Abstract
Objective
Trichoderma species are found in soil and in association with plants. They can act directly or indirectly in the biological control of plant diseases and in the promotion of plant growth, being among the most used fungi in the formulation of bioproducts applied to agricultural systems. The main objective of this study was to characterize at a first-tier level a collection of 67 Trichoderma isolates from various tropical sources, based solely on sequencing of the internal transcribed spacer (ITS) region of the rRNA genes. Our goal was to provide a preliminary idea of the baseline diversity in this collection, to combine this information later with an array of other isolate-specific physiological data. This study provides a required knowledge at molecular level for assessment of this germplasm potential as a source of biotechnological products for beneficial effects in plants.
Results
Sequencing of the ITS region showed that the 67 Trichoderma isolates belonged in 11 species: T. asperellum, T. atroviride, T. brevicompactum, T. harzianum, T. koningiopsis, T. longibrachiatum, T. pleuroticola, T. reesei, T. spirale, T. stromaticum and T. virens. A total of 40.3% of the isolates were very closely related to each other and similar to T. harzianum. The baseline genetic diversity found indicates that the collection has different genotypes, which can be exploited further as a source of bioproducts, aiming at providing beneficial effects to plants of interest to cope with biotic and abiotic stresses.
Keywords: Biological control, Polymerase chain reaction, Internal transcribed spacer, BlastN, Phylogeny, Biotechnological development
Introduction
Filamentous fungi of the genus Trichoderma (Ascomycota) are among the microorganisms most used in biological control of plant diseases [1], displaying mechanisms of action that include direct and indirect antagonism against plant pathogens [2]. Trichoderma can establish endophytic associations with plants, systemically induce resistance against phytopathogens, increase nutrient uptake, and consequently promote plant growth [3]. They are also known for their abilities to grow under diverse environmental conditions and to parasitize other fungi [4]. Due to these features, Trichoderma can colonize an array of niches and compete with other microorganisms for space, nutrients and light, which explains its success as a biological control agent [5]. All these characteristics, associated with a recognized efficacy in the generation of dispersive propagules (conidia), make Trichoderma spp. ideal for the development of bioproducts for beneficial effects on plants [6]. Several abiotic factors (light, temperature, humidity, pH, carbon and nitrogen sources, oxygen) can interact with genetic components (inherent genetic variability of different Trichoderma isolates), producing unique growth and sporulation responses, which tend to contribute to the biocontrol efficacy in a strain-specific manner [7–9]. The internal transcribed spacer (ITS) region of rRNA genes is considered the primary barcode sequence for evolutionary/phylogenetic studies on fungi, due to its relevant features such as (i) ease of amplification in a very reproducible manner (ii) a widespread use among a variety of fungal systems (iii) a tendency of displaying a large barcode gap, (iv) the possibility of alignment across all the kingdom, and (v) an appropriate length for amplification, sequencing and phylogenetic information supply [10, 11]. In this study, direct amplicon sequencing of the ITS region of rDNA was used to preliminarily characterize a tropical collection of 67 Trichoderma isolates from the Atlantic and Amazon rainforests, and ‘caatinga’ (semi-arid) biomes of Brazil. This collection has been evaluated concerning isolate-specific phenotypes related to growth and sporulation levels responsive to biotic and abiotic factors [12]. Based on the molecular characterization of Trichoderma isolates described in the present work, further studies on this germplasm will address its potential as a source of new bioproducts to be used in biological control of plant diseases and other beneficial effects.
Main text
Methods
Trichoderma isolates and growth conditions
The Trichoderma collection studied consisted of 67 isolates obtained from different natural environments (biomes) in different provinces of Brazil, including Bahia (47), Amazonas (9), Rondônia (10) and Minas Gerais (1) (Additional file 1: Table S1). The isolates were cultured in Petri dishes containing PDA medium (Difco™) and incubated at 25 °C under constant light. After 5 days, the conidia were collected and stored in sterile glycerol (50%) at − 80 °C.
