these 25 patients, average progressive was motility seen in 24 patients, and the average normal morphology was seen in all 25 patients. The site-specific patency rate of VE was two out of five (40%) at the head, 18 out of 29 (62.06%) at the body, and five out of six (83.33%) at the tail [Table 2].
Table 2.
Summary of study outcomes (n=40)
| Parameter | n |
|---|---|
| Patency at 3 months, n (%) | 25 (62.5) |
| Site specific patency rate of vasoepididymostomy, n (%) | |
| Head | 2 (40) |
| Body | 18 (62.06) |
| Tail | 5 (83.3) |
| Patency at 1 year, n (%) | 20 (50) |
| Natural pregnancy rate, n (%) | 6 (15) |
| Hematoma, n (%) | 2 (5) |
On follow-up at 1 year, 20 patients had patency of VE as shown by the presence of spermatozoa on semen analysis [Table 2]. No sperm was found in ejaculate at 1 year in 5 patients who had shown patency at 3 months on semen analysis. Only two patients (5%) had developed hematoma at the site of surgery which was managed conservatively. No patients developed sperm granuloma during the follow-up period. Natural pregnancy rate at 1 year without assisted reproductive technique was 15% (six out of 40).
DISCUSSION
Microsurgical intussusception (MIV) VE is well-established treatment option for obstructive azoospermia due to epididymal obstruction. With recent advances in assisted reproductive techniques such as ICSI, which tend to bypass the need to correct male factor infertility, the number of patients undergoing VEA has decreased over the last few years. However, the role of VEA to correct the basic pathology resulting in infertility and giving a long-term solution to patients is undebatable. Hence, microsurgical VEA has made the permanent place in treatment armamentarium for obstructive azoospermia even in the era of IVF/ICSI.
With the use of microsurgical techniques, accurate approximation of vasal lumen with specific epididymal tubule is made possible. Berger introduced the original triangulation end-to-side invagination technique of VE.[7] This microsurgical VE technique was further modified by Marmar and then by Chan.[8,9] Nowadays, robot-assisted microsurgical is also being performed with more precision of anastomosis. As the technique of microsurgical VE is refined over a period of time, the success rate of surgery in terms of patency rate and pregnancy rate has improved.
Marmar was the first to use two suture techniques for invagination VEA.[8] He had achieved excellent patency results of 78% in bilateral VEA and 86% in unilateral VEA. Later in 2005, Chan et al. published their study of 68 cases of intussusceptions' VEA and reported the overall patency rate of VEA as 84%.[9] Also, in another study, McCallum et al. compared the patency rates of conventional end-to-side and MIV VE in an animal model and found MIV is superior to conventional VE in terms of patency rates.[12] Various other preliminary studies have shown comparable patency rates in MIV VEA [Table 3].[7,8,13,14,15,16,17]
Table 3.
Summary of previous studies and patency rates
| Study (year) | Technique | Number of patients | Patency rate |
|---|---|---|---|
| Kumar et al., 2006[13] | Longitudinal VE | 29 | 48% (at 3 months) |
| Neto et al., 2016[14] | Longitudinal intussusception microscopic VE | 754 | 81% (at 3 months) |
| Huang et al., 2017[15] | Two stitch longitudinal intussusception | 96 | 79.5% (at 6 months) |
| Silber, 1989[16] | Specific tubule direct VE | 190 | 78% |
| Berger, 1998[7] | Triangulation end-to-side VE | 12 | 92% (at 3 months) |
| Marmar, 2000[8] | Simultaneous two suture (modified bilateral VE, n=9; combined unilateral VE with unilateral vasovasostomy, n=7) | 16 | 81.3 (at 3 months) |
| Zhao et al., 2013[17] | Modified single-arm suture | 17 | 58.8% (at 3 months) |
| Present study | Intussusception technique of microscopic VE by taking only adventitia of epididymal tubule | 40 | 62.5% (at 3 months) |
VE: Vasoepididymostomy
In our study, we used two-stitch intussusception VE technique with a modification of taking only adventitia of epididymal tubule. With help of these sutures, epididymal tubule was intussuscepted into vasal lumen. This technique has the following advantages.
Since the sutures are not in the lumen of the tubule, the chances of anastomotic stricture are also less
The intussusception of the epididymal tubule in the vas makes anastomosis watertight
Eventually, the distal tubule gets absorbed. Thus, the anastomosis is more physiological. Distal tubule acts as a tamponade
There is no leakage of sperms from the anastomosis; hence, the chances of sperm granuloma are also less.
In our study, patency rate at 3-month follow-up was 62.5%. Body and tail region VE have shown higher patency rates than head region VE. Lower patency rate in our study can be explained by epididymal dysfunction. Testicular sperm is immotile, and it becomes motile and functional after epididymal transits. Long-standing obstruction may lead to epididymal dysfunction and the poor result of microsurgical reconstruction.
In this study, 15% of patients were able to achieve paternity without any assisted reproductive technique. The reasons for achieving low paternity rates despite 62.5% patency rate can be stated as low sperm count, poor-quality sperms, the presence of antisperm antibody, female partner factors contributing to infertility, and short duration of follow-up.
