Abstract
Purpose
Dry eye (DE) is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. Although the pathogenesis of dry eye has not been fully understood, the role of increased tear osmolarity has been established. There is increasing evidence that dry eye is an inflammatory disease. This article aims to investigate the potential pathogenicity of inflammatory cytokine interleukin (IL)-33 and its receptor ST2 in dry eye.
Methods
Human conjunctival epithelial cells (HConECs) were stimulated with hyperosmolality to produce a dry eye cell model. Real-time PCR evaluated the IL-33 mRNA level, and western blotting assessed IL-33 protein expression. Clinical data (sex, age, ocular surface disease index [OSDI] score, tear film breakup time, Schirmer test, and corneal fluorescein staining [CFS]) of patients with DE were collected. Conjunctival impression cytology (CIC) specimens were collected to detect the protein expression of IL-33 and ST2 with western blotting. Tears were collected with Schirmer strips, and analyzed using multiplex assay kits to examine IL-33 and its downstream factors IL-4, IL-5, and IL-13.
Results
The IL-33 mRNA level of the HConECs increased in the hyperosmotic state (relative 4.35-fold upregulation, p<0.001). The IL-33 protein expression of HConECs also showed higher levels in the hyperosmotic state (relative 2.22-fold upregulation, p<0.01). A total of 25 patients with dry eye and 20 healthy subjects were enrolled. There were no statistically significant differences in age and sex between the two groups. The OSDI score, tear film breakup time, Schirmer test, and ocular surface staining of the two groups were statistically significantly different. The IL-33 and ST2 protein levels were increased in patients with DE versus controls (IL-33: relative 9.25-fold upregulation, p<0.001; ST2: relative 4.35-fold upregulation, p<0.05). The concentrations of IL-33, IL-13, and IL-5 in tears increased in patients with DE versus controls (IL-33: 3.00-fold upregulation, p<0.0001; IL-13: 6.65-fold upregulation, p<0.0001; IL-5: 16.54 -fold upregulation, p=0.01). IL-13 and IL-5 were statistically significantly correlated with IL-33. The level of IL-33 was positively correlated with the OSDI score and CFS, but was negatively correlated with the Schirmer I test and the tear film breakup time (TBUT). The level of IL-13 was positively correlated only with the CFS, and was negatively correlated with the Schirmer I test. The level of IL-5 was positively correlated with the OSDI score and CFS. We failed to detect the concentration of IL-4, as most samples were below the detection limit.
Conclusions
The IL-33 mRNA and protein levels of HConECs increased under hyperosmolality. The IL-33 and ST2 protein levels were higher in the CIC of patients with DE, and have correlations with disease severity. Moreover, the concentrations of IL-13 and IL-5 released from activated type 2 helper T (Th2) cells increased in the tears of patients with DE. The IL-33/ST2 pathway might play a priming role in the regulation of inflammation of the ocular surface.
Introduction
The International Dry Eye Workshop (DEWS) defines dry eye as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles” [1]. Although the symptoms of DE usually are mild, it may cause damage on the ocular surface, including conjunctival goblet cell loss, a decline of MUC5AC secretion, modification of the epithelial glycocalyx, and epithelial cell death. DE may result in punctate epitheliopathy, filamentary keratitis, superior limbic keratitis, and visual impairment [2,3], especially in severe cases. As a consequence of this multifactorial pathophysiology, the causes of DE are numerous, including lacrimal secretion insufficiency, meibomian gland dysfunction (MGD), corneal nerve impairment, mucin layer alterations, etc. [4]. However, accumulating evidence highlights the contribution of inflammation to the progression and severity of DE.
