Abstract
Objective
We sought to investigate the experiences of general practitioners (GPs) with an electronic decision support tool to reduce inappropriate polypharmacy in older patients (the PRIMA-eDS [Polypharmacy in chronic diseases: Reduction of Inappropriate Medication and Adverse drug events in older populations by electronic Decision Support] tool) in a multinational sample of GPs and to quantify the findings from a prior qualitative study on the PRIMA-eDS-tool.
Materials and Methods
Alongside the cluster randomized controlled PRIMA-eDS trial, a survey was conducted in all 5 participating study centers (Bolzano, Italy; Manchester, United Kingdom; Salzburg, Austria; Rostock, Germany; and Witten, Germany) between October 2016 and July 2017. Data were analyzed using descriptive statistics and chi-square tests.
Results
Ninety-one (n = 160) percent of the 176 questionnaires were returned. Thirty-two percent of the respondents reported that they did not cease drugs because of the medication check. The 68% who had discontinued drugs comprise 57% who had stopped on average 1 drug and 11% who had stopped 2 drugs or more per patient. The PRIMA-eDS tool was found to be useful (69%) and the recommendations were found to help to increase awareness (86%). The greatest barrier to implementing deprescribing recommendations was the perceived necessity of the medication (69%). The majority of respondents (65%) would use the electronic medication check in routine practice if it was part of the electronic health record.
Conclusions
GPs generally viewed the PRIMA-eDS medication check as useful and as informative. Recommendations were not always followed due to various reasons. Many GPs would use the medication check if integrated into the electronic health record.
Keywords: aged, general practitioners, evidence-based medicine, deprescribing, multimorbidity
INTRODUCTION
Polypharmacy (the concurrent use of several drugs)1 is increasing worldwide, especially in highly developed countries, due to the aging population2 and multimorbidity.3 Physicians often treat each disease of their patient according to disease-specific guidelines, which results in cumulating medications as these guidelines usually do not take multimorbidity into account.4 Even though polypharmacy may be necessary and appropriate in some cases,5 it increases the risk of medication errors and adverse effects.6,7 To optimize prescribing in patients affected by polypharmacy, regular medication reviews are deemed necessary.5
Computerized decision support systems (CDSSs) have the potential to improve prescribing in older, multimorbid people by minimizing risks associated with the use of multiple drugs.8 One such CDSS is the PRIMA-eDS (Polypharmacy in chronic diseases: Reduction of Inappropriate Medication and Adverse drug events in older populations by electronic Decision Support) tool. The PRIMA-eDS tool was developed to support general practitioners (GPs) in treating their older, multimorbid patients. It is currently being tested in an European multicenter cluster randomized controlled trial (RCT) including patients ≥75 years of age and taking at least 8 drugs. The intervention consists of a comprehensive medication review (CMR) that is provided by the PRIMA-eDS tool to GPs of the intervention group of the trial.
Of course, providing access to a CDSS does not implicate its use.9 Thus, it is important to evaluate factors influencing the use of the PRIMA-eDS tool alongside the investigation of its effectiveness. Recently, we carried out a qualitative study with an exploratory design. To check the relevance of the qualitative findings and quantify them, we conducted a survey with all GPs participating in the intervention group of the PRIMA-eDS trial. These are the only GPs currently using the tool.
Furthermore, we wanted to investigate GPs’ experiences in a multinational sample, as the use of the PRIMA-eDS tool might vary between GPs from different countries due to different cultures and healthcare systems.
The specific aims of this survey are the following:
To assess the level of adoption of the PRIMA-eDS tool (within the PRIMA-eDS RCT).
To assess GPs’ opinions about the content of the CMR and their experience with it.
To evaluate and quantify to what extent factors hinder GPs from realizing recommendations provided by the PRIMA-eDS CMR.
To determine the percentage of the study GPs that are likely to implement the PRIMA-eDS tool in daily practice routine in the future.
To investigate the prerequisites for widespread implementation of the PRIMA-eDS tool among GPs.
To examine whether there are country-specific differences in the answers provided by the GPs regarding the extent of usage of the PRIMA-eDS tool and barriers to deprescribing.
MATERIALS AND METHODS
Participants and procedure
The PRIMA-eDS tool is currently only used within the PRIMA-eDS trial and cannot be accessed outside of the study. We invited all GPs from the intervention group (all of them had access to the CMR) of the PRIMA-eDS trial (n = 176) by letter, fax, or email to participate in the survey. The survey was administered online or via paper (fax or letter) between October 2016 and July 2017 with several reminders sent during that period. At the time of the survey, the GPs had been using the CMR for 1-2 years. Participation was voluntary and there were no incentives to participate in the survey.
