Abstract
A common health-care problem worldwide, urinary tract infection (UTI), represents a disease of significant impact on every country's economy, being the most common cause of hospitalization among elderly people and the most common cause of antibiotic prescription in primary care. Diagnosing and managing upper and lower UTI have always been a challenge to physicians, given its high prevalence, risk of recurrence and improper treatment, and the fact of worldwide increase in antibiotic resistance, necessitating implementation of a proper antibiotic stewardship. Urinary infections are twice more likely to occur in females compared to males and its prevalence increases with increasing age. The following is a comprehensive review paper about UTI in females, discussing the various factors leading to a complicated infection. The various etiologies and microbiologies of UTI are also highlighted. In addition to various usual antibiotic regimens for treating UTI, a significant number of nonantimicrobial treatment modalities are highlighted and described in this manuscript, including the novel use of intravesical antibiotics and vaccines for suppression treatment. Finally, a pathway is suggested for the proper diagnosis and treatment that ensures antibiotic stewardship in order to decrease long-term complications.
Keywords: Antibiotics, intravesical, nonantimicrobial therapy, pregnancy, stewardship, urinary tract infection, vaccines, women
INTRODUCTION
Urinary tract infection (UTI) is a common complaint encountered in outpatient setting, whether primary care or specialist clinic. It is imperative for the clinician to have a comprehensive grasp on the epidemiology, physiology, pathophysiology, and treatment strategies of UTIs. Another frequently encountered entity, especially in the specialty clinics, is resistant bacteria causing UTI and recurrent UTIs. This challenging entity is further complicated with the increasing rates of bacterial resistance and the increasing fear of extended spectrum beta-lactamase and multidrug resistant organisms. The authors reviewed UTI in females with emphasis on antimicrobial and non-antimicrobial treatment strategies.
EPIDEMIOLOGY
Urinary tract infection (UTI) is a significant health-care problem worldwide, often seen in outpatient clinics, emergency department visits, as well as in hospitalized patients. Its estimated incidence approaches 150 million new cases per year.[1] The treatment and diagnosis of UTI accounts for an approximate 6 billion US dollars of expenditures. Bladder infections or cystitis alone accounts for >10 million office visits and 1 million emergency department visits[1,2] and >2 billion dollars as annual health-care cost in the US alone due to various prescriptions and diagnostic tests.[3] A UTI is twice more likely to occur in women than men over all age groups[3] and accounts for 1.2% of all office visits by women.[4] A third of women are diagnosed with a UTI before the age of 24 years and half develop at least one episode by 35 years of age.[5] Up to 70% of women will suffer from a UTI during their lifetime, and of those, 30% will have recurrent UTIs (rUTIs).[6]
Urinary infections carry a big toll on an individual's health including his mental health and sense of well-being. More than half of patients with UTI suffer from clinical depression and 38.5% suffer from anxiety, with a significant improvement in the quality of life after proper treatment and prophylaxis.[7]
DEFINITIONS
A UTI is an inflammatory response at the level of the urothelium to fight a bacterial infection. A UTI is almost always associated with bacteriuria, the presence of bacteria in urine, and pyuria, the presence of white blood cells in the urine. Bacteriuria can be present without pyuria, which could be due to bacterial contamination or aseptic technique in the urine collection. On the contrary, pyuria can be present without bacteriuria indicating an inflammatory process of the urothelium such as a urinary stone or a malignancy.[8] The differentiation between complicated [Table 1] and uncomplicated UTI[9] has clinical importance for duration and type of treatment that will be tackled in the discussion part later on. In general, uncomplicated UTI is present in patients with no anatomical or functional abnormalities in their urinary tract system.
Table 1.