Genomic DNA extraction and amplification
For a first-tier molecular identification of Trichoderma species and an assessment of the baseline diversity of isolates within the collection, the ITS region of the rDNA was sequenced. Each isolate was grown in 30 mL of PD medium (inside 50-mL Falcon tubes) at 25 °C under constant light for 4 days in a growth chamber. For genomic DNA extraction, an initial step of physical mycelium break was employed through addition of 7–10 mL of sterile glass beads in each tube and vortexing for 2 min. Afterwards, 13 mL of each grinded mycelium suspension was transferred to clean 50-mL tubes and centrifuged for 5 min at 13,400 rpm. A wet weight of 200 mg for each pellet was then used for total DNA extraction with the DNeasy® Plant Mini Kit (Qiagen™) following the manufacturer’s protocol. The ITS region was amplified by PCR using the ITS-1 [13] and ITS-4 [14] universal primers. The PCR mixture (40 μL) contained 0.5 μL of 2 U µL−1 Taq DNA polymerase (Invitrogen™), 1.2 μL of 1.5 mM MgCl2, 1.67 μL of each forward and reverse primers (both as 20-μM solutions), 1 μL of 1 mM dNTPs (250 µM each), 4 μL of 10 × buffer, 3.4 μL of DNA template (~ 10–30 ng) and 26.56 μL of distilled water. Amplifications were performed in 0.2-mL PCR tubes using an Applied Biosystems Veriti Thermal Cycler. The conditions for PCR amplification were 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, and a final extension at 72 °C for 10 min. PCR products were visualized on 1.5% agarose gel and the amplified DNA was purified from the gel using the PureLink® Quick Gel Extraction Kit (Invitrogen™) following the manufacturer’s instructions.
Internal transcribed spacer (ITS) sequencing
Direct sequencing of the purified amplicons was performed by the Sanger method using the ABI-PRISM® 3100 Genetic Analyzer system. Sequencing reactions in final volumes of 10 μL contained 3 μL of BigDye Terminator v3.1 Cycle Sequencing RR-100, 5 ng μL−1 of DNA template, and 0.25 pmol μL−1 of each ITS1 or ITS4 primers. Sequencing amplifications were performed in a GeneAmp PCR System 9700 thermocycler as follows: 3 min at 96 °C, plus 25 cycles of 10 s at 96 °C, 5 s at 55 °C and 4 min at 60 °C. The reaction products were precipitated with 40 μL 75% isopropanol (3:1, v/v) for 20-30 min at room temperature, centrifuged at 13,000 rpm for 15 min at 4 °C, washed with 200 μL 60% ethanol (quick vortexing), and centrifuged at 13,000 rpm for 5 min at 4 °C. The pellets were air-dried, resuspended in 10 μL of Hi-Di formamide, denatured at 95 °C for 5 min, cooled on ice for 5 min and electro-injected in the automatic sequencer. The sequencing data were collected using the Data Collection v 1.0.1 program. At least two sequencing reactions were performed for each amplicon/isolate.
Phylogenetic and diversity analyses
Sequences from the ITS region of the isolates were compared to those in GenBank (NCBI) through the BlastN program [15]. From these results, reference sequences for the corresponding Trichoderma species (Additional file 2: Table S2) were used to construct multiple sequence alignments (MSA). For the phylogenetic approach, the GUIDANCE2 server [16] with the MAFFT alignment algorithm [17] was used to ensure that only positions with high probabilities of being correctly aligned were used. Phylogenetic analysis was performed with the MEGA7 program [18] using the Maximum Likelihood (ML) method. The analysis involved a total of 80 DNA sequences, 67 for our study and 13 type species from the database. The tree was edited using the FigTree program [19].
