Abstract
Objective:
In this study, we investigated the preventing effects of Curcumin (Cur) against titanium dioxide nanoparticle (NTiO2)-induced mouse testicular damage.
Methods:
We assessed NTiO2-intoxicated mice received 50mg/kg of NTiO2 for 35 days. The Cur + NTiO2 group was pretreated with Cur (200 mg/kg) for 7 days prior to administering NTiO2. Sperm parameters, testosterone concentration, histological criteria, morphometric parameters and Johnsen's scoring.
Results:
NTiO2 significantly reduced testicular weight, testosterone concentration, morphometric parameters, Johnsen's scoring and sperm quality (p<0.01), as well as a significant increase in histological criteria. Pretreatment with Cur reduced testicular weight, ameliorated morphometric parameters, increased Johnsen's scoring, elevated testosterone levels, and increased histological criteria such as vacuolization, detachment, and sloughing of germ cells into the seminiferous tubules. Cur also improved sperm parameters including sperm count, motility, and percentage of abnormality.
Conclusion:
Cur was found to have a potent protective effect against spermatogenesis defects induced by nanoparticles in mice.
Keywords: curcumin, nanoparticle, spermatogenesis, testosterone, sperm quality, mice
INTRODUCTION
Many recent in vivo studies have shown that most nanoparticles (NPs) have toxic effects on male germ cells (Iavicoli et al., 2013; Brohi et al., 2017). NPs have the capacity to cross the blood-testis barrier and induce testicular toxicity (Lan & Yang, 2012). Among the various metal nanomaterials, titanium dioxide NPs (NTiO2) is used in a variety of consumer products such as sunscreens, cosmetics, clothing, electronics, paints and surface coatings (Cox et al., 2016). NTiO2 has been shown to cause reproductive toxicity including spermiation failure, low sperm production, and abnormal sperm morphology in mice (Miura et al., 2014). In vitro studies have also shown that NTiO2 induces cellular toxicity in the reproductive system (Hong et al., 2016). NTiO2 can be absorbed by spermatids, Sertoli cells, and Leydig cells, and induce histological changes in seminiferous tubules, causing damage to sperm production, sperm motility, and Sertoli cell number (Gao et al., 2013).
Since the high-rate use of NPs, such as NTiO2, affects male reproduction or male reproductive function, it seems crucial to find a suitable drug for reducing its toxic effects. Curcumin (Cur), a yellow pigment present in the rhizome of turmeric (Curcuma longa), has several pharmacological properties including anti-inflammatory, anti-carcinogenesis, antioxidant, and hypocholesterolemic activities (Hewlings & Kalman, 2017). Previous studies showed beneficial effects of Cur against testis damages. For example, Cur inhibited the testicular damage induced by alcohol, cisplatin, aflatoxin, metronidazole, ischemia reperfusion and cadmium (Giannessi et al., 2008; Ilbey et al., 2009; Mathuria & Verma, 2007; Noorafshan et al., 2010; Wei et al., 2009; Salama & El-Bahr, 2007). Therefore, we performed the present study to evaluate the preventive effects of Cur on NTiO2-induced testicular damage in the mouse.
MATERIAL AND METHODS
Animals
Thirty-two NMRI mice (6-8 weeks, 25-30 g) were used in this study. The mice were kept under a light-dark cycle of 12:12 at a temperature of 22±3ºC and a humidity of 50%±5%. They were given free access to water and pellets (commercial food). Our study was approved by the Ethics committee of the Ahvaz Jundishapur University of Medical Sciences (approved number: IR.AJUMS.REC. 1393s98).