Factors suggesting a complicated urinary tract infection[9]
| Factors suggesting a complicated UTI (structural/functional abnormality in the urinary tract) |
| Urinary tract obstruction |
| Pregnancy |
| Immunosuppression |
| Fever |
| Catheterization |
| Renal insufficiency |
| Diabetes |
| Men |
| Prolonged symptoms (>1 week) |
| Failure to respond to medical therapy |
| Persistent bacteria after appropriate treatment |
UTI: Urinary tract infection
UTI can be characterized into either first, unresolved, or recurrent infections. A first UTI is one that occurs in a person with no previous or a remote infection. An unresolved UTI is a re-infection with the same bug and similar antibiogram to a previous UTI treated with appropriate antibiotics. A rUTI occurs after resolution of the previous infection.[8] Conflicting definitions for rUTIs exist; however, most specialists agree that to be considered recurrent, the patient needs to have suffered more than two episodes in the last 6 months or more than three episodes in the past year.[10,11]
Another category of UTIs is a catheter-associated UTI (CAUTI). It is defined as the presence of signs or symptoms of UTI in a patient with indwelling urethral, suprapubic, or even intermittent catheterization with a significant presence of bacteriuria. CAUTI is a serious iatrogenic infection and associated with increased morbidity and mortality in hospitalized and nursing home care patients.[12] Elaborating on CAUTI is beyond the spectrum of the manuscript; however, the authors recognize that UTI from indwelling catheters is a serious complication and must be prohibited by decreasing unnecessary catheterization and abiding by a proper sterile technique while catheterizing patients.[13]
ETIOLOGY
For a urinary infection to occur, there are many superimposing factors that interplay including host factors, inoculum size, and the virulence of the infecting bug. The first event that leads to a UTI is the inoculation. The most common theorem for inoculation is the ascending route. Enteric bacteria colonize the perineum and ascend into the urethra and bladder.[14]
As for the recurrence of a urinary infection, multiple factors play a role. On the microbiological level, one theory is the decrease of peroxide-producing lactobacilli, predisposing to increased colonization with pathogenic enteric bacteria.[15] Another theorem is the formation of intracellular clusters of bacteria that are not sensitive to antibiotics, while others postulate a change in the glycosaminoglycan barrier of the urothelium that makes an individual more susceptible to enteropathogenic infection.[16]
On an individual level, the risk factors for rUTI vary among young, middle-aged, and elderly women [Table 2]. In young women, spermicide use and frequency of sexual intercourse are the main risk factors evidenced by increased urethral and vaginal colonization.[17] In contrast, older women's predisposing risk factors are high urinary residue, atrophic vaginitis, and cystocele.[18]
Table 2.
Risk factors associated with urinary tract infections and recurrent urinary tract infections[17,18,19]
| Reduced urine flow | Promoted colonization | Facilitated ascent |
|---|---|---|
| Urinary outflow obstruction (calculus, stricture) | Sexual activity (increases inoculation) | Catheterization |
| Atonic bladder | Spermicide (increases binding) | Urinary incontinence |
| Inadequate fluid intake | Estrogen depletion (increases binding) | Fecal incontinence |
| High urine residue (causes ischemia of the bladder wall) | Antimicrobial (decreases indigenous flora) | Vaginal and urethral mucosal atrophy |
| Genetic factors (allowing better adherence of bacteria to urothelium) |
MICROBIOLOGY OF URINARY TRACT INFECTION
A urinary infection is rarely secondary to an underlying structural abnormality but rather the interaction between uropathogens and the normal urothelium. This interaction occurs as a result of the colonization of the vaginal and periurethral area with uropathogens originating from the gut. For unknown host factors, probably genetic, women who tend to have rUTI have an inclination to have a prolonged and heavier colonization with uropathogens.[19]