The cornea and conjunctiva are important structures of the ocular surface, and play an important role in maintaining tear film stability. The multifactorial pathogenesis of DE consists of chronic inflammation of the conjunctiva, tear film instability, and cornea and conjunctival epithelial damage [4]. According to the DEWS report, the tear hyperosmolarity acts as the core driver of DE, and stimulates a cascade of events to cause damage to the ocular surface epithelial cells, including corneal epithelial cells and conjunctival epithelial cells, by activating the mitogen-activated protein (MAP) kinase and nuclear factor kappa beta (NF-κB) signaling pathways to release inflammatory cytokines [4]. The released inflammatory cytokines then cause epithelial cell damage, leading to tear film instability and aggravating dry eye. Experimentally, the expression of interleukin (IL)-1, tumor necrosis factor- alpha (TNF-α), and IL-6 by ocular surface epithelia is critical to the inflammatory response of DE [2], and is important for enhancing the innate immune response and directing adaptive immunity toward a type 1 T helper- (Th1-) or Th2-dependent response. The levels of other cytokines, including IL-4, IL-5, IL-8, IL-10, IL-12, IL-13, IL-17, IL-23, and interferon gamma (IFN-γ), are also found to be elevated in patients with DE versus controls [3,5-7]. It has also been reported that Th1 and Th17 CD4+ T cells play a pivotal role in dry eye by causing damage to the corneal epithelium.
IL-33, a newly IL-1 family cytokine
In recent years, researchers have found Th2 CD4+ T cells also have an association with dry eye, and have a role in maintaining homeostatic levels of conjunctival goblet cell [2]. One cytokine that stimulates Th2 cell is IL-33, which is a newly discovered cytokine that belongs to the IL-1 family [8]. It has been reported that IL-33 is involved in the pathophysiology of allergic diseases, cardiovascular disease, rheumatoid arthritis, and chronic obstructive pulmonary disease, and plays different roles in these inflammatory diseases [9].
IL-33 can be released through cell death or stress, inducing excessive inflammatory responses. Recent studies have clarified the distinct function of IL-33 as a nuclear factor, in addition to being an extracellular cytokine [10]. IL-33 is consistently expressed in the nucleus of endothelial cells, epithelial cells, fibroblasts, and macrophages [8,10]. Full length IL-33 is bioactive [11], but proteases derived from different cellular sources, such as neutrophils, macrophages, or mast cells, process bioactive full-length IL-33 into N-terminally truncated forms (hIL-3395–270, hIL-3399–270, and hIL-33109–270) that have up to 30-fold higher biologic activity than full-length IL-33. IL-33 is initiated by binding with ST2 [8], a member of the IL-1 receptor family, which is expressed (or induced) on various immune cell types, including mast cells, basophils, eosinophils, neutrophils, macrophages, dendritic cells, Th2 lymphocytes, invariant natural killer T (iNKT), and natural killer (NK) cells [12-14].
ST2 has two different isoforms
ST2L and sST2 are generated by alternative splicing of ST2 mRNA. ST2L is a membrane-bound form that activates the intracellular signaling pathways, while sST2 is a soluble form that inhibits IL-33 signaling by acting as a decoy receptor. After binding with ST2, IL-33 leads to recruitment of MyD88, interleukin-1 receptor-associated kinase (IRAK), and TNF receptor-associated factor 6 (TRAF6), and sequentially activates downstream MAP kinase (MAPK), c-Jun N-terminal kinase (JNK), p38, extracellular signal-regulated kinase (ERK), and NF-κB signaling pathways, and then induces cytokine production in target cells [15]. As Th2 cells are a major target of IL-33, IL-33 binds to the ST2L of Th2 cells to stimulate the secretion of the Th2 cytokines, such as IL4, IL-5, and IL-13 [16].
Because of the diffuse distribution of IL-33 and ST2 in many tissues and organs, the role of IL-33/ST2 signaling has been investigated in various inflammation-related diseases. However, little is known about IL-33 in the pathogenesis of DE. In this study, we demonstrated the robust expression of IL-33 in the tears of patients with DE, which might contribute to the progression of DE.
Methods
Patients
Twenty-five patients with dry eye (18 women and 7 men; mean age: 44.25 ± 10.22) and twenty healthy individuals (14 women and 7 men; mean age: 48.52 ± 10.03) matched for sex and age were enrolled for testing studies between March 2018 and September 2018 in the First Affiliated Hospital of Harbin Medical University. All subjects are in fine physical condition during the recruitment period. Written informed consent was obtained from all subjects before participation.
