Abstract
Background
Distant metastatic disease is frequently observed in inflammatory breast cancer (IBC), with a poor prognosis as a consequence. The aim of this study was to analyze the association of hormone receptor (HR) and human epidermal growth factor receptor-2 (HER2) based breast cancer subtypes in stage IV inflammatory breast cancer (IBC) with preferential site of distant metastases and overall survival (OS).
Methods
For patients with stage IV IBC, diagnosed in the Netherlands between 2005 and 2016, tumors were classified into four breast cancer subtypes: HR+/HER2−, HR+/HER2+, HR−/HER2+, and HR−/HER2−. Patient, tumor, and treatment characteristics and sites of metastases were compared. OS of the subtypes was compared using Kaplan-Meier curves and the log-rank test. Association between subtype and OS was assessed in multivariable models using logistic regression.
Results
In total, 744 eligible patients were included: 340 (45.7%) tumors were HR+/HER2−, 148 (19.9%) HR−/HER2+, 131 (17.6%) HR+/HER2+, and 125 (16.8%) HR−/HER2−. Bone was the most common metastatic site in all subtypes. A significant predominance of bone metastases was found in HR+/HER2− IBC (71.5%), and liver and lung metastases in the HR−/HER2+ (41.2%) and HR−/HER2− (40.8%) subtypes, respectively. In multivariable analysis, the HR−/HER2− subtype was associated with significantly worse OS as compared to the other subtypes.
Conclusion
Breast cancer subtypes in stage IV IBC are associated with distinct patterns of metastatic spread and display notable differences in OS. The use of breast cancer subtypes can guide a more patient-tailored staging directed to metastatic site and extend of disease.
Keywords: Inflammatory breast cancer, Breast cancer subtype, Survival, Metastases
Introduction
Inflammatory breast cancer (IBC) has the clinical appearance of inflammation of the breast with pathological evidence of malignancy. It comprises 1% of all breast cancers and is the most aggressive form of breast cancer [1].
Breast cancer in general can be categorized into four subtypes based on immunohistochemistry of the hormone receptors (HR), subdivided in estrogen receptor (ER) and progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) [2]. HER2-enriched (HR−/HER2+) and triple negative (HR−/HER2−) tumors in non-IBC have a worse breast cancer-specific survival in comparison with the other subtypes, although the introduction of targeted therapy for HER2-positive breast cancer has increased survival for this subtype [3, 4].
We recently demonstrated that HR/HER2-based breast cancer subtypes influence prognosis and treatment response in patients with IBC without distant metastases [5]. However, nearly 40% of patients with IBC are diagnosed with synchronous distant metastases (stage IV disease), and it is unknown what role the HR/HER2-based subtypes play in this stage [6].
Besides histological subtype, site of metastases at time of diagnosis also strongly influences prognosis of metastatic breast cancer, with bone metastases having a better prognosis compared to lung and liver metastases [7]. In patients with stage IV IBC as initial presentation, the correlation between breast cancer subtypes on both the preferential site of metastases and on OS has not been evaluated before.
While progress has been made in recent years, the survival of IBC remains poor. Both the rarity and the aggressiveness contribute to the difficulty in treating IBC [1]. Improving the understanding of distinct patterns of metastatic spread hopefully will lead to a better understanding of this fatal disease. Moreover, it might influence the diagnostic process for patients presenting with IBC and may be supportive to the multidisciplinary discussion which therapies are appropriate once distant disease has been diagnosed. The purpose of this study was to determine the association of breast cancer subtypes (HR/HER2-based) on preferential site of metastatic disease and overall survival (OS) in patients presenting with stage IV IBC.
Materials and methods
Data source
The most important data on cancer in the Netherlands are registered in the nationwide population-based Netherlands Cancer Registry (NCR), hosted by the Netherlands Comprehensive Cancer Organisation (IKNL). The NCR registers all newly diagnosed malignancies in the Netherlands, using the nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA) as main source of notification. Trained registrars from the IKNL directly collect data from the patient’s medical records. Morphology and differentiation are coded according to the International Classification of Diseases for Oncology (ICD-O), third edition [8]. Staging is coded according to the Tumor, Node and Metastasis (TNM) classification system. The specific edition depended on the year of incidence [9, 10]. With respect to IBC, the criteria used in the TNM system have not changed over time. Yearly linkage to the municipal administration database is used to verify the patient’s vital status and, if applicable, date of death. Follow-up has been completed until December 31, 2016. The privacy committee of the NCR has approved this study.
Patients and study variables
Patients, diagnosed from 2005 to 2016, with clinical T4dN0–3 M0 breast cancer were identified: diffuse erythema and edema (peau d’orange) involving a third or more of the skin of the breast. Patients with only a pathological T4d status without clinical T4d status were excluded. Patients were classified into four breast cancer subtypes, based on HR/HER2-status: HR+ (ER+ and/or PR+)/HER2−, HR+(ER+ and/or PR+)/HER2+, HR− (ER− and PR−)/HER2+, and HR− (ER− and PR−)/HER2−. Patients were excluded when data on HR and/or HER2 status were missing.