Experimental design
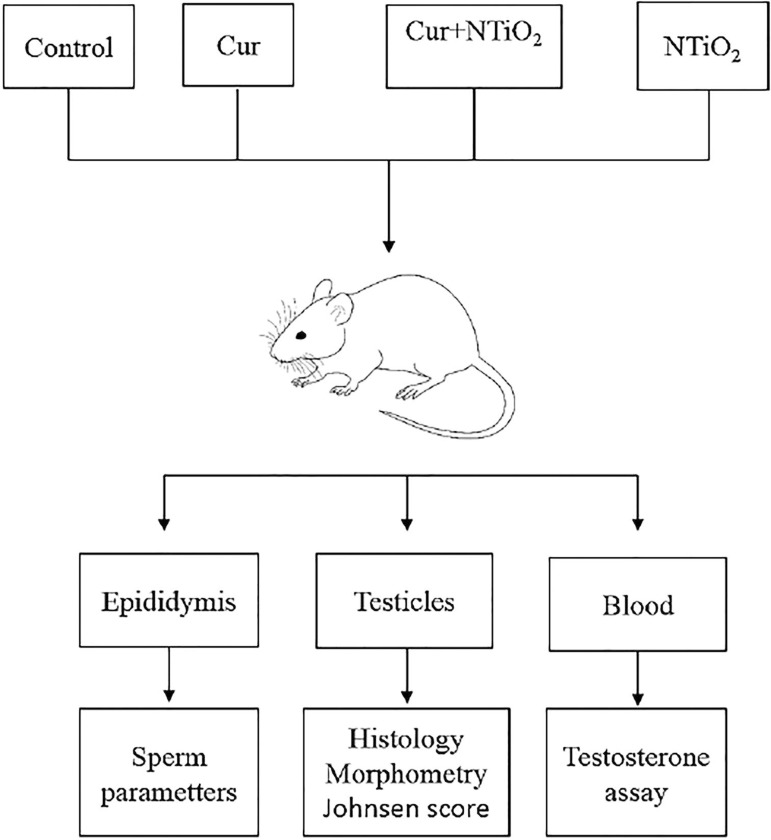
The study mice were randomly assigned to 4 groups (8 animals in each group) described below (Fig. 1):
Figure 1.
Schematic illustration of experimental design.
Group 1 (Control): received normal saline (0.2 ml) for 42 days.
Group 2 (Cur): was treated with Cur (200 mg/kg) for 42 days (Al-Rubaei et al., 2014),
Group 3 (NTiO2): was administered first by normal saline (0.2 ml) for 7 days and then simultaneously with NTiO2 (50mg/kg) for 35 days (Song et al., 2017).
Groups 4 (Cur + NTiO2): received first 200 mg/kg of Cur for 7 days followed by concomitant administration of 50mg/kg NTiO2 for 35 days.
All treatments were given by gavage. Twenty-four hours after the last administration, the mice were sacrificed, and the testicles from each animal were dissected, weighed and maintained in 10% formalin. Paraffin sections (5 µm) were prepared and stained with hematoxylin and eosin (H&E) for histology, Johnsen's scoring and morphometric evaluations.
Nanoparticle preparation
The NTiO2 (Sigma-Aldrich Co.) was diluted by mili-Q water (Song et al., 2017). A dynamic light scattering approach of Zetasizer-Nano-ZSP (Malvern, UK) was used to analyze the mean particle size distribution, polydispersity index (PDI) and zeta potential. The mean particle size was 68.4±5.7. The zeta potential value was measured to be +26.2 mV, high enough to make the NPs repel each other and prevent particle aggregation. The PDI value was calculated to be 0.19, showing an excellent homogeneous ZNP size distribution.
Testosterone assay
The blood samples were collected from the mice heart and centrifuged to obtain serum. Serum testosterone concentration was measured using a commercial Testosterone ELISA kit (SE120089, Sigma, St Louis, MO, USA) according to manufacturer's instructions.
Morphometry
The diameters of the seminiferous tubules and the lumen diameter were measured using the Mutic Images Plus 3.0 software. The height of the seminiferous epithelium was calculated by subtracting the lumen diameter from the tubules' diameter. For each animal, 100 tubules were analyzed.
Histology
Six microscopy slides per animal were examined for signs of testicular damage, including detachment of spermatogenic cells from the basal lamina, sloughing of germ cells and vacuolization in the germinal epithelium. We calculated the average percentage of affected tubules for each feature.
Assessment of spermatogenesis
The maturity of the germinal epithelium was graded using the Johnsen's scoring system (Johnsen, 1970), a simple method for the evaluation of spermatogenesis. In each mouse, 150 tubules were considered and a score ranging from 1 to 10 was given for each tubule. The tubules with complete inactivity were scored as 1 and those with maximum activity (at least 5 or more spermatozoa in the lumen) were scored as 10.
Sperm parameters
Spermatozoa were collected from the right epididymal cauda for examination of sperm motility using the Computer Assisted Semen Analysis (CASA) system (Hamilton Thorne, USA). The sperms were scored as immotile if no movement was detected. The percentages of rapid progressive, slow progressive and no progressive sperms were evaluated in each sample. A suspension of spermatozoa (from the left epididymal cauda) was prepared and counted using a Neubauer hemocytometer. One drop of the suspension was smeared and observed under a light microscope for morphological assessments (Goodson et al., 2011; Hajshafiha et al., 2013).