All subjects were informed of the aims of the study, and their written informed consent was obtained according to the Declaration of Helsinki. The human-related activities were adherent to the ARVO for the Use of Humans in Ophthalmic and Vision Research. We recruited Chinese subjects aged between 18 and 65 years. Dry eye was diagnosed according to criteria reported previously [17]: patients with (1) an ocular surface disease index (OSDI) score≥13, (2) tear film breakup time (TBUT)≤5 s or Schirmer I test≤5 mm, or (3) positive cornea fluorescein staining (CFS) [18]. The following exclusion criteria was used: (1) any intraocular inflammation condition, ocular surgery or trauma within the previous 6 months; (2) present ocular infection or allergy; (3) laser treatment within the previous 6 months; (4) ocular therapies other than artificial tears; (5) contact lens wear; (6) abnormalities in the cornea, conjunctiva, or eyelid; (7) any systemic diseases, such as cancer, AIDS, or hepatic disease.
Tear film breakup time, CFS, and Shirmer I test
TBUT was measured using prepackaged, sterile fluorescein paper strips (Jingming New Technological Development Co. Ltd, Tianjin, China). The strip was wetted with 20 μl saline, and then instilled into the tear film. The patients were asked to blink several times to ensure the dye was well mixed. The time from the last complete blink to the appearance of the first corneal black spot in the stained-tear film was measured three times, and the mean value of the measurements was calculated. After the TBUT was measured, the CFS score was evaluated. CFS scores higher than 3 points were regarded as abnormal. The Schirmer I test was performed without anesthesia. The rounded bulb end of the strip was folded and placed in the lateral canthus, away from the cornea. After wetting for 5 min, the length of the wet filter paper was recorded. The strip was placed in a 0.5-ml tube to be stored immediately at −80 °C.
Tear collection
According to the method described previously [19], tears were extracted from the Schirmer strips as follows: 100 μl PBS (1X; 155 mM NaCl, 3 mM NaHPO4, 1 mM KHPO4, pH 7.4) was added to the strips, and allowed to solubilize for 1 h at 4 °C. Tears were obtained by puncturing the tube at the bottom with a sterilized needle. Then the tube was put into a 1.5-ml tube, and centrifuged at 10,000 ×g at 4 °C for 10 min to obtain the tears.
Conjunctival impression cytology
Conjunctival impression cytology (CIC) samples were collected according to the method described previously [20,21]. The CIC specimens were obtained after administration of topical anesthesia with 0.4% oxybuprocaine. Two separate sterile membrane filters (0.45 μm; Millipore, Boston, MA) were applied to adjacent superior and inferior temporal bulbar conjunctiva, pressed for 10 s gently with a forceps, and then removed. The filter paper collected from both eyes was transferred immediately to an empty 1.5-ml Eppendorf tube for western blotting, to evaluate the protein expression. All samples were immediately placed on ice until they were transferred to −80 °C for storage. The CIC samples were lysed with radioimmunoprecipitation assay buffer (1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) on ice for 1 h, and then centrifuged at 10,000 ×g at 4 °C for 10 min to obtain the supernatant.
Primary culture and cell treatment of human conjunctival epithelial cells
Two pairs of donated normal human conjunctiva samples were preserved in Dulbecco's Modified Eagle Media (DMEM)/F12 (Thermo Fisher, Shanghai, China) before they were transferred to the laboratory. The tissues were washed three times in PBS with penicillin and streptomycin (5,000 U/ml), then cut into explants of 5 mm × 5 mm, and placed on six-well plates with the epithelium side down.
Approximately 15 min were allowed for attachment of the explants, and 100 μl of fetal bovine serum (FBS) was added to cover the explants overnight. The culture medium consisting of DMEM/F12, penicillin/streptomycin (5,000 U/ml), human epidermal growth factor (10 ng/ml), and 10% FBS was supplemented the next day. The primary cell cultures were incubated at 37 °C with 5% CO2, and observed daily. The medium was replaced every 3 days. Sub-culturing was done when the cells reached 70–80% confluence. Sub-confluent cultures were switched to a serum-free medium (DMEM without FBS) for 24 h before treatment. Then the cells were cultured for an additional 4 or 24 h in an equal volume (1.0 ml/well) of serum-free medium with a different osmolarity, ranging from 400 to 500 mOsm, by adding 50, 70, and 90 mM sodium chloride (NaCl) [19]. Cells collected at 4 h were stored for real-time PCR. The IL-33 protein level was measured following 24 h incubation with western blotting.