According to Dutch guidelines, ER/PR status had been determined with immunohistochemistry (IHC). At least 10% positive tumor nuclei were considered as a positive result. In the Netherlands, HER2 status was considered positive with an immunohistochemical score of 3+ (at least 10% of tumor cells with strong complete membrane staining) or amplification of the HER2 gene diagnosed with in situ hybridization (ISH) (at least 10% of tumor cells showing a ratio of HER2 probe to centromere chromosome 17 probe of > 2.2 or with single probe HER2 test when mean > 6 HER2 genes per tumor nucleus were detected) or with other amplification-based techniques, such as multiplex ligation-dependent probe amplification (MLPA). In case of an immunohistochemical score of 2+ (at least 10% of tumor cells with slight to moderate complete membrane staining; considered as an equivocal result), ISH or MLPA was performed. If in this case HER2 was found to be amplified, HER2 was considered positive. HER2 status was considered negative with an immunohistochemical score of 0 or 1+ or if ISH or MLPA showed no amplification of the HER2 gene. In the Netherlands, some variation in determining the HER2 status existed in the period 2005–2016 (especially the cutoff for amplification (> 2.2 or ≥ 2) in the double probe ISH test). For this study, the HER2 status as was registered in the NCR was used.
Metastases diagnosed within 3 months after the date of determination of the treatment plan were considered to be synchronous with the primary tumor and incorporated in initial staging. Different sites of metastases were analyzed: bone, lung, liver, and other and multiple organs affected.
Treatment modalities were analyzed. The use of trimodality treatment (combination of subsequent neoadjuvant chemotherapy, surgery, and adjuvant locoregional radiation therapy) was evaluated. Chemotherapy, endocrine therapy, and targeted therapy (trastuzumab) were reported as administered or not administered.
Statistical analysis
Tumor characteristics, site and number of metastasis, and treatment were compared between the different HR and HER2 subgroups using chi-squared tests for categorical variables and non-parametric approaches (Mann-Whitney U tests) for continuous variables. Fisher’s exact test was used to determine if non-random associations between two categorical variables in case of less than five patients per stratum existed. The p value was not calculated in case there were 0 cases in one or more strata. Follow-up was calculated until time of death or end of observation. OS was determined using Kaplan-Meier curves and breast cancer subtypes, and tumor localizations were compared using the log-rank test. To adjust for patient, tumor, and treatment-related characteristics, a multivariable Cox proportional hazard analysis was performed. Variables included were age, breast cancer subtype, nodal stage, histological tumor type and grade, and trimodality therapy, as these variables were significantly different between breast cancer subtypes and significantly influenced the outcome (p < 0.1). For all other analyses, a p value < 0.05 was considered as statistically significant.
Results
A total of 2235 patients with IBC were diagnosed in the Netherlands between January 2005 and December 2016 of whom 842 patients presented with stage IV IBC (33.3%) at diagnosis. Of these 842 patients, 98 patients were excluded due to an unknown HR or HER2 status, leaving 744 patients for inclusion in the present study. The 98 excluded patients with an unknown HR/HER2 less often underwent any form of treatment and were significantly older (data not shown). In 2005, the first year of registration in the database, 17.3% of patients had an unknown receptor status, but this was in later years low (range 4.1–11.2%). From 2005 onwards, the incidence of stage IV IBC is increasing (data not shown).
Breast cancer subtypes and tumor characteristics
Among the eligible patients, the distribution of breast cancer subtypes was as follows: 340 (45.7%) HR+/HER2−, 148 (19.9%) HR−/HER2+, 131 (17.6%) HR+/HER2+, and 125 (16.8%) HR−/HER2−. In the HR−/HER2− subtype, grade 3 tumors were found most frequently (29.6% versus 12.9–24.3%). HR+/HER2+ and HR−/HER2+ tumors were more often found in ductal cancer (90.8 and 89.9%, respectively) and HR+/HER2− tumors in lobular cancer (14.4%) (Table 1).
Table 1.