Statistical analysis
The data analysis was performed using the SPSS (version 21.0, Chicago, IL, USA) one-way analysis of variance (ANOVA), followed by post-hoc pairwise comparison applying the Bonferroni or Mann-Whitney U test. The variables were tested in SPSS for normality distribution and homogeneity. Furthermore, a p-value of less than 0.05 was considered statistically significant.
RESULTS
Weight change
No significant differences in body weight were seen between the Control and Experimental groups. The weight of the testicles in the Cur group was slightly higher than that of the Control group. A significant reduction in testicular weight was found in the NTiO2 group (p<0.05). In the NTiO2 + Cur-treated animals, a significant increase in testicular weight in comparison with the NTiO2- intoxicated mice (p<0.05) was observed (Table 1).
Table 1.
Testis and body weight of the Control and Experimental groups
| Groups | Body weight (g) | Testisweight (mg) | Testis/body (mg/g) |
|---|---|---|---|
| Control | 30.2±1.7 | 114.6±13.9 | 3.81±0.16 |
| Cur | 30.3±2.2 | 115.1±16. 4 | 3.84±0.21 |
| NTiO2 | 28.3±1.2 | 82.1±17.4* | 2.9±0.20* |
| Cur + NTiO2 | 30.1±1.9 | 107.4±15.5† | 3.56±0.12† |
Values are expressed as mean ± SD for 8 mice.
p<0.05,
p<0.05;
* and † symbols respectively indicate comparison to control and NTiO2-intoxicated groups.
Testosterone assay
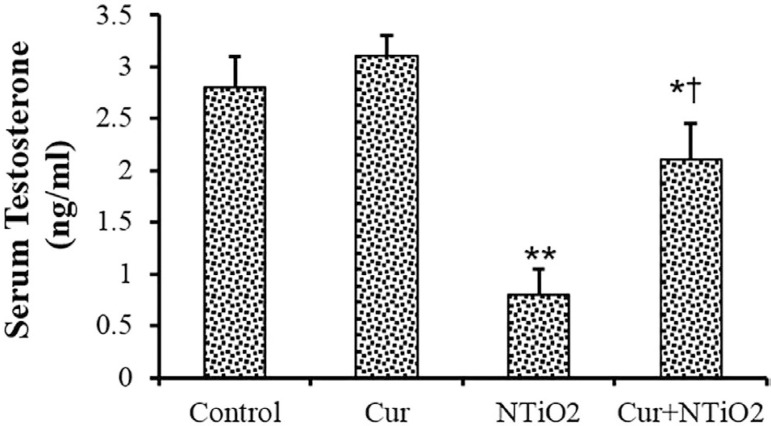
As shown in Figure 2, no significant changes were found between the Control and Cur groups in testosterone concentration. The testosterone concentration was significantly reduced in the NTiO2-intoxicated animals (p<0.01). There was a significant increase in testosterone levels in the Cur + NTiO2 group compared to those treated with NTiO2 (p<0.01).
Figure 2.
Testosterone assessment of control and experimental groups. *p<0.05, **p<0.01, †p<0.01; * and † symbols respectively indicate comparison to the control and NTiO2-intoxicated groups.