Results
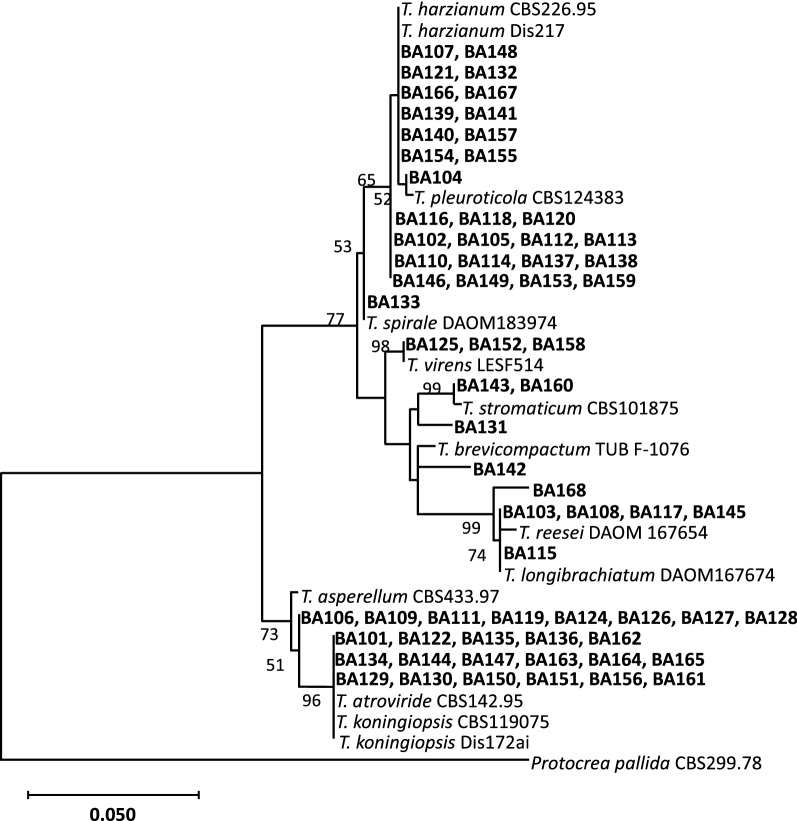
In order to assess the baseline diversity within the Trichoderma collection of this study, a phylogeny-based analysis on the ITS region was performed. The results showed that the 67 isolates from a tropical origin clustered with the reference sequences in variable manners (Fig. 1). The clusters ranged from a pairwise configuration between an isolate and a reference sequence to groups with many isolates and more than one reference sequence. The isolates from the collection were more closely related to 11 species of the Trichoderma genus: T. asperellum, T. atroviride, T. brevicompactum, T. harzianum, T. koningiopsis, T. longibrachiatum, T. pleuroticola, T. reesei, T. spirale, T. stromaticum and T. virens. In this context, the distribution of isolates among the species was heterogeneous. The largest group comprising 27 isolates (40.3%) was related to the T. harzianum species complex; 12 of them (17.9%; BA107, BA121, BA166, BA139, BA140, BA154, BA148, BA132, BA167, BA141, BA157, BA155) grouped within the cluster containing the two T. harzianum references (‘CBS226.95’ and ‘Dis217’), and 15 (22.4%; BA116, BA102, BA110, BA146, BA118, BA105, BA114, BA149, BA120, BA112, BA137, BA153, BA113, BA138, BA159) were more related to this cluster (Fig. 1); due to the not so close relationship to the T. harzianum reference strains, this latter group of 15 isolates were indicated as ‘Trichoderma sp-1’ (Additional file 1: Table S1; also see below). In terms of a first-tier, preliminary identification of the isolates based on the ITS sequences, some noteworthy certainties were verified (Fig. 1; Additional file 1: Table S1): (i) the isolate BA104 was most closely related to the T. pleuroticola species (branching out from the T. harzianum’s complex); (ii) BA133 belonged in the T. spirale species; (iii) BA125, BA152 and BA158, grouped with T. virens; (iv) BA143 and BA160 were most closely related to T. stromaticum; and (v) BA115 belonged in the T. longibrachiatum.
Fig. 1.