Patient, treatment, and tumor-related characteristics of all stage IV IBC patients per breast cancer subtype (n = 744)
| HR+/HER2− (n = 340) |
HR+/HER2+ (n = 131) |
HR−/HER2+ (n = 148) | HR−/HER2− (n = 125) |
p value | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Age, median (IQR) | 61 (52–73) | 60 (50–74) | 57.5 (50–69) | 62 (52–73) | 0.191 |
| Histological grade | |||||
| 1 | 6 (1.8) | 2 (1.5) | 0 (0.0) | 1 (0.8) | NC |
| 2 | 40 (11.8) | 13 (9.9) | 16 (10.8) | 8 (6.4) | |
| 3 | 44 (12.9) | 19 (14.5) | 36 (24.3) | 37 (29.6) | |
| Unknown* | 250 (73.5) | 97 (74.1) | 96 (64.9) | 79 (63.2) | |
| Histological type | |||||
| Ductal | 279 (82.1) | 119 (90.8) | 113 (89.9) | 110 (80.0) | 0.019 |
| Lobular | 49 (14.4) | 7 (5.3) | 10 (6.7) | 8 (6.4) | |
| Other | 12 (3.5) | 5 (3.8) | 5 (3.4) | 7 (5.6) | |
| Metastatic sites | |||||
| 1 | 167 (49.1) | 54 (41.2) | 74 (50.0) | 58 (46.4) | |
| 2 or more | 173 (50.9) | 77 (58.8) | 74 (50.0) | 67 (53.6) | 0.414 |
| Surgery | |||||
| Yes | 60 (17.7) | 24 (18.3) | 40 (27.0) | 25 (20.0) | 0.113 |
| No | 280 (82.4) | 107 (81.7) | 108 (73.0) | 100 (80.0) | |
| ALND | |||||
| Yes | 46 (13.5) | 14 (10.7) | 24 (16.2) | 18 (14.4) | 0.603 |
| No | 294 (86.5) | 117 (89.3) | 124 (83.8) | 107 (85.6) | |
| Chemotherapy | |||||
| Yes | 166 (48.8) | 88 (67.2) | 128 (86.5) | 103 (82.4) | < 0.001 |
| No | 174 (51.2) | 43 (32.8) | 20 (13.5) | 22 (17.6) | |
| Endocrine therapy | |||||
| Yes | 253 (74.4) | 83 (63.4) | 6 (4.1) | 2 (1.6) | < 0.001 |
| No | 87 (25.6) | 48 (36.6) | 142 (96.0) | 123 (98.4) | |
| Radiation therapy | |||||
| Yes | 65 (19.1) | 21 (16.0) | 28 (18.9) | 31 (24.8) | 0.347 |
| No | 275 (80.9) | 110 (84.0) | 120 (81.1) | 94 (75.2) | |
| Anti-HER2 therapy | |||||
| Yes | 21 (6.2) | 90 (68.7) | 111 (75.0) | 11 (8.8) | < 0.001 |
| No | 319 (93.8) | 41 (31.3) | 37 (25.0) | 114 (91.2) | |
| Trimodality therapy | |||||
| Yes | 30 (8.8) | 7 (5.3) | 14 (9.5) | 9 (7.2) | 0.555 |
| No | 310 (91.2) | 124 (94.7) | 134 (90.5) | 116 (92.8) | |
p values indicated in italics are considered as statistically significant (p < 0.05). Abbreviations: IQR interquartile range, ALND axillary lymph node dissection, HR hormone receptor, HER2 human epidermal growth factor receptor-2, NC not calculable.*Histological grade is usually determined postoperatively, and since most patients are not treated with surgery, this variable is unknown in most of the patients. Trimodality therapy: neoadjuvant chemotherapy, surgery, radiation therapy
Site of metastases
In 391 patients (52.6%), metastases were found in multiple organs. In all breast cancer subtypes, bone metastases were most commonly diagnosed, with the highest percentage found in the HR+/HER2− subtype (71.5%). Lung metastases occurred significantly more often in HR−/HER2− IBC (40.8%). Liver metastases were significantly more often found in HER2-enriched (HR−/HER2+) tumors (41.2%) (Table 2). No differences were found with regard to brain metastases.
Table 2.
Frequencies of metastatic sites, divided by molecular subtype (n = 744)
| HR+/HER2− (n = 340) |
HR+/HER2+ (n = 131) |
HR−/HER2+ (n = 148) |
HR−/HER2− (n = 125) |
p value | |
|---|---|---|---|---|---|
| Type of metastasis per subtype | |||||
| Multiple sites | 173 (50.9) | 77 (58.8) | 74 (50.0) | 67 (53.6) | 0.414 |
| Only one site | 167 (49.1) | 54 (41.2) | 74 (50.0) | 58 (46.4) | |
| Bone | 102 (30.0) | 30 (22.9) | 24 (16.2) | 21 (16.8) | < 0.001 |
| Lung | 17 (5.0) | 2 (1.5) | 8 (5.4) | 12 (9.6) | |
| Liver | 12 (3.5) | 8 (6.1) | 20 (13.5) | 8 (6.4) | |
| Other# | 36 (10.6) | 14 (10.7) | 22 (14.9) | 17 (13.6) | |
| All found metastases$ | |||||
| Bones | 243 (71.5) | 90 (68.7) | 75 (50.7) | 52 (41.6) | < 0.001 |
| Lung | 102 (30.0) | 41 (31.3) | 35 (23.7) | 51 (40.8) | 0.023 |
| Liver | 75 (22.1) | 43 (32.8) | 61 (41.2) | 39 (31.2) | < 0.001 |
| Brain | 7 (2.1) | 5 (3.8) | 3 (2.0) | 3 (2.4) | 0.713 |
| Other/unknown | 35 (10.3) | 13 (9.9) | 21 (14.2) | 17 (13.6) | 0.496 |
p values indicated in italics are considered as statistically significant (p < 0.05). Abbreviations: HR hormone receptor, HER2 human epidermal growth factor receptor-2. #Including brain metastasis. $Cumulative percentage per subtype exceeds 100% due to the occurrence of multiple metastases at diagnosis
Treatment
Of the 744 patients with stage IV IBC, 149 patients (20.0%) underwent breast surgery as part of their treatment. Chemotherapy was administered in 485 patients (65.2%), significantly less often in HR+/HER2− tumors compared to the other subtypes. In 253 patients (74.4%) of HR+/HER2− tumors, endocrine treatment was given. HR−/HER2+ and HR−/HER2− received chemotherapy more often compared to the other subtypes (86.5% and 82.4%, respectively). No differences were found between subtypes regarding the frequency of application of trimodality therapy. Overall, just over 70% of HER2-enriched tumors were treated with targeted therapy (Table 1).