One of the main bacterial virulence features is the binding of the uropathogen to the mucosal cellular layer. It has been well studied that Escherichia coli type 1 fimbria is heavily associated with cystitis, and other pathogenic-fimbriated strains are associated with pyelonephritis. Furthermore, these pathogenic fimbriae are associated with persistent colonization of the urothelium and eliciting an inflammatory response.[20,21] It has been also theorized that these bacteria can mature into biofilms in the urothelial barrier to cause recurrence of infections and elude the host immune system.[22]
NONANTIMICROBIAL THERAPY
The rationale behind nonantimicrobial therapy stems from two main drawbacks of the antimicrobial prophylaxis for rUTI. The emergence of resistant strains in the urine and failure to fully eradicate microorganisms are always crucial to take into consideration for any antimicrobial therapy.[23]
Urinary alkalization has been proposed as an intervention to decrease rUTI. It is achieved by administration of alkalinizing agents such as potassium citrate. A Cochrane meta-analysis found multiple trials adopting urinary alkalinization as a method to avoid urinary infection. Nonetheless, none of those trials reached a significant conclusion to adopt such therapy[24]
Another preventive therapy proposed is utilizing Lactobacillus probiotics. The rationale is the formation of vaginal barrier to pathogenic bacteria. Conflicting results have been published with no concrete conclusions[25]
Cranberry has long been associated with the treatment of rUTI. It is composed of shrubs from the subgenus Oxycoccos and possesses antioxidative properties as well as inhibiting the binding of P-fimbriae of uropathogens to the urothelium.[26] A 2012 Cochrane review showed that there was no significant reduction in the incidence of UTI in the subgroup of patients with recurrent cystitis using cranberry products[27]
Topical estrogens have been extensively investigated and have been shown to decrease rates of rUTI. With the decrease in circulating estrogen, physiologically with aging, there is a decrease in vaginal Lactobacillus and increased risk of colonization by uropathogens such as enterococci and Gram-negative rods.[28] Recent Cochrane analysis shows that topical estrogen decreases the risk of rUTI without increasing risk for breast or endometrial cancers in women, but does have local side effects like irritation. Alternatively, systemic estrogens do not decrease rUTI rates.[29] When treating with estrogen, clinicians have to be aware of contraindications including endometrial and breast malignancies, thromboembolic events, or liver dysfunction.[18]
VACCINES
Vaccines have been proposed with the concept that they may trigger a patient's innate and humoral immune response against urinary pathogens.[30] Different formulations have been proposed, both vaginal and oral, with the oral formulation being the most widely used and investigated. Uro-Vaxom, or OM-89, is an oral vaccine, encompassing 18 different strains of lyophilized lysates of E. coli. A relatively recent meta-analysis on Uro-Vaxom has confirmed its efficacy for the treatment of rUTI.[31] The administration protocol adopted is usually 1 capsule per day for 90 days as induction treatment, then stopped for the next 3 months, and when intended as consolidation treatment, will be given 1 capsule per day for the first 10 days of every month, for 3 consecutive months. Vaginal vaccines are not in clinical practice yet and lack sufficient evidence.[25]
SPECIAL CONSIDERATIONS – PREGNANCY
UTI is of paramount importance in pregnant women, given that bacteriuria in the presence of pregnancy could lead to complications and miscarriage. UTI is the second most common pathology affecting pregnant women after anemia and, at the same time, the most common infection in this subgroup of patients. Approximately 5%–10% of women will develop UTI during their pregnancy, and it is estimated that out of all admissions of pregnant women, 5% is attributed to UTIs. UTIs, if mismanaged, can significantly increase the risk for pyelonephritis secondary to pregnancy-related adaptive changes in the urinary tract, resulting in serious maternal and fetal complications such as preterm labor, low birth weight, or maternal systemic infection.[32]
Bacteriuria during pregnancy may be classified as asymptomatic bacteriuria (ASB), infections of the lower urinary tract (cystitis), or infections of the upper urinary tract (pyelonephritis).[33] Our current focus will be on the formal classification.
