Abstract
Background & objectives:
For improved male contraception, a new polymeric drug molecule – Reversible Inhibition of Sperm under Guidance (RISUG) has been synthesized and has been found to be effective, safe and reversible in various animal species. Phase-I and phase-II clinical trials have confirmed its safety and contraceptive efficacy. The present study was undertaken as a multicentric-limited phase-III clinical trial to test the efficacy and safety of RISUG in human volunteers.
Methods:
One hundred and thirty nine young males each having at least two children and living with wife were given 120 μl of RISUG as bilateral vas intraluminal injection. After the single-dose administration, the individuals were followed in respect of general health and semen parameters. Their wives were also followed particularly to determine onset of pregnancy.
Results:
During the six month follow up, the health of male volunteers and their wives was normal with no significant adverse effects. Temporary scrotal enlargement and mild scrotal and inguinal region pain were manifested in most individuals and resolved within one month without any routine activity impairment. In six individuals, there was injection procedure failure and azoospermia was not achieved. The other 133 individuals had either severe oligozoospermia or azoospermia at the first semen examination one month following RISUG injection; 82.7 per cent individuals had continued azoospermia in the month following first semen examination onwards and the rest 17.3 per cent manifested azoospermia within three to six months.
Interpretation & conclusions:
RISUG intravasal injection appears to be a safe clinical procedure with no significant adverse effects and has high sustained contraceptive efficacy. The localized intervention and continued contraceptive action on single-dose administration were significant features of the RISUG technology.
Keywords: Azoospermia, efficacy, intravasal male contraceptive, RISUG, safety, sperm
Despite some scepticism regarding the potential demand for new male methods of contraception1, data of population-based studies of male fertility and contraceptive preferences indicate that globally, a high percentage of men approve of family planning and use some form of contraception themselves2,3,4,5,6. During recent decades, the most important trends in family planning services have been improvement in the quality of care, reaching out to new population groups such as men and adolescents and integrating family planning services into other reproductive health services. Access to a wide range of effective methods of contraception is an important element of reproductive health. However, the contraceptive options available to men are limited. Vasectomy/non-scalpel vasectomy (NSV) and condom are the only contraceptive options for males. Vasectomy/NSV is a terminal method; therefore, the prevalence of vasectomy/ NSV is very low in the country. On the other hand, acceptability of condom use in our country is also very low inspite of its dual protection i.e., prevention of pregnancy and transmission of sexually transmitted diseases7. Hence, development of a new male contraceptive is the need of the hour.
Among all available male contraceptive methods, male hormonal method is at an advanced stage of clinical testing. However, these male hormonal contraceptive regimens have caused two main problems. First, the side effect, particularly in terms of proatherogenic, antiatherogenic action and their association with insulin resistance, obesity and haematopoietic action8,9,10,11, second, a fairly high non-responder rate. This is because of the fact that unlike therapeutic drugs that are used to treat life-threatening illnesses, the use of needle (or frequent injection) for delivering contraceptives has poor acceptance12. Therefore, there is a need to develop a safe, effective and acceptable male contraceptive so that men can use it for a long period without any side effect. Reversible Inhibition of Sperm under Guidance (RISUG®) is a non-hormonal one-time injectable intravasal male contraceptive13. After successful preclinical efficacy and safety studies on various species of animals including primates14, RISUG has also been tested successfully in a limited number of human volunteers during phase-I15, phase-II16 and extended phase-II clinical trials17. In the present investigation, it was proposed to study the efficacy and safety of RISUG in human volunteers in a multicentric mode.
Material & Methods
A clinical trial was conducted at Lok Nayak Jai Prakash Narayan Hospital, Delhi, Deen Dayal Upadhyay Hospital, and Rural Medicare Hospital, New Delhi, India, during 2000-2002. Ethical approval was obtained from the ethical committees of the three participating centres, and also the approval of the Drug Controller General of India.
Reversible Inhibition of Sperm under Guidance (RISUG ®): RISUG® is an injectable drug consisting of styrene maleic anhydride (SMA) copolymer chemically reacted with dimethyl sulfoxide (DMSO)18. The basic component SMA was prepared as per the process described in the US patent number 548807518. The dosage used was 60 mg of SMA in chemical complex with 120 μl of DMSO. Prefilled syringes with RISUG® were supplied to the participating centres.