Context of the intervention
Participating study centers of the PRIMA-eDS trial are Rostock and Witten in Germany, Salzburg in Austria, Bolzano in Italy, and Manchester in the United Kingdom. The PRIMA-eDS tool aims to reduce inappropriate and non–evidence-based polypharmacy in older and chronically ill people by performing a CMR. GPs of the intervention group had access to the CMR, whereas the control group GPs performed care as usual. More information on the PRIMA-eDS trial can be obtained in the study protocol.10 The PRIMA-eDS trial includes 3904 patients and 359 GPs.11
Intervention (PRIMA-eDS tool)
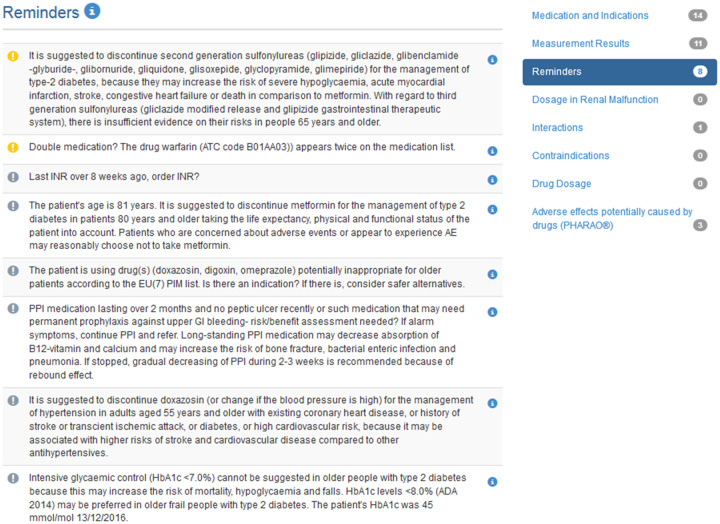
The intervention included the use of the PRIMA-eDS tool, which was developed as part of the European Union–funded project PRIMA-eDS (for details of the project see, www.prima-eds.eu). The tool provided recommendations for drug discontinuation or modification. The PRIMA-eDS tool was supposed to be used at the GP practice during a routine appointment with all study patients at 4 time points (baseline and after 8, 16, and 24 months). Additionally, there was the option to use the PRIMA-eDS tool at any other point during the trial. The PRIMA-eDS tool consisted of 2 parts, the electronic case report form and the CMR. First, relevant patient data (diagnoses, current medications, symptoms, anthropometric measurements, and laboratory values) had to be entered into the electronic case report form to analyze and generate a CMR providing advice for drug discontinuation or modification. Table 1 depicts the components of the CMR and the data sources used. Figure 1 shows excerpts from the CMR. The PRIMA-eDS tool was web based and could be used at any computer with internet access. The CMR was generated on the screen and could also be saved as a PDF and printed. The CMR was intended to support clinical decision-making and not as a substitute for clinical guidelines or clinical judgement. GPs were trained on how to use the PRIMA-eDS tool, and could seek help via a support hotline provided by each study center.
Table 1.
Components of the comprehensive medication review of the PRIMA-eDS tool
| Component | Data source used |
|---|---|
| Check of the indications of current medications | Evidence-Based Medicine Guidelines and evidence summary collection12 |
| Measurement results (laboratory, anthropometric) with alerts | Evidence-Based Medicine Guidelines12 and consensus of the EbMeDS clinical editorial team13 |
| Recommendations about amending current medications based on best available evidence | EbMeDS evidence-based rules14 |
| Systematic reviews on drugs commonly prescribed to older people15 | |
| EU(7)-PIM list16 | |
| Dosage adjustment in renal malfunction | RENBASE database17 |
| Potentially harmful drug-drug interactions | INXBASE database18 |
| Contraindications | Pharmacological literature and summary of medicinal product characteristics by the European Medicines Agency19 |
| Dose warnings | Pharmacological literature and product summaries approved by regulatory authorities |
| Possible adverse drug reactions | RISKBASE database20 |
EbMeDS: Evidence-Based Medicine electronic Decision Support; EU(7)-PIM: European Union (7)-potentially inappropriate medications; PRIMA-eDS: Polypharmacy in chronic diseases: Reduction of Inappropriate Medication and Adverse drug events in older populations by electronic Decision Support.
Figure 1.
Screenshot of the comprehensive medication review tool by Duodecim Medical Publications showing recommendations about amending current medications and recommendations regarding interactions.
Questionnaire
The questionnaire (see Supplementary Appendix 1) was developed incorporating the results of a prior qualitative investigation conducted with 21 GPs of the intervention group cohort of Witten, Germany. This qualitative study had explored the usage of the PRIMA-eDS tool and the adoption of the recommendations provided by the PRIMA-eDS CMR.21
The questionnaire had the aim to quantify the results of the qualitative interviews in a larger multinational sample. Therefore, questions and answer options included in the survey were formulated from the main interview results. The survey enquired about the use of and attitudes toward the CMR, its recommendations, and future use. Besides, demographic data of the participating GPs were gathered. Attitudes were assessed with a 5-point Likert-type scale and additionally respondents had the option to answer “don’t know.” The questionnaire was piloted by 2 physicians and modified according to their feedback. The questionnaire was developed in German and forward-backward translated into English and Italian.
Statistical analysis
We used descriptive statistics to describe the study sample and the GPs’ answers to the questions applying mainly cross tabulation analysis. Chi-square tests were used to test for statistical significance of group differences if applicable. To check for country-specific differences regarding GPs’ attitudes toward the CMR, we compared the results of all Likert-type scales using chi-square statistics. Two-sided tests were used and a significance level of α = .05 was applied. Data were analyzed using the statistical software SPSS version 24.
RESULTS
Characteristics of respondents
In total, 160 of 176 questionnaires were returned from GPs in Austria, Italy, Germany, and the United Kingdom (response rate 91%). Most questionnaires were filled in completely. The n values are reported if there were missing data. Characteristics of the GPs are presented in Table 2.
Table 2.