Morphometry
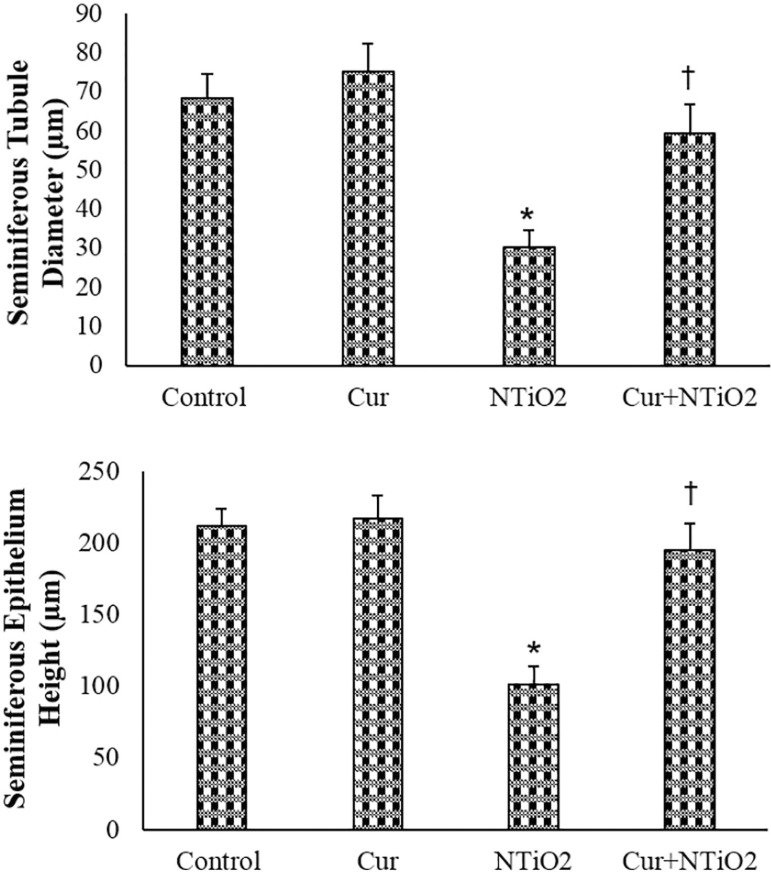
In the Cur-treated mice, the seminiferous tubules' diameter and the seminiferous epithelium height were slightly larger than those in the Control group. The morphometric parameters were significantly decreased in the NTiO2 group (p<0.01). In the Cur + NTiO2 treated animals, the morphometric parameters were significantly increased in comparison with the NTiO2-intoxicated mice (p<0.05), but no significant alteration in parameters was found when compared to the Control group (Figure 3).
Figure 3.
Morphometric parameters of control and experimental groups. *p<0.01, †p<0.05; * and † symbols respectively indicate comparison to the control and NTiO2-intoxicated groups.
Histology
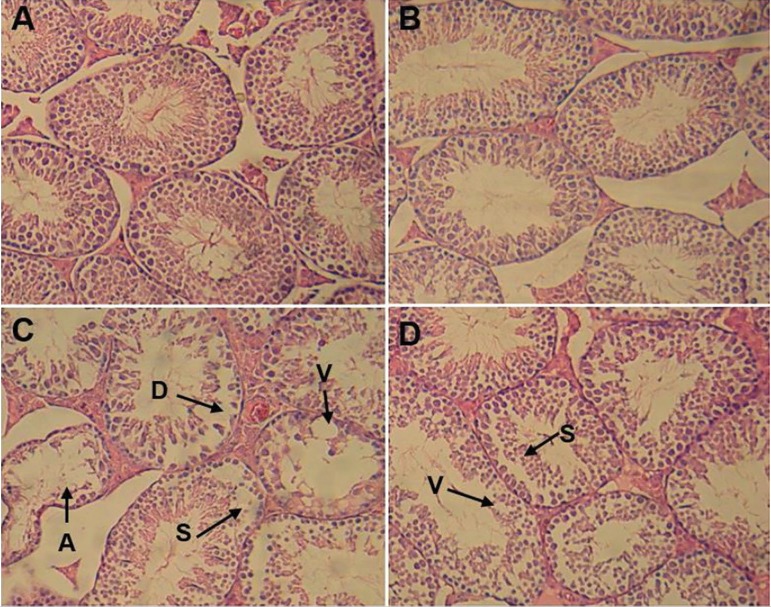
Testicular sections in both the control and Cur groups had a normal appearance. In the NTiO2 group, disorganization of germ cell layers, detachment, sloughing and atrophy was found, and the histologic criteria were significantly increased (p<0.01). Cur administration could attenuate the criteria compared to the NTiO2-intoxicated animals (Table 2 and Figure 4).
Table 2.
Histology assessments in the Control and Experimental groups
| Groups | Percentage of tubules | |||
|---|---|---|---|---|
| Normal | Detached | Sloughed | Vacuolated | |
| Control | 94.5 ±4.9 | 1.3±0.5 | 1.1±0.2 | 4.6±0.5 |
| Cur | 95.7±4.3 | 1.1±0.2 | 0.7 ± 0.08 | 2.5±0.3 |
| NTiO2 | 45.3±3.6** | 23.7±3.1** | 18.9±2.3** | 42.3±3.1** |
| Cur+NTiO2 | 77.6±5.6*† | 9.4±2.6*† | 8.6±1.4*† | 13.1±2.7*† |
Values are expressed as mean ± SD for 8 mice.
p<0.05,
p<0.01,
p<0.01;
* and † symbols respectively indicate comparison to control and NTiO2-intoxicated groups.