Cell proliferation assay
To detect the effect of hyperosmolarity on cell activity, a cell proliferation assay kit (CCK-8; Dojindo, Shanghai, China) was used in accordance with the manufacturer’s instructions. Human conjunctival epithelial cells (HConECs) were seeded in 96-well plates (1 × 104/well). A total of 10 μl CCK-8 solution was added after the cell treatment. Following a 4 h incubation, the plate was measured at 450 nm with a spectrophotometer (Bio-Rad Laboratories, Hercules, CA).
Western blotting
The protein level was determined using the BCA protein assay kit (Beyotime, Shanghai, China). After protein loading buffer was added and the samples were heated at 100 °C for 10 min, all samples were run on a 12% SDS-polyacrylamide gel electrophoresis (SDS–PAGE) gel, blotted onto a polyvinylidene fluoride (PVDF) membrane, and then blocked with 5% non-fat dried milk for 2 h at room temperature and incubated overnight at 4 °C with the following antibodies: anti-beta-actin (Abcam, Cambridge, MA, ab8226), anti-IL-33 antibody (Abcam, ab207737), anti-ST2 antibody (Santa Cruz Biotechnology, Dallas, TX, sc-74297). The membranes were then rinsed six times with Tris-buffered saline containing 0.5% Tween-20 (TBST), and incubated with goat anti-rabbit or anti-mouse immunoglobulin (IgG; Cell Signaling, Danvers, MA) secondary antibodies for 1 h at room temperature. The Odyssey fluorescent scanning system (LI-COR, Biorad) and Quantity One software were used to detect and analyze the immunoreactive proteins.
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Shanghai, China) following the manufacturer’s instructions [22]. The isolated RNA was reverse-transcribed into synthesized cDNA in a 20 μl reaction mixture. Quantification of the IL-33 level with quantitative real-time PCR (RT-PCR) for 95°C, 2 min; 95°C,15s; 60°C, 15s; 72°C,45s; Number of cycles: 40 cycles was performed using the TaqMan miRNA assay system (Applied Biosystems, Foster City, CA). The primers were synthesized by Co Invitrogen Ltd. (Beijing, China). The sequences of primers are shown in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was taken as a loading control. Data were analyzed using the 2-△△Ct method.
Table 1. Sequence of Primers and Probes used for Gene amplification in Real Time–PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
|
IL-33 |
GTGGAAGAACACAGCAAGCA |
AAGGCAAAGCACTCCACAGT |
| GAPDH | AAGAAGGTGGTGAAGCAGGC | TCCACCACCCTGTTGCTGTA |
Multiplexed analyses of cytokines in tears
The concentrations of the tear cytokines were determined measured with a Luminex MAGPIX system (Luminex Corporation, Chicago, IL) with the ProcartaPlex Human Assay kit (Thermo Fisher, Vienna, Austria). The system uses a liquid suspension array of four sets of beads. Each set of beads was combined with a monoclonal antibody raised against IL-33, IL-13, IL-5, and IL-4. The beads were prepared according to the manufacturer’s instructions. Standard curves were generated by using the reference cytokine concentrations supplied by the manufacturer. Results were analyzed with xPONENT software (Luminex Corporation) to obtain the concentration values.
Statistical analysis
Data were shown as mean ± standard deviation. Differences in group means were determined using one-way ANOVA. Statistical analyses were performed using SPSS (version 20.0; SPSS Inc., Chicago, IL), and GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA) was used to generate figures and tables. P values of less than 0.05 were considered statistically significant. Pearson correlation coefficients were used to assess correlations between changes in ocular surface parameters and tear cytokine levels in each group.