Characteristics of general practitioners (n = 160)
| Female | 67 (42) |
|---|---|
| Age (n = 167), y | 55 (35–71) |
| Study center | |
| Bolzano, Italy | 32 (20) |
| Manchester, United Kingdom | 28 (17) |
| Rostock, Germany | 41 (26) |
| Salzburg, Austria | 24 (15) |
| Witten, Germany | 35 (22) |
Values are n (%) or median (range).
Adoption of the PRIMA-eDS tool
At the time of the survey, 18% of the GPs had conducted the CMR for all study patients, as well as additional medication reviews not required according to the protocol of the RCT. Sixty percent had conducted all the CMRs for their study patients as required by the study protocol of the RCT, and 18% for only some of the study patients but not all. Four percent did not answer the question.
GPs did not always follow the recommendations of the CMR. The average numbers of drugs discontinued according to the GPs’ estimate are presented in Table 3. Only 20% of the GPs who discontinued medications reported never having to restart a discontinued drug. All others stated that this was necessary: 35% rarely, 37% occasionally, and 8% often.
Table 3.
Drug discontinuation by country
| Average drug discontinuation per patient (GP estimate) | Germany (%) | United Kingdom (%) | Italy (%) | Austria (%) | All centers (%) |
|---|---|---|---|---|---|
| None | 37 | 39 | 19 | 14 | 32 |
| 1 | 50 | 57 | 72 | 64 | 57 |
| 2 or more | 13 | 4 | 9 | 22 | 11 |
GP: general practitioner.
Of the participating GPs, 66% responded that they had discussed the recommendations with their patients, 30% discussed the recommendations to some extent, and 4% did not discuss the recommendations at all. Out of those who discontinued medication, 74% said that they discussed the recommendations with their patients, with 26% discussing the recommendations to some extent. Out of those who continued prescribing the complete medication despite the recommendations of the tool, 48% had discussed the recommendations with their patients, 40% to some extent, and 12% not at all. The 4% that did not discuss recommendations with the patients did not follow any of the recommendations of the CMR to discontinue a drug.
Attitude toward the CMR
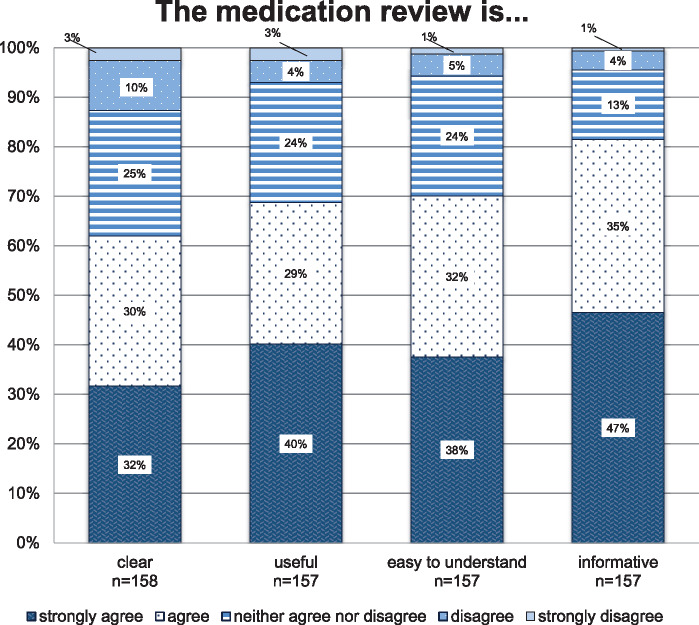
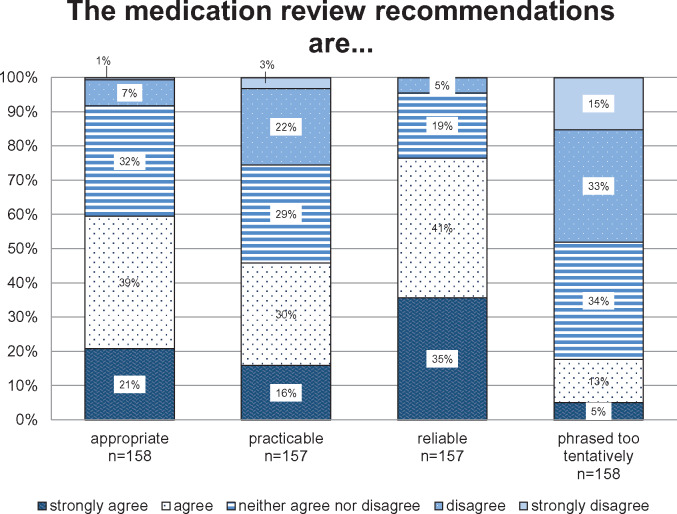
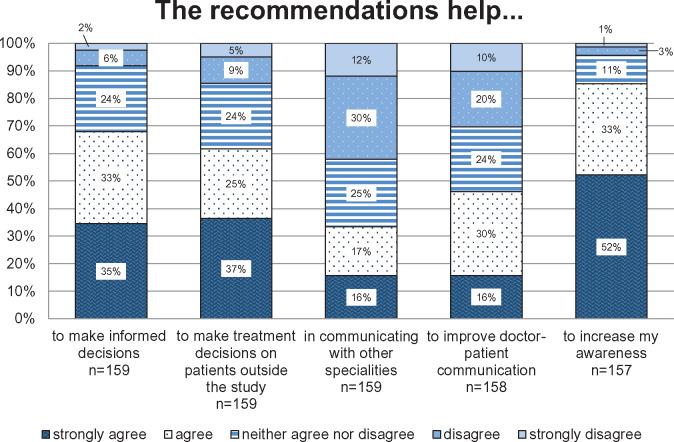
Respondents judged the CMR to be informative (82%), easy to understand (70%), useful (69%), and clear (62%) (details see Figure 2). Recommendations were perceived as reliable by 76% of the respondents, as appropriate by 60%, and as practicable by 46%. Eighteen percent stated that the recommendations were phrased too tentatively (details see Figure 3). Figure 4 outlines to what extent the recommendations provided by the CMR supported the GPs in caring for their older polypharmacy patients. The majority of the GPs agreed or strongly agreed that the recommendations increased their awareness (85%), helped them to make informed decisions (68%), and assisted them in making treatment decisions on patients outside the study (62%). Forty-six percent of the GPs agreed or strongly agreed that the PRIMA-eDS tool serves to improve doctor-patient communication, and 33% agreed or strongly agreed that it helps to better communication with other specialists.