Survival outcomes
Median follow-up was 16.1 months (interquartile range 7.08–30.48 months), with a median OS of the entire cohort of 22.8 months (95% CI 1.68–2.03 months). No significant differences were found with regard to survival between age groups < 60 years and ≥ 60 years.
Stage IV IBC patients with HR+/HER2+ tumors exhibited the most prolonged OS, whereas patients with HR−/HER2− tumors exhibited the worst OS (p < 0.001, Fig. 1): HR+/HER2− 36.5%, HR+/HER2+ 45.8%, HR−/HER2+ 31.8%, and HR−/HER2− 15.2%. Five-year OS for the entire cohort of patients was 33.6%.
Fig. 1.
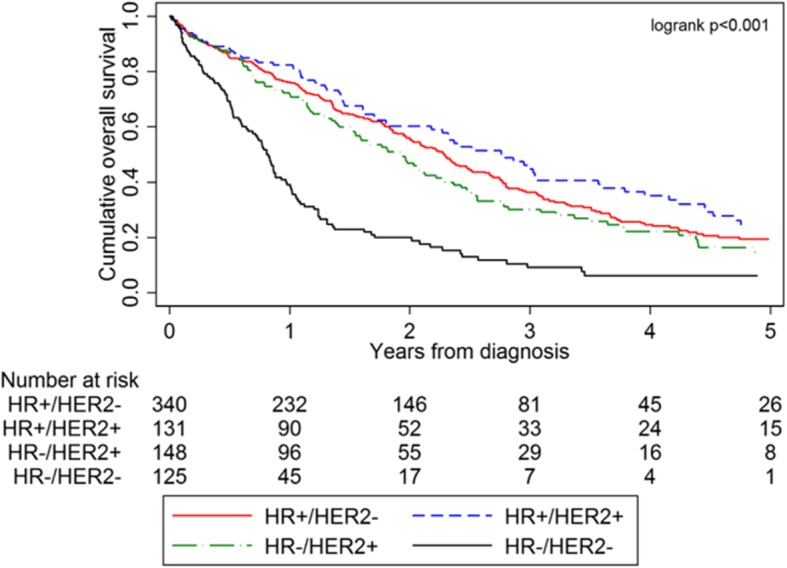
Kaplan-Meier curves displaying OS of all stage IV IBC from 2005 to 2016, presenting with stage IV at diagnosis, divided by breast cancer subtype (n = 744). Abbreviations: HR, hormone receptor; HER2, human epidermal growth factor receptor-2
A worse survival was seen in case of multiple organ involvement, displayed in the Kaplan-Meier curves for OS of stage IV IBC patients according to site of distant metastasis (Fig. 2).
Fig. 2.
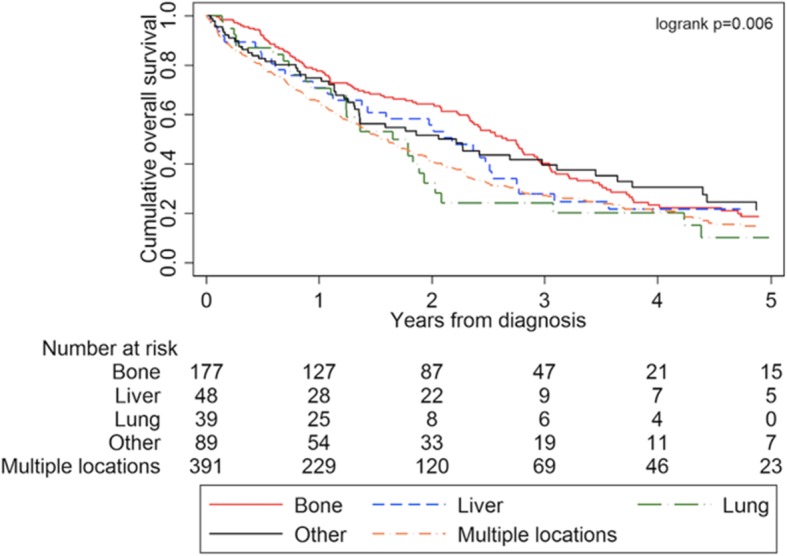
Kaplan-Meier curves displaying OS of all stage IV IBC from 2005 to 2016, presenting with stage IV at diagnosis (n = 744), divided by metastatic site
Multivariable analysis revealed that surgery, chemotherapy, targeted therapy, and antihormonal therapy were all independently associated with better survival (Table 3). Both HR−/HER2+ and HR−/HER2− subtypes were associated with a significantly worse OS compared to the HR+/HER2+ and HR+/HER2− subtypes. Multiple site metastases were associated with a significant worse survival (HR 1.32 [95% CI 1.04–1.68]).
Table 3.