Stability and shelf life of RISUG: The SMA dissolved and reacted with DMSO and loaded in specially prefilled syringes was stable for a period of two years. The shelf life for the prefilled syringes is two years. Good Manufacturing Practice (GMP) procedures as applicable to low-volume parenteral drugs were followed in making RISUG. Quality Control Standards based on physicochemical characteristics and biological properties were established and used to assure consistency from batch to batch at the Ministry of Health & Family Welfare, Government of India-sponsored RISUG Unit, Foundation for Innovation and Technology Transfer (FITT), Indian Institute of Technology (IIT), New Delhi, India.
Study design: Volunteer individuals selected were adult men aged <40 yr, in good health and with the proven fertility record of at least two living children and no history of having failed to induce contraception despite unprotected intercourse. Only those volunteers were enrolled who had no history of any disease having associations with secondary infertility; diabetes and recurrent epididymitis. All volunteers had a normal reproductive system and semen parameters. Wives of the individuals were aged <30 yr, in good health having no pelvic inflammatory disease and no history of intrauterine device implantation or sterilization.
Fifty individuals/centre were to be enrolled in the study. However, in the absence of mandatory requirement imposed due to change in Drugs and Cosmetic Act in the country at that time, the Monitoring Committee of the trial deferred enrolment of new individuals in the end of 2002. Therefore, according to the above mentioned criteria, 141 individuals were recruited from the male family planning unit/urology/surgery outpatient departments of Lok Nayak Jai Prakash Narayan Hospital, Delhi (50), Deen Dayal Upadhyay Hospital (41), and Rural Medicare Center Hospital, New Delhi (50) where the volunteers visited for vasectomy/NSV method. Data from all the enrolled individuals (both husband and wife) were obtained on demographic, clinical and reproductive profile. Ultrasound of scrotum and vital organs in case of males and lower abdominal vital organs in case of females was done. Chest X-ray, blood and urine examination, haemogram, liver function tests (LFTs) and kidney function tests were performed in blood samples of all the enrolled individuals including their wives using standard routine methods. Two semen samples, after three days of abstinence each time, were collected from the enrolled individuals using WHO method manual19. Testicular size was measured and recorded using Prader orchidometer. Of the 141 individuals, two did not come for RISUG injection because of their personal reasons, and hence, only 139 individuals who received RISUG injection, were analysed.
After obtaining written informed consent from the individual volunteers as well as his partner and after clearing all the preparatory medical examinations, the individual was given RISUG® injection (120 μl as bilateral vas intraluminal injection)16. In six individuals, required dose of RISUG could not be injected because of injection procedure failure, that is, either because of leakage from the syringe or because of vas counter punctures. These individuals were advised to go for NSV. However, these individuals continued and were also followed along with other individuals for a period of six months post-injection. Therefore, in the present analysis, only 133 individuals who received complete dose of RISUG injection were considered. Individuals were asked to remain in the hospital under observation for three hours and were advised abstinence for one week after injection. The individuals were also advised to use condom for two months post-injection, and were followed after one week, one month and six months post-injection for all clinical examinations and after six months for laboratory examinations. Extent of post-operative scrotal swelling, if any, was recorded based on a ten-point visual analogue scale. Scrotal pain, if any, was recorded. Semen samples were obtained after one month, one and a half month, two, three, four, five and six months post-injection.
Statistical analysis: Sign test was used to test for significance. The post-injection data were compared with pretreatment values of the respective individual. Epi Info 5.0 (an epidemiology programme, developed by the Centers for Diseases Control and Prevention, Atlanta, Georgia, USA and the WHO Global Programme on AIDS, Geneva, Switzerland) and SPSS12.0 (South Asia Pvt. Ltd., Bengaluru) software packages were used for data management and analysis.
Results
Post-injection clinical evaluation status: No serious side effects were noticed after RISUG injection in the individual volunteers and their partners. Mild scrotal enlargement, on account of diffuse scrotal tissue oedema, was noticed within first week post-injection in majority of individuals. Oedema disappeared within one month period without medication. In 36.2 per cent (n=48) individuals, slight scrotal pain of low grade was also noticed which disappeared within one month. In 23.4 per cent (n=31) individuals, scrotal nodule was noticed at the site of injection which also disappeared during six month period in majority of individuals. On ultrasound examination, after six months post-injection, no abnormality was noticed in any of the individuals, including their partners, except fluid collection around scrotum in two.
Post-injection blood biochemistry, haemogram and urine evaluation: All the parameters of haemogram carried out after six months post-injection were not significantly different than the pretreatment values and were in normal range. All blood biochemistry parameters [serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, alkaline phosphatase, blood urea, serum creatinine and bilirubin, blood sugar (random) and Venereal Disease Research Laboratory (VDRL)] were within normal range, and values were not significantly different from pretreatment values in the male volunteers and their partners.