Figure 2.
Attitudes toward the comprehensive medication review. The y-axis presents the percentage of general practitioners giving answers in each of the Likert-type categories. The x-axis depicts the survey question (in combination with the headline).
Figure 3.
Attitudes toward the recommendations provided by the comprehensive medication review. The y-axis presents the percentage of general practitioners giving answers in each of the Likert-type categories. The x-axis depicts the survey question (in combination with the headline).
Figure 4.
Experiences with the recommendations provided by the comprehensive medication review. The y-axis presents the percentage of general practitioners giving answers in each of the Likert-type categories. The x-axis depicts the survey question (in combination with the headline).
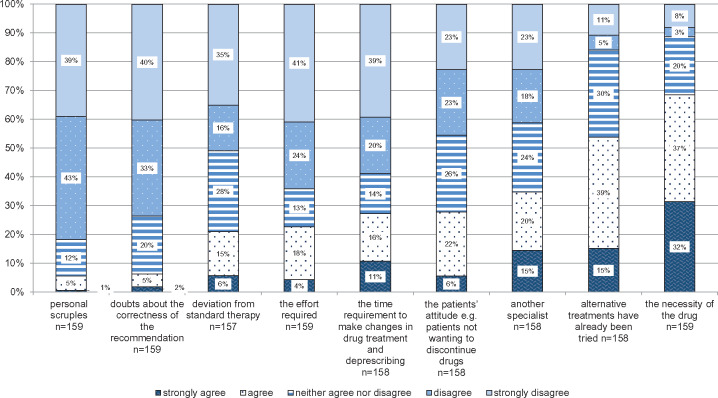
Barriers to implement recommendations
Respondents rated the following aspects as barriers to implementing the recommendations of the CMR: the perceived necessity of the medication (69%), prior trial of suggested alternative medications (54%), and another specialist being involved in prescribing the medication (35%). Only a minority of the GPs considered the patients’ attitude, for example, patients not wanting to discontinue drugs (28%), the time requirement to make changes in drug treatment and deprescribing (27%), the effort required to make changes to the patients’ drug treatment plan (23%), the perceived deviation from standard therapy (21%), doubts about the correctness of the recommendations (7%), or personal scruples (6%), as barriers to implementation of the tool (details see Figure 5).
Figure 5.
Perceived barriers toward implementing recommendations provided by the comprehensive medication review. The y-axis presents the percentage of general practitioners giving answers in each of the Likert-type categories. The x-axis depicts the survey question (in combination with the headline).
Future use
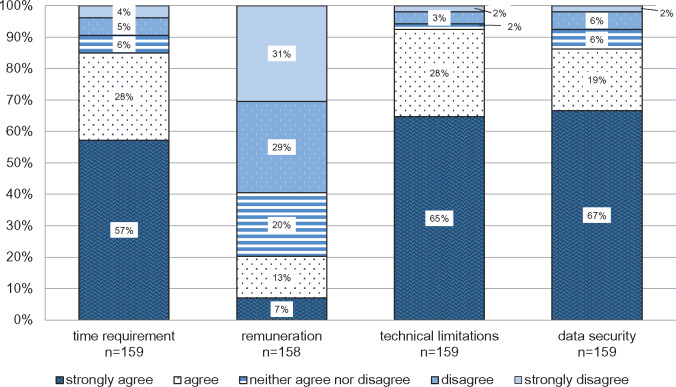
Figure 6 shows prerequisites for the future use of the PRIMA-eDS tool in everyday practice. Technical limitations are rated by 93% of GPs as important for the future use of the PRIMA-eDS tool, data security by 86%, and time requirement by 85%. Remuneration is important only for 20% of the GPs. Sixty-five percent said that they would use the CMR in their own practice if it was part of the electronic health record, another 25% would possibly, and 10% would not use the PRIMA-eDS tool.
Figure 6.
Important factors for the use of the medication review. The y-axis presents the percentage of general practitioners giving answers in each of the Likert-type categories. The x-axis depicts the survey question (in combination with the headline).