Adjusted hazard ratios for 5-year OS in patients with IBC presenting with stage IV disease
| Hazard ratio | (95% CI) | p value | |
|---|---|---|---|
| Age | 1.01 | 1.00–1.01 | 0.168 |
| Year of diagnosis | 0.98 | 0.95–1.02 | 0.352 |
| Clinical nodal stage | |||
| N0 | 1 (ref) | ||
| N1 | 1.15 | 0.78–1.68 | 0.468 |
| N2 | 1.23 | 0.74–2.07 | 0.414 |
| N3 | 1.32 | 0.88–1.99 | 0.174 |
| Surgery | |||
| No | 1 (ref) | ||
| Yes | 0.56 | 0.42–0.74 | < 0.001 |
| Chemotherapy | |||
| Yes | 1 (ref) | ||
| No | 1.62 | 1.24–2.14 | < 0.001 |
| Targeted therapy | |||
| Yes | 1 (ref) | ||
| No | 2.76 | 1.70–3.05 | < 0.001 |
| Antihormonal therapy | |||
| Yes | 1 (ref) | ||
| No | 2.16 | 1.62–2.89 | < 0.001 |
| Radiation therapy | |||
| Yes | 1 (ref) | ||
| No | 1.11 | 0.84–1.46 | 0.457 |
| Molecular subtype | |||
| HR+/HER2− | 1 (Ref) | ||
| HR+/HER2+ | 1.17 | 0.86–1.61 | 0.319 |
| HR−/HER2+ | 1.59 | 1.12–2.24 | 0.009 |
| HR−/HER2− | 1.94 | 1.41–2.67 | < 0.001 |
| Location of metastases | |||
| Bone only | 1 (ref) | ||
| Liver only | 0.86 | 0.56–1.33 | 0.507 |
| Lung only | 1.27 | 0.81–1.99 | 0.292 |
| Other | 0.99 | 0.69–1.40 | 0.934 |
| Multiple organs | 1.32 | 1.04–1.68 | 0.021 |
Abbreviations: ref. refererence, OR odds ratio, CI confidence interval, HR hormone receptor, HER2 human epidermal growth factor receptor-2. p values indicated in italics are considered as statistically significant (p < 0.05)
Discussion
There is limited knowledge on the influence of breast cancer subtypes, based on hormone receptor status and HER2-status, on clinical outcome in stage IV IBC. This large study demonstrates that breast cancer subtypes in stage IV IBC are associated with unique patterns of distant metastatic spread and differences in OS.
To our knowledge, this is the first extensive analysis of the influence of HR/HER2-based breast cancer subtypes on the preferential site of metastases and OS in stage IV IBC. Data of previous studies were derived from patients treated in single institutions [11, 12], whereas our study contains data based on a nationwide population-based cancer registry including unselected and unbiased data of all hospitals (both academic and non-academic) in the Netherlands. Therefore, our study, which includes the largest patient number so far, represents valuable data on current clinical presentation and practice.
Inflammatory breast cancer subtypes and preferential site of metastasis
At first presentation, patients with IBC display significantly higher rates of distant metastases compared to non-inflammatory locally advanced breast cancer (39.7% versus 34.1%) [6].
In our current cohort, 744 patients with stage IV IBC at diagnosis were evaluated, which accounted for 33.3% of all patients diagnosed with IBC in the studied period. Over 50% of patients presented with multiple metastatic sites with different frequencies of the site of distant metastases among the various breast cancer subtypes. Multivariable analysis revealed that metastatic involvement of multiple sites was independently associated with a worse survival.
Bone metastases were most commonly diagnosed in all stage IV IBC subtypes with a significant predominance in the HR+/HER2− group. Liver metastases were more frequently observed in the HER2-enriched group and lung metastases in the HR−/HER2− group. This is not in agreement with a previous SEER analysis which did not show a significant association between IBC subtypes and site of metastases, which may well be attributed to the small sample with only 83 patients with stage IV IBC analyzed in that study [13]. We used data from the NCR to demonstrate that the metastatic patterns of stage IV IBC seem rather comparable to stage IV breast cancer in general [14]. A SEER analysis demonstrated that HR+/HER2+ and HER2-enriched subtypes are prone to abdominal/pelvic metastases and HR+/HER2− and HR+/HER2+ subtypes to bone metastases, while the HR−/HER2− subtype was prone to lung/mediastinal metastases [14]. In the present study, the occurrence of brain metastases was evidently lower than previously reported by Warren et al., who advised to incorporate surveillance brain MRI after diagnosis of extracranial metastatic disease in IBC [15]. However, in their study, no statistically significant association was found between primary tumor subtype and increased risk of development of brain metastases. A US-based single-center analysis in 203 IBC patients showed that the median time to development of brain metastases was 19 months [16]. One of the reasons of the low incidence of brain metastases in our study might be the fact that in the NCR only synchronous metastases are recorded, and as such, we were not able to analyze subsequent brain metastases that occurred more than 3 months after diagnosis.
Treatment of stage IV inflammatory breast cancer
Management of synchronous stage IV IBC includes primary systemic cytotoxic therapies and targeted HER2 therapy in case of HER2 positivity [17]. One area of debate is whether patients with stage IV IBC also should undergo local resection of the breast tumor. In the absence of prospective data, a potential survival benefit from removal of the breast tumor is suggested by retrospective evidence [11, 12, 18].
To our knowledge, there are three prospective trials conducted evaluating the effect of removal of the primary tumor in stage IV breast cancer, in which conflicting results were presented: two studies could not demonstrate a survival benefit [19, 20] and one showed an improved survival after 40 months follow-up (initially not showing any survival benefit of surgery after 36 months of follow-up) [21].