Figure 4.
Light microscopy of testicular tissue in various groups (Hematoxylin and eosin staining). Magnifications ×250.
A. Control Group; B. Cur group; C. NTiO2-intoxicated group; D. Cur + NTiO2 group.
A: atrophy, V: vacuole, S: sloughing, D: detachment
Spermatogenesis Assessment
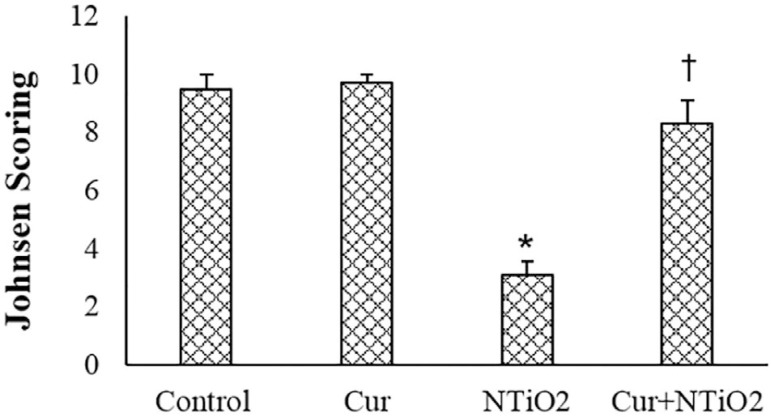
Normal spermatogenesis was see in both control and Cur groups. In the NTiO2 group, abnormal spermatogenesis was found in several tubules of each section and the Johnsen score was significantly decreased when compared to the Control animals (p<0.01). In the Cur + NTiO2 group, a few tubules showed maturity arrest and the mean Johnsen score was slightly lower than that in the Control group (Figure 5).
Figure 5.
Johnsen scoring of control and experimental groups.
*p<0.01, †p<0.01;
and † symbols respectively indicate comparison to the control and NTiO2-intoxicated groups.
Sperm parameters
The number and motility of sperms in the Cur group were significantly higher than those in the Control were (p<0.05), while the abnormality percentage was slightly reduced in this group. NTiO2 induced a significant reduction in all sperm parameters (p<0.01). Pretreatment with Cur substantially attenuated the number, abnormality and motility of the sperms in comparison with the NTiO2-intoxicated animals (Table 3).
Table 3.
Sperm parameters in the Control and Experimental groups
| Parameters | Control | Cur | NTiO2 | NTiO2+ Cur |
|---|---|---|---|---|
| Sperm count (106/ml) | 47.3±4.4 | 56.2±4.1* | 26.4±4.1** | 37.5±8.2*† |
| Abnormality (%) | 23.1±2.4 | 21.3±2.1 | 63.7±5.2** | 39.6±10.3*† |
| Rapid progressive (%) | 71.3±4.5 | 79.9±6.3* | 23.2±3.1 | 68.6±4.5† |
| Slow progressive (%) | 15.7±2.6 | 11.1±1.4 | 29.1±3.8** | 14.9±0.24† |
| No progressive (%) | 7.4±1.4 | 4.9±1.2 | 22.3±3.1** | 10.8±0.36*† |
| Immotile (%) | 5.6±1.2 | 3.1±0.9 | 25.4±2.6** | 5.7±0.16† |
Values are expressed as mean ± SD for 8 mice.
p<0.05,
p<0.05,
p<0.01;
* and † symbols respectively indicate comparison to the Control and NTiO2-intoxicated groups.
DISCUSSION
Data from our study showed that the NTiO2 treatment significantly reduced testicular weight, testosterone concentration, morphometric parameters, and sperm quality. NTiO2 also induced histological changes and maturity arrest. Pretreatment with Cur could effectively attenuate these events.
The reduction in testicular weights of the NTiO2-treated mice indicates the toxic effect of this nanoparticle on mouse testicles. The testicular weight is dependent on the germ cells mass. Thus, the reduction in testicular weight may be a consequence of germ cells death and spermatogenesis defects. In this study, Cur reversed testicular weight loss induced by NTiO2, which is probably caused by the prevention of germ cell death in the seminiferous tubules.