Country-specific differences
A statistically significant difference between the countries of the GPs could be detected regarding the extent of CMR usage (P = .001). Germany showed the highest percentage of GPs (23%) who performed CMRs only in some, but not all, of the study patients. GPs in the United Kingdom scored highest (47%) at conducting CMRs not only for all study patients, but also additional CMRs not required for research. Most of the Austrian GPs (79%) completed only the CMRs that were required for the trial. Furthermore, a significant relationship was detected between the GPs perceiving the patient as a barrier to following the recommendations provided by the CMR and country (P = .003). German GPs rated highest at disagreeing (77%), whereas the United Kingdom had the highest percentage (62%) of GPs agreeing. Another barrier to implementing the recommendations, which differed significantly between the countries, was time requirement (P = .006). German GPs rated highest (81%) at disagreeing, whereas GPs from the United Kingdom rated highest (52%) at agreeing. Only statistically significant differences between study centers are presented in Table 4. Barriers that did not differ between the countries were the perceived necessity of the medication, the absence of therapeutic alternatives, deviation from standard therapy, and prescription of the medication by another specialist (data not shown).
Table 4.
Associations between country of GP and performance of medication review, the patient as a barrier, and the time requirement as a barrier
| Topic; survey question | Answer choice | Germany (n = 79) | United Kingdom (n = 28) | Italy (n = 32) | Austria (n = 14) | Total (n = 153) | Significance X2 test |
|---|---|---|---|---|---|---|---|
| Performance of medication review; For PRIMA-eDS patients I performed the study medication review… | For some study patients | 23% | 14% | 16% | 7% | 18% | 0.001 |
| For all study patients, but only the medication reviews required for research | 70% | 39% | 59% | 79% | 63% | ||
| For all study patients, and additional medication reviews not required for research | 7% | 47% | 25% | 14% | 19.0% | ||
| Total respondents | 79 | 28 | 32 | 14 | 153 | ||
| Barriers; A barrier to implementing the recommendations is the patient. | Strongly agree | 5% | 14% | 9% | 11% | 8% | 0.009 |
| Agree | 18% | 48% | 43% | 45% | 30% | ||
| Disagree | 31% | 38% | 29% | 22% | 31% | ||
| Strongly disagree | 46% | 0% | 19% | 22% | 31% | ||
| Total respondents | 65 | 21 | 21 | 9 | 116 | ||
| Barriers; A barrier to implementing the recommendations is the time requirement. | Strongly agree | 11% | 5% | 10% | 42% | 13% | 0.000 |
| Agree | 8% | 47% | 35% | 0% | 19% | ||
| Disagree | 50% | 48% | 38% | 33% | 45% | ||
| Strongly disagree | 31% | 0% | 17% | 25% | 23% | ||
| Total respondents | 74 | 21 | 29 | 12 | 136 |
GP: general practitioner; PRIMA-eDS: Polypharmacy in chronic diseases: Reduction of Inappropriate Medication and Adverse drug events in older populations by electronic Decision Support.
DISCUSSION
The majority of the GPs participating in our survey made use of the PRIMA-eDS tool as proposed by the study protocol and followed at least some recommendations provided by the CMR. Most found the recommendations to be appropriate, reliable, and helpful to make informed decisions, even regarding treatment of patients outside the study. Despite the positive ratings, GPs did not always follow the recommendations due to various reasons such as that the drug was seen as necessary. The most important factor for future use is time requirement, and the majority of GPs would use the CMR in routine care if it was part of their electronic health record.
In our survey, the majority of GPs reported that they used the PRIMA-eDS CMR at least to a certain extent, but only few used it extensively, and some apparently did not even use it for all of the study patients. So we have had similar experiences as Clyne et al,22 who observed in a study in Ireland that GPs did not do all medication reviews as required by the protocol.
The rate of additional CMRs conducted in addition to those required for the study was especially high in the United Kingdom and lowest in Germany. We had instructed the GPs to conduct CMRs at an appointment that would have happened anyway so that no extra visit for the patient needed to be scheduled. However, this might have limited the number of conducted CMRs as well as the usage of the CMR results. Previous research has shown that physicians ignored alerts when this was not the reason for the patients’ visit, as often there was not enough time to deal with both.23,24
Recommendations provided by the PRIMA-eDS CMR were not always implemented. Disregarding recommendations is common and rates vary per study. A systematic review (SR) on computerized physician order entry systems that include drug safety alerts showed that depending on the kind of alert 27%-96% of alerts were overridden.25 Clyne et al22 found that 61% of potentially inappropriate prescriptions were changed, whereas Tamblyn et al26 reported an implementation rate of only 12%, and Hammar et al27 reported that only 11% of alerts were followed by physicians.
In this survey, only those GPs that stated to have discussed the recommendations with their patients discontinued drugs, suggesting that patient involvement is central to deprescribing. Even though there is no evidence so far that shared decision-making improves clinically relevant outcomes,28 it has been shown that pharmaceutical care can be improved.29 We do not know why GPs did not discuss recommendations at all or only to some extent, but it could be that those GPs wanted to maintain control or did not take the time for discussion.30 Besides, we do not know how GPs conducted the shared decision-making process, as they can influence with their behavior the extent to which patients are actually involved.31
In an SR on the usage of CDSSs, Moxey et al highlighted that apart from the integration with workflow, CDSS specific factors such as the CDSS content (ie, relevance, quality, and type of information) and its presentation are factors determining use.9 Similarly, perceived usefulness,24 reliability, relevant information, and readability of alerts are enhancing usage,23 while physicians dislike alerts showing already known or repetitive information.32 In our study, the overall judgement of usefulness and reliability of the CMR and the recommendations provided was quite positive and should thus support usage. Our results are in a similar range as the CMR judgments reported by Kortteisto et al.24 Perceived usefulness was judged slightly higher in the PRIMA-eDS tool, and the perceived reliability of the recommendations was almost the same for both. Besides, GPs judged our CMR as informative, appropriate, and easy to understand, increasing their awareness and supporting in making informed decisions. Furthermore, a learning effect seems to have taken place, as many GPs stated that they were able to transfer the recommendations to patients that did not participate in the study.