Routine urine examination did not show any variation from their pretreatment value. Traces of albumin were noticed in 14 male individuals in comparison to 11 male individuals where traces of albumin were noticed at the time of enrolment. Of these 11 male individuals, eight showed traces of albumin in urine six months post-injection. However, to rule out any sign of renal and hepatic toxicity in those individuals where traces of albumin were noticed after RISUG injection, renal function tests and LFTs were conducted monthly for the next six months duration, but no renal and hepatic abnormalities were observed.
Contraceptive efficacy: Except six individuals in whom required dose of RISUG could not be injected, azoospermia was achieved in all other individuals ranging from one month to six months post-injection period. At the first semen sampling one month after injection, the impact of RISUG was noticed in all individuals. In 82.7 per cent individuals, azoospermia was achieved within two months post-injection whereas in the remaining 17.3 per cent individuals, azoospermia was achieved during three to six months post-injection period (Table). The time scale of getting to the contraceptive status is shown in the Figure. In those six individuals where complete dose of RISUG could not be injected, azoospermia could not be achieved even after six months post-injection. Of these six individuals, pregnancies were observed in the partners of two individuals.
Table.
Month-wise distribution of individuals achieving azoospermia after RISUG (Reversible Inhibition of Sperm under Guidance) injection
| Months | Cumulative number of individuals achieving azoospermia, n (%) |
|---|---|
| 1 | 30 (22.55) |
| 1.5 | 68 (51.12) |
| 2 | 110 (82.70) |
| 3 | 127 (95.48) |
| 4 | 130 (97.74) |
| 5 | 132 (99.24) |
| 6 | 133 (100) |
Figure.
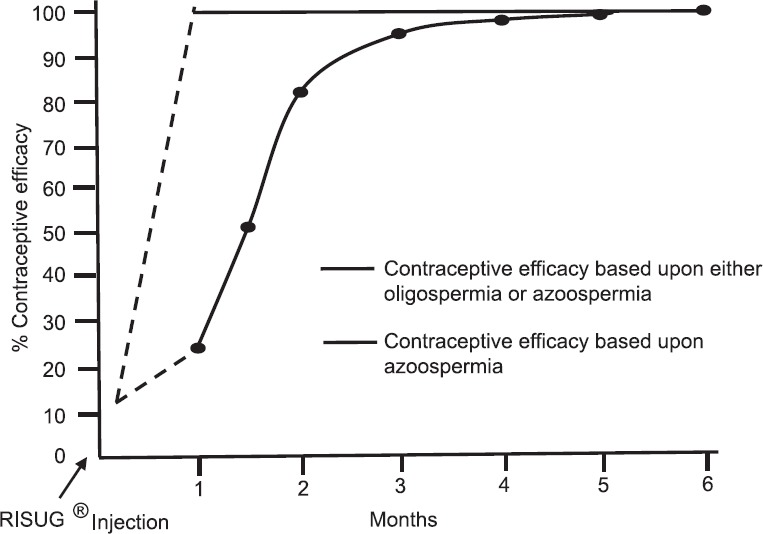
Time scale of getting to the contraceptive status by achieving severe oligozoospermia or azoospermia.
No adverse effects on libido and on any physical activities of the individuals were reported.
Discussion
The findings of the study indicated that 120 μl of RISUG was an effective and safe male contraceptive. The presence of mild scrotal enlargement, slight low-grade scrotal pain and presence of nodules at the site of injection, which disappeared within a few days and weeks, were similar to the observations made during phase-I and phase-II clinical trials15,16.
RISUG molecule has been designed to be non-tissue adherent and non-sclerosing for having reversal potential. To ensure retention even under strong vas peristalsis, by molecular design, a swelling of RISUG following intra vas injection has been incorporated20. The polymer volume increases within the vas lumen and leads to short-duration perivasal lymphatic compression. Lymphatic pressure presumably increases with consequent water transudate formation from the lymphatic channels leading to non-inflammatory scrotal enlargement. Painless nodule formation around the vas at the site of the injection is a form of vasitis nodosa observed in any form of vas intervention21. There is no nerve entrapment and vascular invasion and therefore, no pain. There was only a mild tenderness on firm pressure application.