ASB is the presence of a positive urine culture without any clinical manifestation. ASB incidence is estimated to be 2%–13% and similar in both pregnant and nonpregnant women.[34] One in three pregnant patients suffering from acute cystitis was found to be previously diagnosed with ASB.[35]
Unlike the general female population, ASB in pregnant women always requires treatment in order to reduce the possible maternal and fetal risks. Of particular interest and concern is the presence of Group B Streptococcus (GBS), specifically the Streptococcus agalactiae strain in urine culture, which is present in 2%–10% of all ASB cultures. Such strain carries a high risk for premature rupture of membranes, preterm labor, and significantly increases the risk for neonatal infection by 25-folds.[32]
In a recent meta-analysis conducted in Iran including 31 studies involving 20,309 patients, the prevalence of ASB in pregnant women was estimated to be 8.7% (95% confidence interval [CI]: 7.2–10.4), with the lowest and highest prevalence noted in the third trimester (6.1% [95% CI: 2.1–16.4]) and first trimester (11.7% [95% CI: 7.9–16.9]), respectively. The most common isolated microorganism involved in the etiology of UTI (61.6% [95% CI: 51.6–70.7]) and ASB (63.22% [95% CI: 51.2–73.8]) was E. coli.[36]
As such, European and North American clinical practice guidelines recommend screening for ASB as a routine pregnancy test. Antibiotic treatment of ASB in pregnant women is supposed to reduce maternal upper UTIs, precluding preterm labor.[37]
The present guidelines recommend the identification of GBS from swab samples collected from the vaginal vestibule and rectum of every pregnant lady at the 35–37th week of gestation; unless previous urine cultures during pregnancy showed S. agalactiae, then a follow-up culture is not recommended. Once identified at any stage during pregnancy, a peripartum antibiotic prophylaxis is needed, even in patients with negative swab cultures later on.[38]
ANTIMICROBIAL THERAPIES
Overview of antibacterial agents
The cornerstone of treatment of any bacterial infection, including a UTI, is antimicrobial therapy. Since UTIs are very common, especially in women, it is imperative that controlled use of antibiotics is initiated for treatment. It is the role of the clinician to treat his patients adequately while practicing proper antibiotic stewardship by adhering to practice guidelines. The type and duration of antimicrobial therapy depend on the site and severity of infection and host/bacterial factors. For example, in cases of prostatitis and epididymo-orchitis, proper tissue concentration of antimicrobials has to be taken into consideration. General treatment strategies to eliminate an infection are the judicious use of antibiotics, ensuring adequate hydration, and relieving any urinary obstruction or foreign bodies.
For acute uncomplicated UTI, the Infectious Diseases Society of America (IDSA) advocates the use of nitrofurantoin, trimethoprim-sulfamethoxazole (TMP-SMX), or fosfomycin for 3–5 days.[39]
Nitrofurantoin is a well-known medication to clinicians with a tolerable side effect. A well-known eradicator of E. coli, nitrofurantoin, is otherwise less active against other Gram-negative rods such as Klebsiella and Pseudomonas.[8] Although their incidences are rare, ordering physicians must keep in mind the possibility of detrimental, but rather infrequent, risk of pulmonary fibrosis and interstitial pneumonitis, especially in predisposing patients such as those with chronic obstructive pulmonary disease[40]
TMP-SMX has an excellent eradication rate of UTIs. The caveat in using this agent in clinical practice is the antibiogram and the resistance pattern noted in a specific region/institution being used in a sense that it is only recommended when the resistance rate is <20%.[39] Patients with sulfa allergy cannot receive this drug due to the SMX component
Fosfomycin is appropriate for treatment of an uncomplicated UTI; however, its activity might be inferior to other antimicrobials[39]
Quinolones have long been associated with the treatment of UTIs. However, for uncomplicated UTIs, specialists prefer refraining from its use due to increasing resistance rates in communities and the need to preserve it for more complicated UTIs.[39]
Failure of bacterial eradication
Follow-up urinary culture and urological evaluation are usually unneeded for patients who respond to first-line treatment of an uncomplicated UTI.[8] If a patient does not respond to first-line antimicrobials prescribed, many causes must be considered. The causes for inability to eradicate a simple UTI are (1) initial or acquired bacterial resistance, (2) different bacterial species infecting the urothelium, (3) azotemia, and (4) urologic structural abnormalities.[8] The most common cause, however, is bacterial resistance. In these cases, clinicians can obtain a second urine culture and might consider quinolones or cephalosporins.[8] Recently, extended-spectrum β-lactamase (ESBL)-producing bacteria have been emerging even in the ambulatory setting, with up to 7% of community-acquired UTIs due to ESBL-producing bacteria. In this situation, antibiotics must be tailored according to the urinary culture antibiogram, with utilization of carbapenems in certain situations.[40]
INTRAVESICAL ANTIBIOTICS
The fact behind intravesical use of antibiotics is novel and been extrapolated from other routes of administration, other than the usual oral or intravenous administration. It has been noted that the number of multidrug-resistant bacterial strains are increasing worldwide, and this poses a threat to clinicians along with the lack of development of new antibiotics. This has shifted attention to utilizing older class of drugs that were abandoned due to their toxicity when administered intravenously. The presence of those old class antibiotics in high concentration in body fluids is considered otherwise safe and has been made utilized in the form of aerosols to treat lung infections in patients with cystic fibrosis and intrathecal or even intraventricular administration to treat meningitis.