The weight changes after NTiO2 administration were accompanied by alterations in the morphometric parameters. In agreement with our results, Moridian et al. (2015) showed that zinc oxide NPs decreased seminiferous tubules' diameter and seminiferous epithelium height. The decrease in seminiferous diameter may indicate germ cell loss induced by NTiO2. The Cur-pretreatment could attenuate morphometric parameters indicated its beneficial effects on male germ cells. Mahmoudi et al. (2017) showed that Cur attenuated morphometric parameters of seminiferous tubules in sodium metabisulfite-treated mice. Cur ameliorated morphometric parameters in gentamicin-induced reproductive toxicity (Fetouh & El-Saied Azab, 2014). Ema et al. (2010) showed that NTiO2 altered spermatogenesis and induced histological changes in the offspring's' testicles.
The remarkable decrease in morphometric parameters after NTiO2 treatment was accompanied by histological changes to the seminiferous tubules, such as epithelial vacuolization, sloughing, detachment, and atrophy. These histological features are commonly observed in Sertoli cell damage after exposure to various toxicants (Russell & Griswold, 1995). Gao et al. (2013) showed that NTiO2 aggregated in the Leydig cells, the Sertoli cells, and spermatids, which led to disruption of seminiferous tubules, reduction on the number of mature sperms, as well as decreased numbers of Sertoli cells in the mice. Zhao et al. (2013) demonstrated that NTiO2 induces apoptosis in Sertoli cells. Hong et al. (2016) showed that NTiO2 induced cytotoxicity in primary cultured Sertoli cells of mice.
The Cur pretreatment effectively reversed vacuolization in the germinal epithelium, detachment of germ cells from basal lamina and sloughing of immature germ cells, indicating improvements in Sertoli cell functions. Cur increased the total number of Sertoli cells in gentamicin-induced reproductive toxicity in adult male guinea pigs (Fetouh & El-Saied Azab, 2014). Cur inhibited DNA fragmentation, apoptosis and cell cycle arrest induced by sodium arsenite in cultured murine Sertoli cells (Khan et al., 2013). Mahmoudi et al. (2017) also reported that Cur prevents structural changes in rat testicles induced by sodium meta-bisulfite.
The results of Johnsen's scoring also showed poor spermatogenesis in NTiO2-treated animals. Alterations in the Johnsen scoring may relate to germ cell degeneration. The decreasing Johnsen score by Cur indicated the preventive effect of Cur on germ cell damage caused by NTiO2.
The administration of NTiO2 significantly reduced testosterone concentrations, which was accompanied by a significant increase in histological criteria. Blanco-Rodríguez & Martínez-García (1998) reported that testosterone withdrawal induced detachment of germ cells from the seminiferous epithelium. Testosterone is required for the attachment of round spermatids to Sertoli cells (Smith & Walker, 2014). Komatsu et al. (2008) reported that NTiO2 was absorbed by mouse Leydig TM3 cells and it affected testosterone secretion.
Pretreatment with Cur could raise the testosterone concentration by about 2.6-fold, higher than the NTiO2-intoxicated animals. Cur ameliorated testosterone serum levels in metronidazole-treated mice (Karbalay-Doust & Noorafshan, 2011). Cur protected the Leydig cells of mice against chronic alcohol administration (Giannessi et al., 2008). Fetouh & El-Saied Azab (2014) also showed that Cur increased the total number of Leydig cells in gentamicin-treated animals. The improved testicular tissue and increased testicular weights in the Cur-pretreated mice may be the result of restoring testosterone levels.
As shown in the results, NTiO2 induced a significant reduction in sperm count and sperm motility and a significant increase in sperm abnormality. In accordance with our results, Gao et al. (2013) reported that the administration of NTiO2 resulted in a decrease in sperm count and motility, increase in sperm abnormalities, and apoptosis of germ cells in rats. In the study by Ema et al. (2010), NTiO2 caused a decrease in daily sperm production and a decrease in sperm mobility.