Majority of the individuals, who received complete dose of RISUG injection, achieved either oligozoospermia or azoospermia within two months after injection. The remaining individuals achieved azoospermia within six months post-injection. Inspite of unprotected sexual relationship, no pregnancy was reported among the 133 individuals who received complete dose. The data indicated that RISUG was an effective male contraceptive. RISUG affects the sperm membrane including the acrosome which may have resulted in the sperm losing its fertilizing ability22,23,24. Successful reversal of RISUG injection has been observed in rat, rabbit and langur monkeys’ studies without any side effects25,26,27,28,29,30,31,32. However, reversibility of fertilizing ability of sperms was not attempted in this study.
Based on the present observations, it may be suggested that RISUG, which is non-hormonal and once injectable, is a safe, and effective male contraceptive. Small sample size and short duration of follow up were the major limitations of the study. Further studies need to be done with a large sample, long duration of follow up and including reversibility studies.
Acknowledgment
Authors acknowledge Shri Subhash Gautam for technical assistance.
Footnotes
Financial support & sponsorship: The financial support from Ministry of Health & Family Welfare, Government of India, New Delhi, is acknowledged.
Conflicts of Interest: None.
References
- 1.Potts M. The myth of a male pill. Nat Med. 1996;2:398–9. doi: 10.1038/nm0496-398. [DOI] [PubMed] [Google Scholar]
- 2.Posner JK, Mbodji F. Men's attitudes about family planning in Dakar, Senegal. J Biosoc Sci. 1989;21:279–91. doi: 10.1017/s0021932000017983. [DOI] [PubMed] [Google Scholar]
- 3.Ezeh AC, Seroussi M, Raggers H. DHS comparative studies No. 18. Calverton. Maryland: Macro International Inc; 1996. Men's Fertility, contraceptive use, and reproductive preferences. [Google Scholar]
- 4.Grady WR, Tanfer K, Billy JO, Lincoln-Hanson J. Men's perceptions of their roles and responsibilities regarding sex, contraception and childrearing. Fam Plann Perspect. 1996;28:221–6. [PubMed] [Google Scholar]
- 5.Ringheim K. Whither methods for men. Emerging gender issues in contraception? Reprod Health Matters. 1996;7:79–89. [Google Scholar]
- 6.Drennan M. Reproductive health. New perspectives on men's participation. Popul Rep J. 1998;46:1–35. [PubMed] [Google Scholar]
- 7.International Institute of Population Sciences. National Family Health Survey (NFHS-2): 1998-99. Mumbai: 2000. [accessed on January 10, 2018]. Available from: http://rchiips.org/nfhs/data/india/indch5.pdf . [Google Scholar]
- 8.Büchter D, Behre HM, Kliesch S, Chirazi A, Nieschlag E, Assmann G, et al. Effects of testosterone suppression in young men by the gonadotropin releasing hormone antagonist cetrorelix on plasma lipids, lipolytic enzymes, lipid transfer proteins, insulin, and leptin. Exp Clin Endocrinol Diabetes. 1999;107:522–9. doi: 10.1055/s-0029-1232561. [DOI] [PubMed] [Google Scholar]
- 9.Buchter D, Von Eckardstein S, Von Eckardstein A, Kamischke A, Simoni M, Behre HM, et al. Clinical trial of transdermal testosterone and oral levonorgestrel for male contraception. J Clin Endocrinol Metab. 2006;84:1244–9. doi: 10.1210/jcem.84.4.5594. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Wang XH, Nelson AL, Lee KK, Cui YG, Tong JS, et al. Levonorgestrel implants enhanced the suppression of spermatogenesis by testosterone implants: Comparison between Chinese and non-Chinese men. J Clin Endocrinol Metab. 2006;91:460–70. doi: 10.1210/jc.2005-1743. [DOI] [PubMed] [Google Scholar]
- 11.Gu YQ, Wang XH, Xu D, Peng L, Cheng LF, Huang MK, et al. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab. 2003;88:562–8. doi: 10.1210/jc.2002-020447. [DOI] [PubMed] [Google Scholar]
- 12.Wong Elissa WP, Mruk DD, Cheng CY. Delivery of contraceptives to men: Lesson from other therapeutic drugs. Immun Endoc Metab Agents Med Chem. 2008;8:91–4. [Google Scholar]
- 13.Guha SK. A process for the preparation of an injectable copolymer for use as a contraceptive by a male. Indian Patent No. 179093. 1991 [Google Scholar]