Instillation in the bladder for UTIs has been reported to be effective. An example would be intravesical instillation of colistin in treating UTI caused by Acinetobacter baumannii or Pseudomonas aeruginosa. Although the type of publications about intravesical instillation are either case reports or case series, intravesical instillation has shown to be effective in decreasing the rate of recurrent infections.[41,42]
Another antibiotic used in a similar fashion is gentamicin. A recent prospective trial from The Netherlands was published, encompassing an overnight intravesical instillation of gentamicin for 6 months in 63 adults with rUTIs caused by multidrug-resistant pathogens. The results were a reduction in the number of UTIs from 4.8 to 1.0 episodes, during the intravesical treatment. The resistance rate of the uropathogens decreased from 78% to 23%, and no clinically relevant side effects were observed.[43]
Also reported is a significant decrease in frequency of symptomatic urinary infections, along with respective emergency department visits and hospital admissions, for those with neurogenic bladder maintained on clean intermittent catheterization, with the use of intravesical antibiotic instillation.[44]
In general, intravesical antimicrobial instillation seems effective in treating rUTIs, especially in the short-term period, and would help clinicians in finding alternatives to treatment, especially in those who developed resistance to other forms of systemic treatment.[45]
ANTIMICROBIAL PROPHYLAXIS
The authors advocate the treatment of a UTI in patients with recurrent episodes only after taking a pretreatment urine culture. Choice of antibiotic therapy should be tailored according to the patient's previous cultures, local resistance patterns, patient's allergy status, side effect profile, as well as cost. When a patient is diagnosed with rUTI, we advocate the initiation of a 6–12-month course of low-dose antimicrobial agents. The IDSA advocates the use of a single strength TMP-SMX (40/200) or nitrofurantoin 50–100 mg daily. Even though quinolones (ciprofloxacin 250 mg daily) is proposed by the IDSA, we suggest preserving quinolones for complicated cases due to increased resistance pattern and its association with ESBL Enterobacteriaceae.[46] Behavioral modifications are as well of paramount importance in this population such as limiting use of spermicides, postcoital voiding, increasing fluid intake, and avoiding distention of the bladder.[25]
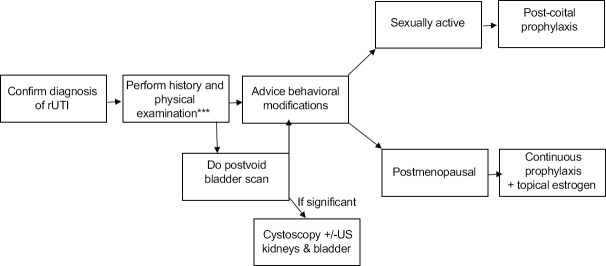
The authors propose a workup to identify causes of rUTI [Figure 1]. History taking can identify modifiable behaviors that can decrease the risk of recurrence [Table 3].[13,47,48,49] A physical examination can identify if there is a significant vaginal atrophy, which is a known cause of rUTI. Postvoid residual urine can be a nidus for bacterial overgrowth and inadequate eradication of bacteria.[50] Therefore, a postvoid bladder scan can identify a significant postvoid residual urine. If present, we suggest extending the workup with either a formal ultrasound examination of the kidneys and bladder and/or a diagnostic cystoscopy under local anesthesia.
Figure 1.
Recommended Algorithm for the Management of urinary tract infections in Women
Table 3.