Phylogenetic tree of Trichoderma isolates from tropical sources based on sequences of the ITS region. The tree was constructed by using the Maximum Likelihood method with 425 aligned nucleotides from the ITS region of the rDNA. The nucleotide substitution model used was GTR + G to model evolutionary rate differences among sites [18]. The bootstrap analysis was performed with a 1000 resamplings and values above 50% are shown on the appropriate branches. The ITS sequence from Protocrea pallida was used as the outgroup. The list of (i) ITS sequences with accession numbers of the ‘BA’ isolates (Additional file 1: Table S1) and (ii) type species/ITS sequences with accession numbers used in the alignments (Additional file 2: Table S2) are shown in additional material. The scale bar represents the number of expected substitutions per site
The other isolates, on their turn, showed a pattern of uncertain relationship with the reference isolates, with variable levels of proximity. For instance, a cluster with 17 isolates (25.4%) could not be distinguished from three reference sequences, the ‘CBS142.95’ of T. atroviride and the ‘CBS119075’ and ‘Dis172ai’ of T. koningiopsis (Fig. 1; bottom part of the dendrogram). BA131, BA142 and BA168, and a group of eight isolates (including BA106 to BA128; Fig. 1) did not form a cluster with any reference sequence. For an intra-collection differentiation, those isolates with uncertain species assignment were given an ‘sp’ indication, followed by specific extension numbers to represent distinct locations in the dendrogram topolgy (Additional file 1: Table S1). The T. brevicompactum, T. asperellum and T. reseei reference sequences (‘TUB F-1076’, ‘CBS433.97’, ‘DAOM 167654’, respectively) did not specifically clustered with any isolate, although the second one appeared more closely related to BA142, and the last one to BA103, BA108, BA117 and BA145 isolates (Fig. 1).
Discussion
The development of Trichoderma-based bioproducts begins with screening procedures over a hopefully variable genetic material available from collection-assembly efforts [6, 20], as the identification of novel genotypic variations among isolates allows to increase the options for beneficial-effects applications [21–23]. The tropical collection of Trichoderma here studied bears some genetic diversity on an isolate-specific level, based on a phylogenetic assessment with ITS sequences (Fig. 1). Despite that ITS is the primary barcode sequence for fungi, bearing several relevant features for phylogenetic/evolutionary studies in fungi [10, 11], alone it is not a sufficient marker for full species discrimination in highly speciose genera, such as Trichoderma [24]. Therefore, not unexpectedly, the level of similarities among all the ITS sequences was high, with differences within a range of 0.05 to 0.10 substitutions per site. However, the clustering pattern provided by the dendrogram topology suggest that the tropical isolates of this collection may be possibly assigned to more than the 11 species indicated by the reference strains (Fig. 1). The fact that more than a third of the isolates were genetically related to T. harzianum may be explained by the recognized condition of this taxon as a complex of cryptic species [25], in which the analysis based on ITS sequences is not able to distinguish closely related species in this complex [26]. The 11 species of Trichoderma identified/characterized in this study, coupled with the isolate-specific phenotypic aspects related to growth and sporulation responses to biotic and abiotic factors [12], suggest that the genetic diversity associated with this germplasm (at the genotypic level) will be useful for further work aiming at developing novel bioproducts with an array of possible applications. Subsequent characterization steps based on assays at laboratory and agronomic scales are needed to evaluate which isolates and conditions stand out, such as for biocontrol of phytopathogens or plant-growth promotion for distinct plant species. Once particular strains of this collection are revealed as promising sources for beneficial-effects related bioproducts, fine-tuned species definition can be achieved by characterizing other genes [24], as an aid to the ITS sequence database here provided.
Limitations
The main limitation of this study is the fact that the information contained in the ITS region is not sufficient to separate some of the isolates at the species level within the genus Trichoderma. However, since full, unequivocal taxonomy was not the main goal of this study, we claim that ITS-based preliminary characterization of Trichoderma isolates, coupled with phenotypic differences detected through specific comparative assays (e.g. [12, 21, 23]), was sufficiently appropriate to provide information on the existing diversity in this collection at the isolate (genotypic) level.