- 14.Guha SK, Ansari S, Anand S, Farooq A, Misro MM, Sharma DN, et al. Contraception in male monkeys by intra-vas deferens injection of a pH lowering polymer. Contraception. 1985;32:109–18. doi: 10.1016/0010-7824(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 15.Guha SK, Singh G, Anand S, Ansari S, Kumar S, Koul V, et al. Phase I clinical trial of an injectable contraceptive for the male. Contraception. 1993;48:367–75. doi: 10.1016/0010-7824(93)90082-i. [DOI] [PubMed] [Google Scholar]
- 16.Guha SK, Singh G, Ansari S, Kumar S, Srivastava A, Koul V, et al. Phase II clinical trial of a vas deferens injectable contraceptive for the male. Contraception. 1997;56:245–50. doi: 10.1016/s0010-7824(97)00142-x. [DOI] [PubMed] [Google Scholar]
- 17.Guha SK, Singh G, Srivastava A, Das HC, Bhardwaj JC, Mathur V, et al. Two years clinical efficacy trial with dose variations of a vas deferens injectable contraceptive for the male. Contraception. 1998;58:165–74. [Google Scholar]
- 18.Guha SK. Contraceptive for use by a male. US Patent. 1996:5488705. [Google Scholar]
- 19.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. New York: Cambridge University Press; 1999. [Google Scholar]
- 20.Roy S, Ghosh D, Guha SK. Polyelectrolyte polymer properties in relation to male contraceptive RISUG action. Colloids Surf B Biointerfaces. 2009;69:77–84. doi: 10.1016/j.colsurfb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Hirschowitz L, Rode J, Guillebaud J, Bounds W, Moss E. Vasitis nodosa and associated clinical findings. J Clin Pathol. 1988;41:419–23. doi: 10.1136/jcp.41.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhury K, Bhattacharyya AK, Guha SK. Studies on the membrane integrity of human sperm treated with a new injectable male contraceptive. Hum Reprod. 2004;19:1826–30. doi: 10.1093/humrep/deh332. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Chaudhury K, Sen P, Guha SK. Topological alterations in human spermatozoa associated with the polyelectrolytic effect of RISUG. Micron. 2006;37:526–32. doi: 10.1016/j.micron.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Guha SK. Biophysical mechanism-mediated time-dependent effect on sperm of human and monkey vas implanted polyelectrolyte contraceptive. Asian J Androl. 2007;9:221–7. doi: 10.1111/j.1745-7262.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- 25.Lohiya NK, Manivannan B, Mishra PK, Pathak N, Balasubramanian SP. Intravasal contraception with styrene maleic anhydride and its noninvasive reversal in langur monkeys (Presbytis entellus entellus) Contraception. 1998;58:119–28. doi: 10.1016/s0010-7824(98)00073-0. [DOI] [PubMed] [Google Scholar]
- 26.Lohiya NK, Manivannan B, Mishra PK. Repeated vas occlusion and non-invasive reversal with styrene maleic anhydride for male contraception in langur monkeys. Int J Androl. 2000;23:36–42. doi: 10.1046/j.1365-2605.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 27.Lohiya NK, Manivannan B, Mishra PK, Sriram S, Bhande SS, Panneerdoss S, et al. Preclinical evaluation for noninvasive reversal following long-term vas occlusion with styrene maleic anhydride in langur monkeys. Contraception. 2005;71:214–26. doi: 10.1016/j.contraception.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Lohiya NK, Suthar R, Khandelwal A, Goyal S, Ansari AS, Manivannan B, et al. Sperm characteristics and teratology in rats following vas deferens occlusion with RISUG and its reversal. Int J Androl. 2010;33:e198–206. doi: 10.1111/j.1365-2605.2009.00992.x. [DOI] [PubMed] [Google Scholar]
- 29.Ansari AS, Hussain M, Khan SR, Lohiya NK. Relative suitability of DMSO and naHCO3 for reversal of RISUG® induced long-term contraception. Andrology. 2016;4:306–13. doi: 10.1111/andr.12155. [DOI] [PubMed] [Google Scholar]
- 30.Ansari AS, Badar A, Balasubramanian K, Lohiya NK. Contraception with RISUG® and functional reversal through DMSO and naHCO3 in male rabbits. Asian J Androl. 2017;19:389–95. doi: 10.4103/1008-682X.185000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ansari AS, Badar A, Lohiya NK. Safety evaluation through genotoxicity and apoptotic markers following RISUG® induced contraception and its reversal in male rabbits. Reprod Toxicol. 2018;81:84–92. doi: 10.1016/j.reprotox.2018.07.083. [DOI] [PubMed] [Google Scholar]
- 32.Ansari AS, Hussain M, Khan SR, Badar A, Lohiya NK. Toxicity and mutagenicity evaluation following RISUG contraception reversal in rats. Int J Toxicol. 2018;37:457–65. doi: 10.1177/1091581818809473. [DOI] [PubMed] [Google Scholar]