Risk factors and Myths associated with rUTIs
| Pre-menopausal | |
|---|---|
| Modifiable | Nonmodifiable |
| Contraceptive use | Genetic |
| Frequent sexual intercourse | Congenital urinary tract anomalies |
| Family history of UTI | |
| Post-menopausal | |
| Atrophic vaginitis | Residual urine |
| Incontinence | Catheterization |
| Cystocele | History of UTI |
| Risk factors not proven to be associated with UTI (myths) | |
| Douching | |
| Hot baths | |
| History of sexually transmitted diseases | |
| Type of underwear | |
| Caffeine consumption | |
UTI: Urinary tract infection
ANTIBIOTIC STEWARDSHIP: THE PATHWAY TOWARD ADEQUATE TREATMENT AND PREVENTION
Antimicrobial stewardship compliance is of the utmost importance when treating patients with recurrent infection. First, the physician must abide by the duration of therapy of not >5 days for the treatment of an uncomplicated cystitis, since overtreatment not only increases resistance rates but also increases recurrences due to loss of protective flora.[51] We strongly discourage treatment of asymptomatic bacteriuria in the general population due to the risk of increased antimicrobial resistance.[52,53] More and more, we are encountering in our practice-resistant bugs that are mainly due to the misuse and overuse of antibiotics. Clostridium difficile infections of the colon have also been implicated in antibiotics for UTIs, more commonly cephalosporins and fluoroquinolones.[25] The IDSA does not recommend fluoroquinolones nor cephalosporins for the treatment of uncomplicated cystitis as first-line agents. These classes are associated with resistance- and ESBL-producing organisms and increase recurrence rates by changing the periurethral and vaginal protective flora.[51] However, the authors do acknowledge that these medications are indispensable for resistant bugs and in cases of complicated UTI. Therefore, it is the responsibility of all prescribers of antibiotics to take into consideration not only the eradication of infection but also the collateral damage in choosing the antibiotic duration.
SUMMARY AND RECOMMENDATIONS
Antimicrobial prophylaxis
Behavioral modifications are advised before initiation of continuous prophylaxis
Confirmatory with negative urine culture before starting continuous prophylaxis
Trial of nonantimicrobial measures should be tried prior to initiation
Choice of antimicrobial should be individualized
Postcoital prophylaxis is encouraged in sexually active women.
Nonantimicrobial interventions
Cranberry products have some efficacy in decreasing UTI rates
Intravaginal probiotics can be used
Uro-Vaxom has proven efficacy and can be tried
Avoid the use of spermicide for contraception
Vaginal estrogen is well studied and should be used for postmenopausal vaginal atrophy
Proper perineal care and wiping front to back.[54]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2006. Natl Health Stat Report. 2008;8:1–29. [PubMed] [Google Scholar]
- 2.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat. 13 2;11(169):1–38. [PubMed] [Google Scholar]
- 3.Foxman B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28:1–3. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Schappert SM. Ambulatory care visits of physician offices, hospital outpatient departments, and emergency departments: United States, 1995. Vital Health Stat. 1997;13(129):1–38. [PubMed] [Google Scholar]
- 5.Foster RT., Sr Uncomplicated urinary tract infections in women. Obstet Gynecol Clin North Am. 2008;35:235–48, viii. doi: 10.1016/j.ogc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Albert X, Huertas I, Pereiro, Sanfelix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;3:Cd001209. doi: 10.1002/14651858.CD001209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renard J, Ballarini S, Mascarenhas T, Zahran M, Quimper E, Choucair J, et al. Recurrent Lower Urinary Tract Infections Have a Detrimental Effect on Patient Quality of Life: A Prospective, Observational Study. Infect Dis Ther. 2015;4:125–35. doi: 10.1007/s40121-014-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDougal W, Wein A, Kavoussi L, Novick A, Partin A, Peters C, et al. Campbell-Walsh Urology. (10th Edition Review) 2012 [Google Scholar]
- 9.Dielubanza EJ, Mazur DJ, Schaeffer AJ. Management of non-catheter-associated complicated urinary tract infection. Infect Dis Clin North Am. 2014;28:121–34. doi: 10.1016/j.idc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Epp A, Larochelle A Urogynaecology Committee, Family Physicians Advisory Committee. Recurrent urinary tract infection. J Obstet Gynaecol Can. 2010;32:1082–90. doi: 10.1016/S1701-2163(16)34717-X. [DOI] [PubMed] [Google Scholar]