A further aspect expressed in the prior qualitative investigation was the criticism that the recommendations are phrased too tentatively21; however, this was not confirmed by this quantitative survey.
Deprescribing is “the process of withdrawal of an inappropriate medication, supervised by a healthcare professional with the goal of managing polypharmacy and improving outcomes.”33 In our study, the greatest barrier to deprescribing appeared to be the perceived necessity of the medication when alternative treatments had already been tried. This is also reflected by the fact that less than halve of the GPs perceive the recommendations as practicable. Indeed, Anderson et al34 reported that poor acceptance of alternatives and limited availability of alternatives are barriers to reducing potentially inappropriate medication.
Furthermore, our GPs stated that specialists are a barrier to implementing recommendations provided by the PRIMA-eDS CMR. Conflicts or disagreement with specialists have previously been described in the literature.35,36 Care for multimorbid patients is often not well coordinated and communicated between the various providers.37,38 Thus, GPs are not always aware of the specialists’ motivation in prescribing and find this information difficult to get.39 Good communication between GPs and the specialists is therefore important to facilitate deprescribing.40
GPs mentioned that at times the patient hampered them from deprescribing. The patient as a barrier has also been described previously.34 Nowadays many GPs recognize that patients’ believes and ideas about drug treatment play an important role in the deprescribing process.41 However, if there are conflicts between the patients’ and the physicians’ treatment goals this can make treatment decisions more difficult.35 Patients can have a multitude of reasons why they do not agree with ceasing a medication such as due to fear or due to finding the medication necessary.42 We found national differences in how far GPs experience the patient as a barrier. In Germany, GPs rarely expressed that patients hindered deprescribing. On the contrary, GPs in the United Kingdom more frequently perceived the patient as a barrier. Schoen et al38 showed that the percentage of patients who feel that within GP consultations they are encouraged to ask questions and receive in-depth explanations of choices is highest in the United Kingdom compared with Germany. It could be that German GPs have a more paternalistic approach and advise rather than discuss in detail with their patients, thus giving the patient less of a chance to object, which in turn lets them experience the patient less as a barrier.
In the literature, time pressure has been described as hindering deprescribing.34 Fried et al35 reported that time and effort needed for decision-making poses a barrier for deprescribing and, similarly, Moen et al39 reported that time constraints impede patient conversation, leading to medications not being ceased. In our study, more GPs from the United Kingdom thought time to be a barrier to deprescribing than those in other countries. This is interesting, given that the consultations in the United Kingdom are generally longer compared with the other countries involved in this study. An SR of international variations in primary care physician consultation time showed that the mean duration of a consultation in the United Kingdom ranges from 8.65 to 11.7 minutes. In contrast, the mean consultation time is estimated to be 5 minutes in Austria and 7.6 minutes in Germany.43 Possibly GPs in the United Kingdom experienced the time required for deprescribing in the PRIMA-eDS study higher because they conducted CMRs more often and experienced a larger time requirement for communication with the patient and the shared decision-making process.
Some barriers to deprescribing brought up in a prior qualitative investigation were only mentioned by a minority of the surveyed GPs, such as the deviation from standard therapy.21 Guidelines usually advise GPs to provide standard therapy, but guidelines rarely take old age or multimorbidity into account.4 In a qualitative SR on perceived barriers and enablers to reducing potentially inappropriate medications, physicians found guidelines to negatively impact on deprescribing, as they often feel compelled to adhere to guidelines.34
Furthermore, we investigated whether personal scruples could hinder deprescribing, which GPs denied in this survey. A similar barrier namely that “prescribing is kind and meets needs”34 was found by Anderson et al,34 reflecting cultural barriers to not giving a drug. It looks like GPs in our study did not have the feeling of needing to prescribe and did not have any cultural or personal barriers toward deprescribing in these older polypharmacy patients.
Previously, we discussed the importance of adequate and reliable recommendations as an important factor for CDSS acceptance. Apart from hindering the use of the CDSS, incorrect recommendations can obviously also directly impact on deprescribing. Even though in the prior qualitative investigation doubts were expressed as to the correctness of the recommendations,21 this barrier was not confirmed by this survey.
Regarding the future implementation, the issue of time was an essential component for the GPs. The prior qualitative investigation revealed that the PRIMA-eDS tool as it is now is too time-consuming.21 Interestingly, GPs did not rate reimbursement as important, though complaints about insufficient reimbursement for caring for multimorbid older patients were brought up in other studies.4,35 One has to note though that time and reimbursement are closely linked, as increased reimbursement could enable GPs to take more time for their patients.34
Implications for future implementation and use
Although the PRIMA-eDS CMR can support GPs in reviewing medications, the process of deprescribing still takes time. This study underlines the importance of a shared decision-making process in deprescribing, which is particularly time-consuming. The PRIMA-eDS CMR supports GPs in reducing drugs and in quickly obtaining relevant, useful, and reliable information about medications for complex patients. Future developments of the PRIMA-eDS CMR should focus on how to reduce time to conduct the CMR and link it to electronic health records. Additionally, healthcare systems should allocate enough resources for deprescribing, so that CMRs like PRIMA-eDS can be conducted and properly used to provide high-quality care for older multimorbid patients. In addition, efforts should be made to enhance coordination and communication between the various physicians involved in caring for a patient so that all relevant information is known to the GP.