Sperm abnormalities are usually accompanied by sperm inactivation. In NTiO2-intoxicated mice, 26 and 63% of sperms were immobilized and had abnormal morphology, respectively. Our results showed that sperm abnormality in Cur pretreated mice was approximately 1.6 times less than that of the NTiO2-intoxicated animals. Thus, Cur pretreatment may decrease genotoxicity and, consequently, reduce sperm abnormality. Karbalay-Doust & Noorafshan (2011) showed that Cur improved sperm abnormality in metronidazole-treated mice. The group pretreated with Cur also showed a significant increase in the percentage of rapid progressive and slow progressive sperms, and a significant decrease in the percentage of non-progressive and immotile sperms. On the other hand, the number of sperms in the Cur-pretreated groups was approximately 1.4-fold higher than those treated with NTiO2. These findings demonstrate that Cur pretreatment can improve sperm quality. Cur reduced metronidazole-induced testicular toxicity and improved the quality of sperms in mice (Noorafshan et al., 2010). Cur improved daily sperm production in gentamicin-induced reproductive toxicity in adult male guinea pigs (Fetouh & El-Saied Azab, 2014). The improved sperm quality in Cur pretreated mice was considered a result of improving testicular histology and elevation of testosterone concentration.
CONCLUSION
This study demonstrated that Cur improved the spermatogenesis defects in NTiO2-treated mice. This study suggests that Cur can protect germ cells by enhancing testosterone secretion. Further experiments are needed to clarify the mechanisms of the Cur effect on NP-induced toxicity. This study was conducted on mice and cannot be recommended for human clinical use. However, Cur has been suggested as a possible treatment for male reproductive disorders in humans exposed to high doses of nanoparticles.
ACKNOWLEDGEMENT
This paper was supported by a grant (93s98) from the student research committee council of the Ahvaz Jundishapur University of Medical Sciences.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Al-Rubaei ZM, Mohammad TU, Ali LK. Effects of local curcumin on oxidative stress and total antioxidant capacity in vivo study. Pak J Biol Sci. 2014;17:1237–1241. doi: 10.3923/pjbs.2014.1237.1241. [DOI] [PubMed] [Google Scholar]
- Brohi DD, Wang L, Talpur HS, Wu D, Khan FA, Bhattarai D, Rehman Z, Farmanullah F, Huo L. Toxicity of Nanoparticles on the Reproductive System in Animal Models: A Review. Front Pharmacol. 2017;8:606. doi: 10.3389/fphar.2017.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Rodríguez J, Martínez-García C. Apoptosis precedes detachment of germ cells from the seminiferous epithelium after hormone suppression by short-term oestradiol treatment of rats. Int J Androl. 1998;21:109–115. doi: 10.1046/j.1365-2605.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- Cox A, Venkatachalam P, Sahi S, Sharma N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol Biochem. 2016;107:147–163. doi: 10.1016/j.plaphy.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Ema M, Kobayashi N, Naya M, Hanai S, Nakanishi J. Reproductive and developmental toxicity studies of manufactured nanomaterials. Reprod Toxicol. 2010;30:343–352. doi: 10.1016/j.reprotox.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Fetouh FA, El-Saied Azab A. Ameliorating effects of curcumin and propolis against the reproductive toxicity of gentamicin in adult male guinea pigs: Quantitative analysis and morphological study. Am J Life Sci. 2014;2:138–149. doi: 10.11648/j.ajls.20140203.13. [DOI] [Google Scholar]
- Gao GD, Ze Y, Zhao X, Sang X, Zheng L, Ze X, Gui S, Sheng L, Sun Q, Hong J, Yu X, Wang L, Hong F, Zhang X. Titanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male mice. J Hazard Mater. 2013;258-259:133–143. doi: 10.1016/j.jhazmat.2013.04.046. [DOI] [PubMed] [Google Scholar]
- Giannessi F, Giambelluca MA, Grasso L, Scavuzzo MC, Ruffoli R. Curcumin protects Leydig cells of mice from damage induced by chronic alcohol administration. Med Sci Monit. 2008;14:BR237–BR242. [PubMed] [Google Scholar]