- 11.Nosseir SB, Lind LR, Winkler HA. Recurrent uncomplicated urinary tract infections in women: A review. J Womens Health (Larchmt) 2012;21:347–54. doi: 10.1089/jwh.2011.3056. [DOI] [PubMed] [Google Scholar]
- 12.Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307:637–42. doi: 10.1056/NEJM198209093071101. [DOI] [PubMed] [Google Scholar]
- 13.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–63. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 14.Foxman B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 15.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–9. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohsin R, Siddiqui KM. Recurrent urinary tract infections in females. J Pak Med Assoc. 2010;60:55–9. [PubMed] [Google Scholar]
- 17.Beerepoot MA, ter Riet G, Nys S, van der Wal WM, de Borgie CA, de Reijke TM, et al. Lactobacilli vs. antibiotics to prevent urinary tract infections: A randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704–12. doi: 10.1001/archinternmed.2012.777. [DOI] [PubMed] [Google Scholar]
- 18.Raz R. Urinary tract infection in postmenopausal women. Korean J Urol. 2011;52:801–8. doi: 10.4111/kju.2011.52.12.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfau A, Sacks T. The bacterial flora of the vaginal vestibule, urethra and vagina in premenopausal women with recurrent urinary tract infections. J Urol. 1981;126:630–4. doi: 10.1016/s0022-5347(17)54661-3. [DOI] [PubMed] [Google Scholar]
- 20.Wullt B. The role of P fimbriae for Escherichia coli establishment and mucosal inflammation in the human urinary tract. Int J Antimicrob Agents. 2003;21:605–21. doi: 10.1016/s0924-8579(02)00328-x. [DOI] [PubMed] [Google Scholar]
- 21.Bahrani-Mougeot FK, Buckles EL, Lockatell CV, Hebel JR, Johnson DE, Tang CM, et al. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol. 2002;45:1079–93. doi: 10.1046/j.1365-2958.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- 22.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–7. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 23.Franco AV. Recurrent urinary tract infections. Best Pract Res Clin Obstet Gynaecol. 2005;19:861–73. doi: 10.1016/j.bpobgyn.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 24.O'Kane DB, Dave SK, Gore N, Patel F, Hoffmann TC, Trill JL, et al. Urinary alkalisation for symptomatic uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2016;4:CD010745. doi: 10.1002/14651858.CD010745.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith AL, Brown J, Wyman JF, Berry A, Newman DK, Stapleton AE. Treatment and prevention of recurrent lower urinary tract infections in women: A Rapid review with practice recommendations. J Urol. 2018;200:1174–91. doi: 10.1016/j.juro.2018.04.088. [DOI] [PubMed] [Google Scholar]
- 26.Micali S, Isgro G, Bianchi G, Miceli N, Calapai G, Navarra M. Cranberry and recurrent cystitis: More than marketing? Crit Rev Food Sci Nutr. 2014;54:1063–75. doi: 10.1080/10408398.2011.625574. [DOI] [PubMed] [Google Scholar]
- 27.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raz P. Urinary tract infection in elderly women. Int J Antimicrob Agents. 1998;10:177–9. doi: 10.1016/s0924-8579(98)00024-7. [DOI] [PubMed] [Google Scholar]
- 29.Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;2:Cd005131. doi: 10.1002/14651858.CD005131.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Bauer HW, Alloussi S, Egger G, Blümlein HM, Cozma G, Schulman CC. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur Urol. 2005;47:542–8. doi: 10.1016/j.eururo.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Naber KG, Cho YH, Matsumoto T, Schaeffer AJ. Immunoactive prophylaxis of recurrent urinary tract infections: A meta-analysis. Int J Antimicrob Agents. 2009;33:111–9. doi: 10.1016/j.ijantimicag.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Szweda H, Jóźwik M. Urinary tract infections during pregnancy – An updated overview. Dev Period Med. 2016;20:263–72. [PubMed] [Google Scholar]
- 33.Glaser AP, Schaeffer AJ. Urinary tract infection and bacteriuria in pregnancy. Urol Clin North Am. 2015;42:547–60. doi: 10.1016/j.ucl.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Matuszkiewicz-Rowińska J, Małyszko J, Wieliczko M. Urinary tract infections in pregnancy: Old and new unresolved diagnostic and therapeutic problems. Arch Med Sci. 2015;11:67–77. doi: 10.5114/aoms.2013.39202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delzell JE, Jr, Lefevre ML. Urinary tract infections during pregnancy. Am Fam Physician. 2000;61:713–21. [PubMed] [Google Scholar]