Results
Validation of IL-33 in HConECs
To detect the response of HConECs to hyperosmolarity, CCK-8 was used to detect the cell viability. The mean of the cell viabilities was 86.401±6.3620, 72.517±11.382, and 69.930±5.5710 in 400, 450, and 500 mOsm, respectively (Figure 1A). The mean decreased with the increase in hyperosmolarity, but had no statistical significance. Real-time PCR was performed to analyze the mRNA expression of IL-33; western blotting was used to measure the protein level. According to the results, the expression of IL-33 mRNA seemed osmolality-dependent in the HConECs treated with hypertonic medium, and was 4.35-fold higher in the 500 mOsm medium compared with the 400 mOsm medium (p<0.001; One-way ANOVA; Figure 1B). Moreover, the production of the IL-33 protein was consistent with the transcription, and was 2.22-fold higher in the 500 mOsm medium compared with the 400 mOsm medium (p<0.01; One-way ANOVA; Figure 1C, D). The data shown are representative of the results in three independent experiments.
Figure 1.
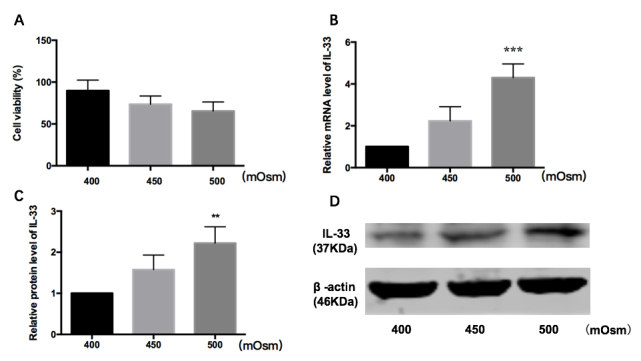
Response of HConECs to hyperosmolarity. A: Cell viability decreased as hyperosmolarity increased, but showed no statistical significance. B: The level of IL-33 mRNA increased in 500 mOsm compared with 400 mOsm (4.35-fold upregulation, p<0.001, one-way ANOVA). C, D: IL-33 protein increased in 500 mOsm compared with 400 mOsm (2.22-fold upregulation, p<0.01, one-way ANOVA). The data shown are representative of the results in three independent experiments.
Demographics and clinical examination parameters
After discovering the phenomenon at the cellular level, we hoped to verify it on the human eye surface. A total of 45 subjects were enrolled in this study, including 25 patients with dry eye and 20 healthy controls. The ocular surface parameters are presented in Table 2. There were no statistically significant differences in the sex or mean age between the two groups. The mean OSDI score and CFS were statistically significantly higher in the DE group (p<0.0001; Student’s t-test), while the Schirmer test and the TBUT were much lower in the DE group than in the control group (p<0.0001; Student’s t-test).
Table 2. Summary of clinical data.
| Parameters | Control | DE | p-Values |
|---|---|---|---|
| Age (years) |
48.52 ± 10.03 |
44.25 ± 10.22 |
0.9178 |
| Sex, female/male (%) |
70/30 |
72/28 |
|
| OSDI score (points) |
5.050±4.774 |
37.80±17.58 |
<0.0001 |
| Schirmer I test (mm) |
13.23±3.759 |
7.060±3.738 |
<0.0001 |
| TBUT (s) |
11.92±1.999 |
7.916±3.926 |
<0.0001 |
| CFS score (points) | 0.1000±0.3078 | 1.560±1.083 | <0.0001 |
DE=dry eye; OSDI=ocular surface disease index; TBUT=tear film break-up time; CFS=fluorescein score. Student’s t-test.
IL-33/ST2 protein expression in conjunctival impression cytology
Because the IL-33/ST2 axis has a vital function in regulating inflammation and immune responses, we detected the expression in patients with dry eye. The samples were not pooled together, but were extracted and tested individually. With the use of western blotting, the relative expression of IL-33 (17 kDa) and ST2 (60 kDa) protein from CIC was higher in the DE group compared with the control group (IL-33: relative 9.25-fold upregulation, p<0.001; 17 and 15 samples in DE and controls respectively; ST2: relative 4.35-fold upregulation, p < 0.05, 15 and 19 samples in DE and controls respectively; Kruskal-Wallis test; Figure 2). All CIC results can be found in the Appendix 1.
Figure 2.