However, sperms from ejaculated semen of patients with patent VE can be used for intrauterine insemination or ICSI. This will help to achieve pregnancy even if low count, poor-quality sperms are present in the semen or coexisting female infertility factors are present. As fresh sperms are retrieved in each ejaculate after successful VE, the painful surgical retrieval of sperms by other methods for each IVF cycle is avoided. Long-term patency rates of VE are not available; thus, sperms can be isolated and cryopreserved if required. Previous studies on the microsurgical technique of VE are suggestive of advantages such as less number of sutures, less operative time, less bleeding, and fewer occurrences of resulting complications, which are comparable to results in our study.
Authors also acknowledge the following limitation of the study. The long-term follow-up in microsurgical VE was difficult as many patients do not follow up if they achieve paternity either naturally or by assisted reproduction technique. Hence, the long-term patency rate of MIV VE is not available in previous studies and also in our study.
CONCLUSION
For men with obstructive azoospermia, surgical reconstruction is an acceptable management option. It is an effective treatment option for patients with epididymal obstruction when compared with other techniques. Our modified technique of microsurgical longitudinal intussusception VE using epididymal adventitial stitch showed a reasonable patency rate after surgery. This technique helps to overcome the problem of tubular leakage and collapse.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertil Steril. 2002;77:873–82. doi: 10.1016/s0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Sexual and Reproductive Health: Infertility is a Global Public Health issue. World Health Organization; [Last accessed on 2018 May 25]. Available from: http://www.who.int/reproductivehealth/topics/infertility/perspective/en/ [Google Scholar]
- 3.Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A, Weidner W, et al. EAU guidelines on male infertility. Eur Urol. 2005;48:703–11. doi: 10.1016/j.eururo.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Jarow J, Sigman M, Kolettis PN, Lipshultz LR, McClure RD, Nangia AK, et al. The Management of Obstructive Azoospermia: AUA Best Practice Statement. 2010. [Last accessed on 2018 May 25]. Available from: https://www.auanet.org/documents/education/clinical-guidance/Male-Infertility-c.pdf .
- 5.Shin DH, Turek PJ. Sperm retrieval techniques. Nat Rev Urol. 2013;10:723–30. doi: 10.1038/nrurol.2013.262. [DOI] [PubMed] [Google Scholar]
- 6.Silber SJ. Microscopic vasoepididymostomy: Specific microanastomosis to the epididymal tubule. Fertil Steril. 1978;30:565–71. [PubMed] [Google Scholar]
- 7.Berger RE. Triangulation end-to-side vasoepididymostomy. J Urol. 1998;159:1951–3. doi: 10.1016/S0022-5347(01)63205-1. [DOI] [PubMed] [Google Scholar]
- 8.Marmar JL. Modified vasoepididymostomy with simultaneous double needle placement, tubulotomy and tubular invagination. J Urol. 2000;163:483–6. [PubMed] [Google Scholar]
- 9.Chan PT, Brandell RA, Goldstein M. Prospective analysis of outcomes after microsurgical intussusception vasoepididymostomy. BJU Int. 2005;96:598–601. doi: 10.1111/j.1464-410X.2005.05691.x. [DOI] [PubMed] [Google Scholar]
- 10.Monoski MA, Schiff J, Li PS, Chan PT, Goldstein M. Innovative single-armed suture technique for microsurgical vasoepididymostomy. Urology. 2007;69:800–4. doi: 10.1016/j.urology.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 11.Meng MV, Turek PJ. Comparison of standard and invagination vasoepididymostomy techniques. Fertil Steril. 2000;74:S88. [Google Scholar]
- 12.McCallum S, Li PS, Sheynkin Y, Su LM, Chan P, Goldstein M. Comparison of intussusception pull-through end-to-side and conventional end-to-side microsurgical vasoepididymostomy: Prospective randomized controlled study in male wistar rats. J Urol. 2002;167:2284–8. [PubMed] [Google Scholar]
- 13.Kumar R, Gautam G, Gupta NP. Early patency rates after the two-suture invagination technique of vaso-epididymal anastomosis for idiopathic obstruction. BJU Int. 2006;97:575–7. doi: 10.1111/j.1464-410X.2006.05952.x. [DOI] [PubMed] [Google Scholar]
- 14.Neto F, Ayangbesan A, Najari B, Bach P, Gottesdiener A, Li P, et al. Comparing vasoepididymostomy technique outcomes: Longitudinal intussusception vasoepididymostomy (LIVE) versus other techniques. J Urol. 2016;195:e223. [Google Scholar]
- 15.Huang W, Lu C, Huang I, Lin AT, Chen K. Scrotal exploration and microsurgical vasoepididymostomy in azoospermic patients due to non-vasectomy, non-traumatic etiologies. Transl Androl Urol. 2017;6(Suppl 3):AB023. [Google Scholar]
- 16.Silber SJ. Results of microsurgical vasoepididymostomy: Role of epididymis in sperm maturation. Hum Reprod. 1989;4:298–303. doi: 10.1093/oxfordjournals.humrep.a136892. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Deng CH, Sun XZ, Chen Y, Wang WW, Zhao LY, et al. A modified single-armed technique for microsurgical vasoepididymostomy. Asian J Androl. 2013;15:79–82. doi: 10.1038/aja.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]