- 36.Azami M, Jaafari Z, Masoumi M, Shohani M, Badfar G, Mahmudi L, et al. The etiology and prevalence of urinary tract infection and asymptomatic bacteriuria in pregnant women in Iran: A systematic review and meta-analysis. BMC Urol. 2019;19:43. doi: 10.1186/s12894-019-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angelescu K, Nussbaumer-Streit B, Sieben W, Scheibler F, Gartlehner G. Benefits and harms of screening for and treatment of asymptomatic bacteriuria in pregnancy: A systematic review. BMC Pregnancy Childbirth. 2016;16:336. doi: 10.1186/s12884-016-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verani JR, McGee L, Schrag SJ Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) Prevention of perinatal group B streptococcal disease – Revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 39.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–20. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 40.Syed H, Bachuwa G, Upadhaya S, Abed F. Nitrofurantoin-induced interstitial pneumonitis: Albeit rare, should not be missed. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-213967. pii: bcr2015213967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assis RE, Coelho I, Real A, Franca L, Araujo A, Pereira T, et al. Intra-Vesical Colistin for Pseudomonas aeruginosa Urinary Tract Infections. European journal of case reports in internal medicine. 2019;6:000996. doi: 10.12890/2019_000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giua R, Pedone C, Cortese L, Antonelli Incalzi R. Colistin bladder instillation, an alternative way of treating multi-resistant acinetobacter urinary tract infection: A case series and review of literature. Infection. 2014;42:199–202. doi: 10.1007/s15010-013-0507-y. [DOI] [PubMed] [Google Scholar]
- 43.Stalenhoef JE, van Nieuwkoop C, Menken PH, Bernards ST, Elzevier HW, van Dissel JT. Intravesical gentamicin treatment for recurrent urinary tract infections caused by multidrug resistant bacteria. J Urol. 2019;201:549–55. doi: 10.1016/j.juro.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Huen KH, Nik-Ahd F, Chen L, Lerman S, Singer J. Neomycin-polymyxin or gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on clean intermittent catheterization. J Pediatr Urol. 2019;15:178.e1–7. doi: 10.1016/j.jpurol.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Pietropaolo A, Jones P, Moors M, Birch B, Somani BK. Use and effectiveness of antimicrobial intravesical treatment for prophylaxis and treatment of recurrent urinary tract infections (UTIs): A systematic review. Curr Urol Rep. 2018;19:78. doi: 10.1007/s11934-018-0834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalhoff A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip Perspect Infect Dis. 2012;2012:976273. doi: 10.1155/2012/976273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316–22. doi: 10.5489/cuaj.11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raz R, Gennesin Y, Wasser J, Stoler Z, Rosenfeld S, Rottensterich E, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152–6. doi: 10.1086/313596. [DOI] [PubMed] [Google Scholar]
- 49.Quinlan JD, Jorgensen SK. Recurrent UTIs in women: How you can refine your care. J Fam Pract. 2017;66:94–9. [PubMed] [Google Scholar]
- 50.Bergamin PA, Kiosoglous AJ. Non-surgical management of recurrent urinary tract infections in women. Transl Androl Urol. 2017;6:S142–S152. doi: 10.21037/tau.2017.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneeberger C, Stolk RP, Devries JH, Schneeberger PM, Herings RM, Geerlings SE. Differences in the pattern of antibiotic prescription profile and recurrence rate for possible urinary tract infections in women with and without diabetes. Diabetes Care. 2008;31:1380–5. doi: 10.2337/dc07-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai T, Nesi G, Mazzoli S, Meacci F, Lanzafame P, Caciagli P, et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin Infect Dis. 2015;61:1655–61. doi: 10.1093/cid/civ696. [DOI] [PubMed] [Google Scholar]
- 53.Bartoletti R, Cai T, Wagenlehner FM, Naber K, Bjerklund Johansen TE. Treatment of Urinary Tract Infections and Antibiotic Stewardship. Eur Urol Suppl. 2016;15:81–7. [Google Scholar]
- 54.Ignatavicius DD, Workman ML. Medical-Surgical Nursing: Critical Thinking for Collaborative Care. (4th Edition) 2002 Single Volume. [Google Scholar]