Methodological considerations
To achieve a high response rate, we kept the questionnaire short, using only a minimum number of questions and measuring each item by 1 variable only. This was good for the conduct of the survey and the response rate, but it lowered measurement specificity. We structured the online questionnaire the way that physicians were obliged to answer completely or to stop, whilst in the paper-based version questions could be skipped. In Germany and Austria, only the paper version of the survey was used, as the study centers believed that this would achieve the highest response rate. In the United Kingdom and Italy, the survey was mainly conducted online. We sent the paper version to those that did not fill in the survey online. GPs in this study were already preselected as voluntary participants of the randomized controlled PRIMA-eDS trial and do not necessarily represent the whole population, as they are more likely to be interested in polypharmacy and the use of CDSS. Nonetheless, they are an international population and are highly important for future implementation. Furthermore, one has to note that the analysis was done exploratory without correcting for multiple testing. Thus, significant results need to be interpreted with caution.
CONCLUSION
The PRIMA-eDS CMR was used by the majority of GPs. Generally, GPs regarded the CMR as useful for GPs’ decision making, providing reliable and appropriate recommendations. However, recommendations were not always perceived as practicable and a large proportion of the recommendations were not implemented. Those who did not discuss medications with their patients probably did not cease any drugs. There are several barriers that hinder GPs in following the CMR recommendations such as that the medication is perceived as necessary or that alternative treatments have been tried. Time was considered to be the most important factor for future implementation; thus, further development should focus on how the PRIMA-eDS tool could become more time efficient. Despite the difficulties in deprescribing, many GPs would like to use the CMR in the future, if it was part of their electronic health record.
FUNDING
This study is funded by the 7th framework programme of the European Union, theme Health-2012-Innovation-1-2.2.2-2, grant agreement no. 305388-2.
AUTHOR CONTRIBUTIONS
AR and AS conceptualized and designed the study. AR designed the questionnaire, supported by all authors. A-LT, ED, CK, and GP collected the data, which was entered by A-LT. AR performed the analysis, supported by A-LT. AR drafted the manuscript. All authors critically reviewed the manuscript and read and approved the final manuscript.
ETHICS APPROVAL
The PRIMA-eDS study has been approved by the 5 local ethics committees: 1. Ethikkomission der Universität Witten/Herdecke, 3 December 2013, ref. 103/2013; 2. NRES Committee North West Greater Manchester East, 6 June 2014, ref. 14/NW/0197; 3. Ethikkommission für das Bundesland Salzburg, 15 September 2013, ref. 08.04.2014 (415-E/1509/20-2014); 4. Ethikkommission der Universitätsmedizin Rostock, 3 February 2014, ref. A 2014-0020; and 5. Comitato etico di Belluno (Azienda ULSS), 19 June 2013, ref. 305388-2. The need for an additional ethics approval for this survey which is part of the process evaluation of the PRIMA-eDS-trial has been waived by the ethics committee of Witten/Herdecke University.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
Supplementary Material
ACKNOWLEDGMENTS
We would like to express our gratitude to all participating general practitioners. Furthermore, we would like to thank the PRIMA-eDS team for their support in collecting data. The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest statement None declared.
REFERENCES
- 1. Bushardt RL, Massey EB, Simpson TW, Ariail JC, Simpson KN.. Polypharmacy: misleading, but manageable. Clin Interv Aging 2008; 3: 383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eurostat. Population Structure and Ageing. http://ec.europa.eu/eurostat/statistics-explained/index.php/Population_structure_and_ageing#Further_Eurostat_information. Accessed October 4, 2018.
- 3. Violan C, Foguet-Boreu Q, Flores-Mateo G, Salisbury C, Blom J, Freitag M, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One 2014; 97: e102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW.. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA 2005; 2946: 716–24. [DOI] [PubMed] [Google Scholar]
- 5. Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T.. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995-2010. BMC Med 2015; 13: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rollason V, Vogt N.. Reduction of polypharmacy in the elderly. Drugs Aging 2003; 20: 817–32. [DOI] [PubMed] [Google Scholar]
- 7. Viktil KK, Blix HS, Moger TA, Reikvam A.. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol 2007; 632: 187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molokhia M, Majeed A.. Current and future perspectives on the management of polypharmacy. BMC Fam Pract 2017; 181: 70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moxey A, Robertson J, Newby D, Hains I, Williamson M, Pearson S-A.. Computerized clinical decision support for prescribing: provision does not guarantee uptake. J Am Med Inform Assoc 2010; 171: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sönnichsen A, Trampisch US, Rieckert A, et al. Polypharmacy in chronic diseases–reduction of inappropriate medication and adverse drug events in older populations by electronic decision support (PRIMA-eDS): study protocol for a randomized controlled trial. Trials 2016; 17: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rieckert A, Trampisch US, Klaaßen-Mielke R, et al. Polypharmacy in older patients with chronic diseases: a cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam Pract 2018; 19: 113.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duodecim. Evidence-based medicine guideline. https://www.duodecim.fi/english/products/ebmg. Accessed May 3, 2018.