Supplementary information
Additional file 1: Table S1. Internal Transcribed Spacer (ITS) sequences from the 67 tropical Trichoderma isolates from the ‘BA’ series and the corresponding GenBank accession numbers.
Additional file 2: Table S2. List of ITS sequences from type species used in this study.
Acknowledgements
The authors thank the State University of Santa Cruz (UESC) and the Post-graduation Program in Genetics and Molecular Biology (PPG-GBM/UESC) for the infrastructure provided.
Abbreviations
- ITS
internal transcribed spacer
- PDA
potato dextrose agar
- PCR
polymerase chain reaction
- dNTPs
deoxynucleosides triphosphate
- MSA
multiple sequence alignment
- NCBI
National Center for Biotechnology Information
Authors’ contributions
YBF performed the experiments, collected all the data, and helped with the analyses. YBF, LLL, RCAF and JTdS designed the project. LLL and RCAF supervised the execution of all project’s steps. VC-M performed the phylogenetic analysis; VC-M and YBF wrote the draft manuscript; JTdS and LLL corrected it and provided the final MS version prior to submission. All authors contributed to the final manuscript. All authors read and approved the final manuscript.
Funding
This study was financially supported by the following Brazilian Government funding agencies: CNPq (National Council for Scientific and Technological Development) and CAPES (Coordination for the Improvement of Higher Education Personnel). CNPq funded consumables, collection of isolates, sequencing services and post-doctoral and doctoral fellowships (Project no. 402973/2012-7). CAPES funded a master’s fellowship - Finance Code 001. The infrastructure was provided by the Agroindustry-applied microbiology Laboratory (LABMA) and the Center for Genetics and Biotechnology (CBG) from State University of Santa Cruz (UESC), Ilhéus-BA, Brazil.
Availability of data and materials
The data sets and working sheets are available upon request to Dr. Valter Cruz-Magalhães (E-mail: valter.magalhães@ufla.br) and Dr. Leandro L. Loguercio (E-mail: leandro@uesc.br).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yara Barros Feitosa, Email: yarabfeitosa@usp.br.
Valter Cruz-Magalhães, Phone: +55 35 3829 4527, Email: valter.magalhaes@ufla.br.
Ronaldo Costa Argolo-Filho, Email: ronaldoargolo@yahoo.com.br.
Jorge Teodoro de Souza, Email: jorge.souza@ufla.br.
Leandro Lopes Loguercio, Email: leandro@uesc.br.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13104-019-4694-1.
References
- 1.Woo SL, Ruocco M, Vinale F, Nigro M, Marra R, Lombardi N, et al. Trichoderma- based products and their widespread use in agriculture. Open Mycol J. 2014;8:71–126. doi: 10.2174/1874437001408010071. [DOI] [Google Scholar]
- 2.Holmes KA, Schroers H-J, Thomas SE, Evans HC, Samuels GJ. Taxonomy and biocontrol potential of a new species of Trichoderma from the Amazon basin of South America. Mycol Prog. 2004;3:199–210. doi: 10.1007/s11557-006-0090-z. [DOI] [Google Scholar]
- 3.Schuster A, Schmoll M. Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol. 2010;87:787–799. doi: 10.1007/s00253-010-2632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species—opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 5.Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kenerley CM, Monte E, et al. Trichoderma: the genomics of opportunistic success. Nat Rev Microbiol. 2011;9:749–759. doi: 10.1038/nrmicro2637. [DOI] [PubMed] [Google Scholar]
- 6.Glare T, Caradus J, Gelernter W, Jackson T, Keyhani N, Köhl J, et al. Have biopesticides come of age? Trends Biotechnol. 2012;30:250–258. doi: 10.1016/J.TIBTECH.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Steyaert JM, Weld RJ, Mendoza-Mendoza A, Stewart A. Reproduction without sex: conidiation in the filamentous fungus Trichoderma. Microbiology. 2010;156:2887–2900. doi: 10.1099/mic.0.041715-0. [DOI] [PubMed] [Google Scholar]