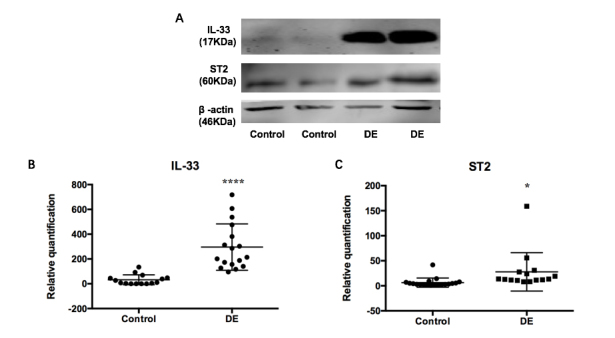
Upregulation of IL-33 and ST2 expression in the CIC samples of patients with dry eye. A: Western blotting for interleukin (IL)-33 and ST2 protein expression in parts of conjunctival impression cytology (CIC) specimens. B: Relative quantification of IL-33 showed statistically significant upregulation in the DE group compared with the controls (relative 9.25-fold upregulation, ****p≤0.0001; Kruskal-Wallis test, 17 and 15 samples in DE and controls respectively). C: Relative quantification of ST2 showed statistically significant upregulation in the DE group compared with the controls (relative 4.35-fold upregulation, *p≤0.05, Kruskal-Wallis test, 15 and 19 samples in DE and controls respectively). DE, dry eye.
Tear cytokine concentrations
The concentrations of IL-33, IL-13, IL-5, and IL-4 in the tears of each group were measured by Luminex. The mean levels of IL-33 in tears were 2.4850±0.6379 in controls, and 7.467±1.379 in the DE group (3.00-fold upregulation, p<0.0001; Figure 3A). The mean levels of IL-13 in tears were 0.2265±0.1223 in controls, and 1.5070±0.1048 in the DE group (6.65-fold upregulation, p<0.0001; Figure 3B). The mean levels of IL-5 in tears were 0.7905±0.3563 in controls, and 13.07±4.039 in the DE group (16.54 -fold upregulation, p=0.01; Figure 3C). As IL-33 can stimulate Th2 cells to release IL-13 and IL-5, we performed correlation analysis between IL-13, IL-5, and IL-33. The IL-13 and IL-5 levels were statistically significantly correlated with the IL-33 concentration (IL-33 with IL-5: R=0.9224, p<0.0001; IL-33 with IL-13: R=0.7263, p<0.0001; Figure 4). There were 21 and 17 samples in DE and control group respectively. We failed to detect the concentration of IL-4 as most samples were below the detection limit. Kruskal-Wallis test and Pearson correlation coefficients were used to analyze the data.
Figure 3.
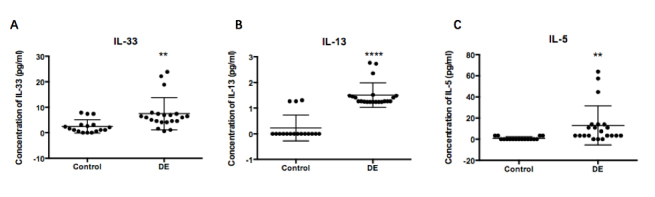
Upregulation of tear cytokine concentrations in patients with dry eye. A: Tear concentrations of interleukin (IL)-33 showed statistically significant upregulation in the DE group compared with controls (3.00-fold upregulation, **p≤0.01, Kruskal–Wallis test, 21 and 17 samples in DE and controls respectively). B: Tear concentrations of IL-13 showed statistically significant upregulation in the DE group compared with controls (6.65-fold upregulation, **** p≤0.0001, Kruskal–Wallis test, 21 and 17 samples in DE and controls respectively). C: Tear concentrations of IL-5 showed statistically significant upregulation in the DE group compared with controls (16.54 -fold upregulation, p=0.01, Kruskal–Wallis test, 21 and 17 samples in DE and controls respectively). DE, dry eye.
Figure 4.