- 13. Duodecim. Evidence-Based Medicine Electronic Decision Support. https://www.ebmeds.org/en/. Accessed June 26, 2019.
- 14. Duodecim. EBMeDS Clinical Decision Support. http://www.ebmeds.org. Accessed May 3, 2018.
- 15. Martinez YV, Renom-Guiteras A, Reeves D, et al. A set of systematic reviews to help reduce inappropriate prescribing to older people: study protocol. BMC Geriatr 2017; 17 suppl 1: 231. doi: 10.1186/s12877-017-0570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Renom-Guiteras A, Meyer G, Thurmann PA.. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol 2015; 717: 861–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Medbase. Renbase-Analysis of Adverse Drug Reactions. http://www.medbase.fi/en/professionals/renbase. Accessed May 3, 2018.
- 18. INXBASE Interaction Database. http://www.medbase.fi/en/professionals/inxbase. Accessed May 3, 2018.
- 19. European Medicines Agency. http://www.ema.europa.eu/ema. Accessed May 3, 2018.
- 20. Medbase. RISKBASE–Analysis of Adverse Drug Reactions. http://www.medbase.fi/en/professionals/riskbase. Accessed May 3, 2018.
- 21. Rieckert A, Sommerauer C, Krumeich A, Sönnichsen A.. Reduction of inappropriate medication in older populations by electronic decision support (the PRIMA-eDS study): a qualitative study of practical implementation in primary care. BMC Fam Pract 2018; 19: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clyne B, Cooper JA, Hughes CM, Fahey T, Smith SM.. A process evaluation of a cluster randomised trial to reduce potentially inappropriate prescribing in older people in primary care (OPTI-SCRIPT study). Trials 2016; 17: 386.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lugtenberg M, Weenink J-W, van der Weijden T, Westert GP, Kool RB.. Implementation of multiple-domain covering computerized decision support systems in primary care: a focus group study on perceived barriers. BMC Med Inform Decis Making 2015; 15: 1339.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kortteisto T, Komulainen J, Mäkelä M, Kunnamo I, Kaila M.. Clinical decision support must be useful, functional is not enough: a qualitative study of computer-based clinical decision support in primary care. BMC Health Serv Res 2012; 12: 349.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Sijs H, Aarts J, Vulto A, Berg M.. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 2006; 132: 138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamblyn R, Huang A, Taylor L, et al. A randomized trial of the effectiveness of on-demand versus computer-triggered drug decision support in primary care. J Am Med Inform Assoc 2008; 154: 430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hammar T, Lidström B, Petersson G, Gustafson Y, Eiermann B.. Potential drug-related problems detected by electronic expert support system: physicians' views on clinical relevance. Int J Clin Pharm 2015; 375: 941–8. [DOI] [PubMed] [Google Scholar]
- 28. Willeboordse F, Hugtenburg JG, Schellevis FG, Elders P.. Patient participation in medication reviews is desirable but not evidence-based: a systematic literature review. Br J Clin Pharmacol 2014; 786: 1201–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geurts MME, Talsma J, Brouwers J, De Gier JJ.. Medication review and reconciliation with cooperation between pharmacist and general practitioner and the benefit for the patient: a systematic review. Br J Clin Pharmacol 2012; 741: 16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belcher VN, Fried TR, Agostini JV, Tinetti ME.. Views of older adults on patient participation in medication-related decision making. J Gen Intern Med 2006; 214: 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stevenson FA, Cox K, Britten N, Dundar Y.. A systematic review of the research on communication between patients and healthcare professionals about medicines: the consequences for concordance. Health Expect 2004; 73: 235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hume AL, Quilliam BJ, Goldman R, Eaton C, Lapane KL.. Alternatives to potentially inappropriate medications for use in e-prescribing software: triggers and treatment algorithms. BMJ Qual Saf 2011; 2010: 875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reeve E, Gnjidic D, Long J, Hilmer S.. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol 2015; 806: 1254–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson K, Stowasser D, Freeman C, Scott I.. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open 2014; 412: e006544.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fried TR, Tinetti ME, Iannone L.. Primary care clinicians' experiences with treatment decision making for older persons with multiple conditions. Arch Intern Med 2011; 1711: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schuling J, Gebben H, Veehof LJG, Haaijer-Ruskamp FM.. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract 2012; 13: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sinnott C, Mc Hugh S, Browne J, Bradley C.. GPs' perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open 2013; 39: e003610.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schoen C, Osborn R, Squires D, Doty M, Pierson R, Applebaum S.. New 2011 survey of patients with complex care needs in eleven countries finds that care is often poorly coordinated. Health Aff (Millwood) 2011; 3012: 2437–48. [DOI] [PubMed] [Google Scholar]
- 39. Moen J, Norrgård S, Antonov K, Nilsson JLG, Ring L.. GPs' perceptions of multiple-medicine use in older patients. J Eval Clin Pract 2010; 161: 69–75. [DOI] [PubMed] [Google Scholar]
- 40. Milton JC, Hill-Smith I, Jackson S.. Prescribing for older people. BMJ 2008; 3367644: 606–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ailabouni NJ, Nishtala PS, Mangin D, Tordoff JM.. Challenges and enablers of deprescribing: a general practitioner perspective. PLoS One 2016; 114: e0151066.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD.. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging 2013; 30: 793–807. [DOI] [PubMed] [Google Scholar]
- 43. Irving G, Neves AL, Dambha-Miller H, et al. International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open 2017; 710: e017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.