- Goodson SG, Zhang Z, Tsuruta JK, Wang W, O'Brien DA. Classification of mouse sperm motility patterns using an automated multiclass support vector machines model. Biol Reprod. 2011;84:1207–1215. doi: 10.1095/biolreprod.110.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajshafiha M, Ghareaghaji R, Salemi S, Sadegh-Asadi N, Sadeghi-Bazargani H. Association of body mass index with some fertility markers among male partners of infertile couples. Int J Gen Med. 2013;6:447–451. doi: 10.2147/IJGM.S41341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlings SJ, Kalman DS. Curcumin: A Review of Its' Effects on Human Health. Foods. 2017;6 doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F, Zhao X, Chen M, Zhou Y, Ze Y, Wang L, Wang Y, Ge Y, Zhang Q, Ye L. TiO2 nanoparticles-induced apoptosis of primary cultured Sertoli cells of mice. J Biomed Mater Res A. 2016;104:124–135. doi: 10.1002/jbm.a.35548. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Fontana L, Leso V, Bergamaschi A. The effects of nanomaterials as endocrine disruptors. Int J Mol Sci. 2013;14:16732–16801. doi: 10.3390/ijms140816732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilbey YO, Ozbek E, Cekmen M, Simsek A, Otunctemur A, Somay A. Protective effect of curcumin in cisplatin-induced oxidative injury in rat testis: mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways. Human Reprod. 2009;24:1717–1725. doi: 10.1093/humrep/dep058. [DOI] [PubMed] [Google Scholar]
- Johnsen SG. Testicular biopsy score count - A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- Karbalay-Doust S, Noorafshan A. Ameliorative effects of curcumin on the spermatozoon tail length, count, motility and testosterone serum level in metronidazole-treated mice. Prague Med Rep. 2011;112:288–297. [PubMed] [Google Scholar]
- Khan S, Telang AG, Malik JK. DNA fragmentation, apoptosis and cell cycle arrest induced by sodium arsenite in cultured murine Sertoli cells: prevention by curcumin. Toxicol Env Chem. 2013;95:1006–1018. doi: 10.1080/02772248.2013.828883. [DOI] [Google Scholar]
- Komatsu T, Tabata M, Kubo-Irie M, Shimizu T, Suzuki K, Nihei Y, Takeda K. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol in Vitro. 2008;22:1825–1831. doi: 10.1016/j.tiv.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Lan Z, Yang WX. Nanoparticles and spermatogenesis: how do nanoparticles affect spermatogenesis and penetrate the blood-testis barrier. Nanomedicine (Lond) 2012;7:579–596. doi: 10.2217/nnm.12.20. [DOI] [PubMed] [Google Scholar]
- Mahmoudi R, Honarmand Z, Karbalay-Doust S, Jafari-Barmak M, Nikseresht M, Noorafshan A. Using curcumin to prevent structural impairments of testicles in rats induced by sodium metabisulfite. EXCLI J. 2017;16:583–592. doi: 10.17179/excli2017-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathuria N, Verma RJ. Curcumin ameliorates aflatoxin-induced lipid peroxidation in liver, kidney and testis of mice--an in vitro study. Acta Pol Pharm. 2007;64:413–416. [PubMed] [Google Scholar]
- Miura N, Ohtani K, Hasegawa T, Hojo R, Yanagiba Y, Suzuki T, Suda M, Wang RS. Hazardous effects of titanium dioxide nanoparticles on testicular function in mice. Fund Toxicol Sci. 2014;1:81–85. doi: 10.2131/fts.1.81. [DOI] [Google Scholar]
- Noorafshan A, Karbalay-Doust S, Valizadeh A, Aliabadi E, Mirkhani H. Ameliorative effects of curcumin on the seminiferous epithelium in metronidazole-treated mice: a stereological study. Toxicol Pathol. 2010;38:366–371. doi: 10.1177/0192623310362248. [DOI] [PubMed] [Google Scholar]
- Russell L, Griswold M. Sertoli cell toxicants. In: Russell L, Griswold M, editors. . The Sertoli Cell. 1st ed. Clearwater: Cache River Press; 1995. pp. 552–575. [Google Scholar]
- Salama FA, El-Bahr SM. Effect of curcumin on cadmium-induced oxidative testicular damage in rats. J Med Res Inst. 2007;28:167–173. [Google Scholar]
- Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Lin L, Liu L, Ding Y, Niu Q, Mu L, Wang H, Shen H, Guo S. Toxic effects of anatase titanium dioxide nanoparticles on spermatogenesis and testicles in male mice. Pol J Environ Stud. 2017;26:2739–2745. doi: 10.15244/pjoes/70807. [DOI] [Google Scholar]
- Wei SM, Yan ZZ, Zhou J. Curcumin attenuates ischemia reperfusion injury in rat testis. Fertil Steril. 2009;91:271–277. doi: 10.1016/j.fertnstert.2007.10.082. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ze Y, Gao G, Sang X, Li B, Gui S, Sheng L, Sun Q, Cheng J, Cheng Z, Hu R, Wang L, Hong F. Nanosized TiO2-induced reproductive system dysfunction and its mechanism in female mice. PLoS One. 2013;8:e59378. doi: 10.1371/journal.pone.0059378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]