Just over 20% of all patients in our analysis underwent surgical resection of the primary tumor and just over 8% received trimodality treatment. This combination of neoadjuvant chemotherapy, surgery, and adjuvant radiation therapy is considered to be the most effective treatment regimen in stage III IBC [17]. Our numbers indicate that, also in the metastatic setting, surgery is used for locoregional management.
Inflammatory breast cancer subtypes and survival
An important finding of the present study is the highly variable prognosis among the different breast cancer subtypes in stage IV IBC. Patients with HR+/HER2+ IBC had the best survival among the four subgroups, whereas HR−/HER2− IBC is an independent prognostic factor for decreased survival, compared to the other subtypes. These results are consistent with previous studies in stage III IBC as well as stage IV non-IBC, both showing that HR−/HER2− tumors have the worst prognosis [5, 22, 23]. The 5-year OS of HR+/HER2+ IBC patients is 3.5-fold higher than that of patients with HR−/HER2− IBC, whereas HR+/HER2− and HR−/HER2+ subtypes display similar survival rates to each other but evidently lower compared to the HR+/HER2+ subtype. These ratios are comparable to a recent SEER analysis of metastatic breast cancer. Improved survival of the HR+/HER2+ subtype most likely reflects the use of HER2-targeted therapies. After the development of HER2-targeted therapies for the treatment of metastatic breast cancer in general, the survival of patients with HER2-positive tumors was greatly improved. This effect was irrespective of the HR status of the tumor [24]. Data were collected from patients who presented with stage IV IBC in the period 2005–2016. The last years, trastuzumab emtansine and pertuzumab enlarged the therapeutic arsenal for HER-2 positive patients, and as a consequence, the prognosis for survival will be even better nowadays than in the study period [25].
In general, the present study confirmed that compared with other primary tumor characteristics, breast cancer subtypes based on the HR and HER2 status of the primary tumor in stage IV IBC are important predictors of OS.
Several limitations of the present study have to be discussed. Firstly, the NCR does not register cause of death, and therefore, breast cancer-specific survival could not be determined. However, since all included patients already were diagnosed with stage IV disease at diagnosis, and since metastatic disease is the major cause of cancer-related deaths among breast cancer patients, the cause of death in our population is most likely to be breast cancer specific [26]. Secondly, it should be noted that 98 patients who were excluded with an unknown HR/HER2 less often underwent any form of treatment. These patients were significantly older and represent a specific subgroup of stage IV IBC patients. Reasons why older patients with cancer accept or decline treatment vary considerably, but the most consistent determinant found in the literature is physician recommendation [27]. Unfortunately, we are not able to draw firm conclusions on the absence of HR/HER2 data, since reasons for the waiver of treatment modalities could not be investigated in this database. These factors, as well as comorbidity, were not registered in the NCR and could not be accounted for in our study. Moreover, local therapy of the sites of metastatic disease (for example, resection of metastases and/or radiation therapy) could not be analyzed in this study.
We chose to only analyze clinical T4d breast cancers, instead of analyzing both clinical and pathological T4d breast cancers. However, since IBC is typically diagnosed clinically (dermal lymphatic invasion without typical clinical findings is not sufficient for a diagnosis of IBC), analysis of clinical T4d breast cancers seems to be the most accurate approach. As with any information obtained retrospectively from the abstraction of medical records, we acknowledge the dependency on the availability of data and reporting accuracy.
Furthermore, a central pathology review during treatment was not conducted, which might have led to an altered HR/HER2 status in several patients. Therefore, the potential impact of inter-institutional discordance was not evaluated. However, our current analysis reflects daily clinical practice in which local laboratories do not send all samples to a central laboratory and limited discordance was found in previous analyses which addressed the possibility of potential discordance [28–30]. Furthermore, discordances in ER/PR/HER2 test results between tumor core needle biopsy taken at the time of diagnosis and tumor resection material are low, also in patients receiving any form of neoadjuvant therapy [31].
Finally, information regarding the type of diagnostic modalities is lacking. Given the high rate of metastatic disease at presentation, patients with IBC undergo extensive staging, including whole body bone scintigraphy, ultrasonography of the liver, and a chest X-ray. Some institutions might have used other modalities such as 18-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT), which might influence the detection of distant metastases compared to traditional modalities [32]. More than 20% of patients appear to have distant metastases after FDG-PET scanning in comparison with conventional staging in locally advanced breast cancer [33]. However, with regard to subtypes, no difference is expected, since there is no guideline of which breast cancer subtype should receive a specific type of diagnostic modality and a potential diagnostic bias would be present for all breast cancer subtypes and this will not affect the differences we report.
Clinical relevance
IBC is diagnosed at a younger age with survival rates which are clearly inferior to the average rates for patients with non-IBC [6]. Similar to IBC in general, the incidence of stage IV IBC seems to be increasing (data not shown) [6]. This might, among others, be due to increased use of improved staging modalities [32]. Knowledge of the biology of IBC has to increase to achieve improvement on the treatment of IBC. This applies for both stage III and IV IBC. Stratification of breast cancer subtypes in stage IV IBC is of clinical use for estimating prognosis, since OS differed significantly between the subtypes with the worst OS for HR−/HER2− IBC. These data might aid physicians in patient counseling regarding prognosis and underscribe the need of new systemic (targeted) therapies to improve OS in stage IV IBC and HR−/HER2− disease in particular.