- 8.Steyaert JM, Weld RJ, Stewart A. Ambient pH intrinsically influences Trichoderma conidiation and colony morphology. Fungal Biol. 2010;114:198–208. doi: 10.1016/J.FUNBIO.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Steyaert JM, Weld RJ, Loguercio LL, Stewart A. Rhythmic conidiation in the blue-light fungus Trichoderma pleuroticola. Fungal Biol. 2010;114:219–223. doi: 10.1016/J.FUNBIO.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci USA. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindahl BD, Nilsson RH, Tedersoo L, Abarenkov K, Carlsen T, Kjøller R, et al. Fungal community analysis by high-throughput sequencing of amplified markers—a user’s guide. New Phytol. 2013;199:288–299. doi: 10.1111/nph.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feitosa YB. Evaluation of biotic and abiotic factors on growth and sporulation of tropical genotypes of Trichoderma spp. State University of Santa Cruz (UESC), Ilhéus-BA, Brazil. 2016. [M.Sc. dissertation in Portuguese].
- 13.Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 14.White T, Bruns T, Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. San Diego: Academic Press; 1990. [Google Scholar]
- 15.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 16.Sela I, Ashkenazy H, Katoh K, Pupko T. GUIDANCE2: accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Res. 2015;43:W7–W14. doi: 10.1093/nar/gkv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rambaut. FigTree. 2012.
- 20.Montesinos E. Development, registration and commercialization of microbial pesticides for plant protection. Int Microbiol. 2003;6:245–252. doi: 10.1007/s10123-003-0144-x. [DOI] [PubMed] [Google Scholar]
- 21.Loguercio LL, de Carvalho AC, Niella GR, De Souza JT, Pomella AWV. Selection of Trichoderma stromaticum isolates for efficient biological control of witches’ broom disease in cacao. Biol Control. 2009;51:130–139. doi: 10.1016/J.BIOCONTROL.2009.06.005. [DOI] [Google Scholar]
- 22.Loguercio LL, Santos LS, Niella GR, Miranda RAC, de Souza JT, Collins RT, et al. Canopy-microclimate effects on the antagonism between Trichoderma stromaticum and Moniliophthora perniciosa in shaded cacao. Plant Pathol. 2009;58:1104–1115. doi: 10.1111/j.1365-3059.2009.02152.x. [DOI] [Google Scholar]
- 23.Hoyos-Carvajal Lilliana, Bissett John. The Dynamical Processes of Biodiversity - Case Studies of Evolution and Spatial Distribution. 2011. Biodiversity of Trichoderma in Neotropics. [Google Scholar]
- 24.Raja HA, Miller AN, Pearce CJ, Oberlies NH. Fungal identification using molecular tools: a primer for the natural products research community. J Nat Prod. 2017;80:756–770. doi: 10.1021/acs.jnatprod.6b01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaverri P, Branco-Rocha F, Jaklitsch W, Gazis R, Degenkolb T, Samuels GJ. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia. 2015;107:558–590. doi: 10.3852/14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbertse B, Strope PK, Chaverri P, Gazis R, Ciufo S, Domrachev M, et al. Improving taxonomic accuracy for fungi in public sequence databases: applying ‘one name one species’ in well-defined genera with Trichoderma/Hypocrea as a test case. Database. 2017 doi: 10.1093/database/bax072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Internal Transcribed Spacer (ITS) sequences from the 67 tropical Trichoderma isolates from the ‘BA’ series and the corresponding GenBank accession numbers.
Additional file 2: Table S2. List of ITS sequences from type species used in this study.
Data Availability Statement
The data sets and working sheets are available upon request to Dr. Valter Cruz-Magalhães (E-mail: valter.magalhães@ufla.br) and Dr. Leandro L. Loguercio (E-mail: leandro@uesc.br).