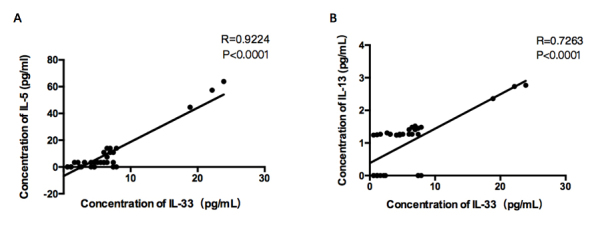
Correlation of IL-13 and IL-5 levels with IL-33 concentration. A: Interleukin (IL)-5 was positively correlated with IL-33 (R=0.9224, p<0.0001, Pearson correlation coefficients). B: IL-13 was positively correlated with IL-33 (R=0.7263, p<0.0001, Pearson correlation coefficients, n=38).
Correlations between cytokines and ocular surface parameters
The correlations between the levels of IL-33, IL-13, and IL-5 and ocular surface parameters, including the OSDI score, TBUT, Schirmer I test, and CFS, were evaluated in the DE group and healthy controls. In the DE group, the level of IL-33 was positively correlated with the OSDI score and CFS, but was negatively correlated with the Schirmer I test and the TBUT (IL-33 with OSDI: R=0.4483, p=0.0415; IL-33 with Shirmer I test: R=-0.4448, p=0.0433; IL-33 with the TBUT: R=-0.4459, p=0.0427; IL-33 with CFS: R=0.5657, p=0.0075; n=21; Figure 5A–D). The level of IL-13 was positively correlated only with CFS, and was negatively correlated with the Schirmer I test (IL-13 with Shirmer I test: R=-0.4348, p=0.0433; IL-13 with CFS: R=0.5005, p=0.0208; n=21; Figure 5E,F). The level of IL-5 was positively correlated with the OSDI score and CFS (IL-5 with OSDI: R=0.4551, p=0.0382; IL-5 with CFS: R=0.5261, p=0.0143; n=21; Figure 5G,H). IL-13 and IL-5 and the other ocular surface parameters showed no statistical significance. In the control group, the levels of IL-33, IL-13, and IL-5 in tears showed no correlation with the ocular surface parameters. Pearson correlation coefficients were used for analyzing,
Figure 5.
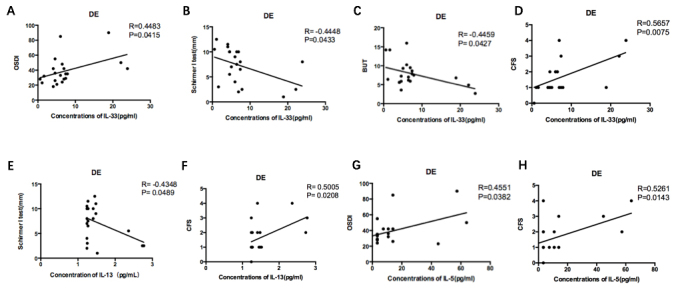
Correlation of IL-33, IL-13, and IL-5 levels with ocular surface parameters. Only the eight graphs that indicated statistically significant correlation are presented. A–D: In the DE group, the level of IL-33 positively correlated with the OSDI score (R=0.4483, P=0.0415, n=21) and CFS (R=0.5657, P=0.0075, n=21) while negatively correlated with the Schirmer I test (R=-0.4448, P=0.0433, n=21) and BUT (R=-0.4459, P=0.0427, n=21). E–F: The level of IL-13 positively correlated with the CFS (R=0.5005, P=0.0208, n=21) while negatively correlated with the Schirmer I test (R=-0.4348, P=0.0433, n=21). G–H: The level of IL-5 positively correlated with both OSDI score (R=0.4551, P=0.0382, n=21) and CFS (R=0.5261, P=0.0143, n=21). The levels of IL-33, IL-13, IL-5 in tears showed no correlation with ocular surface parameters in controls. Pearson correlation coefficients. DE, dry eye.