Moreover, the differences seen in sites of metastases between breast cancer subtypes can guide a more patient-tailored staging directed to metastatic sites. Since metastatic disease remains the principal cause of cancer-related deaths [34], this tailored staging could lead to the identification of more effective prognostication and, hopefully in the future, individualized targeted approaches to treat these patients. Some evidence suggests a potential role for metastasis-specific local treatment (e.g., metastasectomy and radiation therapy) in the prolongation of survival, especially in oligometastatic disease, although prospective data are lacking [35]. Consequently, in patients with multiple organ metastases, locoregional treatment of metastases should be discussed and potentially omitted. This will prevent potential morbidity of non-beneficial treatments [36].
Conclusion
This study demonstrates important differences in distant metastatic behavior and overall survival between breast cancer subtypes, as defined by HR/HER2 status, and contributes to an expanding knowledge of prognostic markers in stage IV IBC. Consequently, more focused and patient-tailored staging should be based on breast cancer subtypes in order to achieve the most accurate information on the site and extend of disease and to discuss potential treatment options in case of metastatic disease.
Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation for the collection of data for the Netherlands Cancer Registry and the scientific staff of the Netherlands Cancer Registry.
Authors’ contributions
All authors read and approved the final manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript. DvU wrote the main paper. MvM contributed to the analysis of the results and to the writing of the manuscript. PB discussed the results and implications and commented on the manuscript at all stages. LS discussed the results and implications and commented on the manuscript at all stages. KvdH discussed the results and implications and commented on the manuscript at all stages. SS gave technical support and conceptual advice and commented on the manuscript at all stages. CB conceived the original idea and commented on the manuscript at all stages. HdW supervised the project and commented on the manuscript at all stages.
Funding
The author(s) received no specific funding for this work.
Availability of data and materials
The data that support the findings of this study are available from the NCR but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the NCR.
Ethics approval and consent to participate
The privacy committee of the NCR has approved this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van Uden D.J.P., van Laarhoven H.W.M., Westenberg A.H., de Wilt J.H.W., Blanken-Peeters C.F.J.M. Inflammatory breast cancer: An overview. Critical Reviews in Oncology/Hematology. 2015;93(2):116–126. doi: 10.1016/j.critrevonc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 3.Engstrøm M. J., Opdahl S., Hagen A. I., Romundstad P. R., Akslen L. A., Haugen O. A., Vatten L. J., Bofin A. M. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Research and Treatment. 2013;140(3):463–473. doi: 10.1007/s10549-013-2647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haque R, Ahmed SA, Inzhakova G, Shi J, Avila C, Polikoff J, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomark Prev. 2012;21:1848–1855. doi: 10.1158/1055-9965.EPI-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Uden D, Van Maaren M, Bult P, Strobbe L, Siesling S, De Wilt H, et al. Molecular subtypes in inflammatory breast cancer: a descriptive analysis using the Netherlands cancer registry. Eur J Cancer. 2018;92:S120–S121. doi: 10.1016/S0959-8049(18)30583-5. [DOI] [Google Scholar]
- 6.van Uden DJP, Bretveld R, Siesling S, de Wilt JHW, Blanken-Peeters CFJM. Inflammatory breast cancer in the Netherlands; improved survival over the last decades. Breast Cancer Res Treat. 2017;162:365–374. doi: 10.1007/s10549-017-4119-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen MT, Sun HF, Zhao Y, Fu WY, Yang LP, Gao SP, et al. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-017-10166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz AG. International Classification of Diseases for Oncology (ICD-O). World Heal Organ. 2000;240.
- 9.Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed. Cancer. 2010;116:5336–5339. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 10.Sobin LH. TNM, sixth edition: new developments in general concepts and rules. Semin Surg Oncol. 2003;21:19–22. doi: 10.1002/ssu.10017. [DOI] [PubMed] [Google Scholar]
- 11.Takiar V, Akay CL, Stauder MC, Tereffe W, Alvarez RH, Hoffman KE, et al. Predictors of durable no evidence of disease status in de novo metastatic inflammatory breast cancer patients treated with neoadjuvant chemotherapy and post-mastectomy radiation. Springerplus. 2014;3:166. doi: 10.1186/2193-1801-3-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akay CL, Ueno NT, Chisholm GB, Hortobagyi GN, Woodward WA, Alvarez RH, et al. Primary tumor resection as a component of multimodality treatment may improve local control and survival in patients with stage IV inflammatory breast cancer. Cancer. 2014;120:1319–1328. doi: 10.1002/cncr.28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Xia Y, Wu Q, Zhu S, Chen C, Yang W, et al. Outcomes of patients with inflammatory breast cancer by hormone receptor- and HER2-defined molecular subtypes: a population-based study from the SEER program. Oncotarget. 2017;8:49370–49379. doi: 10.18632/oncotarget.17217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun S, Chen C, Wu J, Liu Q, Zhu S, Wei W, et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget. 2017;8:27990–27996. doi: 10.18632/oncotarget.15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren LEG, Guo H, Regan MM, Nakhlis F, Yeh ED, Jacene HA, et al. Inflammatory breast cancer and development of brain metastases: risk factors and outcomes. Breast Cancer Res Treat. 2015;151:225–232. doi: 10.1007/s10549-015-3381-8. [DOI] [PubMed] [Google Scholar]
- 16.Dawood S, Ueno NT, Valero V, Andreopoulou E, Hsu L, Lara J, et al. Incidence of and survival following brain metastases among women with inflammatory breast cancer. Ann Oncol. 2010;21:2348–2355. doi: 10.1093/annonc/mdq239. [DOI] [PubMed] [Google Scholar]
- 17.Dawood S, Merajver SD, Viens P, Vermeulen PB, Swain SM, Buchholz TA, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22:515–523. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawood S, Ueno NT, Valero V, Woodward WA, Buchholz TA, Hortobagyi GN, et al. Identifying factors that impact survival among women with inflammatory breast cancer. Ann Oncol. 2012;23:870–875. doi: 10.1093/annonc/mdr319. [DOI] [PubMed] [Google Scholar]
- 19.Badwe R, Hawaldar R, Nair N, Kaushik R, Parmar V, Siddique S, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 2015;16:1380–1388. doi: 10.1016/S1470-2045(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 20.Fitzal Florian, Bjelic-Radisic Vesna, Knauer Michael, Steger Günther, Hubalek Michael, Balic Marija, Singer Christian, Bartsch Rupert, Schrenk Peter, Soelkner Lidija, Greil Richard, Gnant Michael. Impact of Breast Surgery in Primary Metastasized Breast Cancer. Annals of Surgery. 2019;269(6):1163–1169. doi: 10.1097/SLA.0000000000002771. [DOI] [PubMed] [Google Scholar]
- 21.Soran A, Ozmen V, Ozbas S, Karanlik H, Muslumanoglu M, Igci A, et al. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast cancer at presentation: protocol MF07-01. Ann Surg Oncol. 2018;25(11):3141–3149. doi: 10.1245/s10434-018-6494-6. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Chen K, Jiang W, Mao K, Li S, Kim MJ, et al. Chemotherapy response and survival of inflammatory breast cancer by hormone receptor- and HER2-defined molecular subtypes approximation: an analysis from the National Cancer Database. J Cancer Res Clin Oncol. 2016;143:161–168. doi: 10.1007/s00432-016-2281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Y, Liu YR, Ji P, Hu X, Shao ZM. Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci Rep. 2017;7:1–10. doi: 10.1038/srep45411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiteni F, Villanueva C, Bazan F, Perrin S, Chaigneau L, Dobi E, et al. Long-term follow-up of patients with metastatic breast cancer treated by trastuzumab: impact of institutions. Breast. 2014;23(2):165–169. doi: 10.1016/j.breast.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Ocaña A, Amir E, Pandiella A. Dual targeting of HER2-positive breast cancer with trastuzumab emtansine and pertuzumab: understanding clinical trial results. Oncotarget. 2018;9(61):31915–31919. doi: 10.18632/oncotarget.25739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeSantis Carol, Ma Jiemin, Bryan Leah, Jemal Ahmedin. Breast cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 27.Puts MTE, Tu HA, Tourangeau A, Howell D, Fitch M, Springall E, et al. Factors influencing adherence to cancer treatment in older adults with cancer: a systematic review. Ann Oncol. 2014;25(3):564–577. doi: 10.1093/annonc/mdt433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlando L, Viale G, Bria E, Lutrino ES, Sperduti I, Carbognin L, et al. Discordance in pathology report after central pathology review: implications for breast cancer adjuvant treatment. Breast. 2016;30:151–155. doi: 10.1016/j.breast.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30(6):593–599. doi: 10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Rooijen JM, De Munck L, De Graaf JC, Siesling S, De Vries EG, Boers JE. Limited human epidermal growth factor receptor 2 discordance in metastatic breast cancer patients treated with trastuzumab, a population based study. Eur J Cancer. 2014;50(5):885–891. doi: 10.1016/j.ejca.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Berghuis AMS, van Deurzen CHM, Koffijberg H, Terstappen LWMM, Sleijfer S, IJzerman MJ. Real-world data on discordance between estrogen, progesterone, and HER2 receptor expression on diagnostic tumor biopsy versus tumor resection material. Breast Cancer Res Treat. 2019;175(2):451–458. doi: 10.1007/s10549-019-05141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang WT. Advances in imaging of inflammatory breast cancer. Cancer. 2010;116:2755–2757. doi: 10.1002/cncr.25170. [DOI] [PubMed] [Google Scholar]
- 33.Van Der Hoeven JJM, Krak NC, Hoekstra OS, Comans EFI, Boom RPA, Van Geldere D, et al. 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography in staging of locally advanced breast cancer. J Clin Oncol. 2004;22:1253–1259. doi: 10.1200/JCO.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 34.Redig AJ, Mcallister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274(2):113–126. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102(7):456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardona-Morrell M, Kim JCH, Turner RM, Anstey M, Mitchell IA, Hillman K. Non-beneficial treatments in hospital at the end of life: a systematic review on extent of the problem. Int J Qual Heal Care. 2016;28(4):456–469. doi: 10.1093/intqhc/mzw060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the NCR but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the NCR.