Discussion
There is increasing evidence that immune-based inflammation on the ocular surface underpins the pathological damage of dry eye. Various cytokines have been identified in the tear film of patients with DE, and lead to inflammation of the ocular surface. The IL-33/ST2 pathway can activate a signaling cascade that participates in inflammation, apoptosis, and pyroptosis that play important roles in dry eye [2]. IL‐33 may be involved in Th2 cytokine‐mediated immune responses based on the capacity to induce Th2 cytokine production from various immune cells, such as Th2 cells, mast cells, basophils, and eosinophils. Much valuable work has implicated the importance of Th2 cytokine production in the onset and development of many inflammation-related diseases [9,12-16]. Previous studies have shown that the IL‐33/ST2 axis is involved in the pathogenesis of Sjogren syndrome [23,24], a disease that can cause dry eye. We speculated that the IL-33/ST2 axis may be involved in the regulation of ocular surface inflammation in dry eye. To study the potential involvement of the IL-33/ST2 in dry eye, a hyperosmolarity stress model of ocular epithelial cells was performed to induce inflammation, and to verify the mRNA and protein expression of IL-33 after different degrees of hyperosmolarity stimulation. When the HConECs were treated with different osmolality in vitro, the levels of IL-33 and relative cytokines were released in an osmolality-dependent manner. Meanwhile, we prospectively recruited patients with dry eye, and collected tear and conjunctival impression cytology samples to investigate the protein expression of IL-33 and ST2, and its downstream cytokines IL-4, IL-5, and IL-13. The results showed a statistically significant increase in IL-33 mRNA and protein expression in HConECs following hyperosmolarity stimulation. There was a statistically significant increase in IL-33 and ST2 protein expression in the CIC and tears of patients with DE compared with the controls. The results imply an association between IL-33 and the regulation of dry eye ocular surface inflammation. The full-length IL-33 protein is 37 kDa [25], while the western blotting results showed IL-33 appeared at 37 kDa in the HConECs and 17 kDa in CIC. This result may be due to inflammatory serine proteases from neutrophils and mast cells from the ocular surface that can cleave IL-33 into shorter mature forms of 18–21 kDa with higher biologic activity [26-29]. Previous studies confirmed that IL-33 activates Th2 cells to release Th2 cytokines, including IL-4, IL-5, and IL-13 [30,31], while Th2 CD4+ T cells maintain homeostatic levels of conjunctival goblet cells, and regulate inflammation [32-34]. The corresponding cytokines released from activated Th2 cells, such as IL-13 and IL-5, were also tested. They were statistically significantly higher in the DE group compared with the control subjects. These results were consistent with the IL-33 upregulation that we observed. These results suggested that increased IL-33 expression might be associated with activation of Th2 CD4+ T cells and regulated inflammation in patients with dry eye.
In this study, the OSDI, Schirmer I test, TBUT, and CFS values all were statistically significantly worse in the DE group than in the control group. We proposed that the level of IL-33 expression in patients with DE may be associated with the DE severity. Therefore, we analyzed the correlations between ocular surface parameters and IL-33 and its downstream cytokines. The results showed IL-33 was positively correlated with the OSDI score and CFS, and negatively correlated with the Schirmer I test and the TBUT, but IL-13 and IL-5 were correlated only with some of the clinical parameters. These results were consistent with those of another study [35], which verified the correlation between IL-33, IL-4, and IL-5, and the OSDI, Schirmer I test, and TBUT. However, the present study showed the correlation was weak according to the r value, which might possibly be associated with the small sample size. It would be better to enlarge the sample size to validate the correlation analysis in future experiments. There are also limitations in this study. First, the sample sizes were relatively small. In addition, although we demonstrated upregulation of IL-33/ST2, their specific roles in DE were not fully interpreted in this study. Further investigations are essential to elucidate the molecular mechanism of IL-33 expression control, and its role in dry eye.
In summary, we detected the IL-33 expression of HConECs under different osmolality and the IL-33 level in the tears and ocular surface of patients with dry eye. We found IL-33 in HConECs increased under hyperosmolality. Consistent with that result, IL-33 expression was higher in patients with DE. Moreover, IL-5 and IL-13 released from activated Th2 cells were also increased. These results suggested that IL-33 might play a pivotal role in inducing ocular surface inflammation via activation of Th2 cells. It is recognized that therapeutic targets on locally ocular inflammation hold promise for relieving symptoms and preventing the progression of DE. The correlation between IL-33 expression levels and DE severity may allow the development of targeted therapies for DE, aimed to relieve symptoms and prevent disease progression of DE.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81671844), Distinguished Young Scientists Foundation of Heilongjiang Province (JC2016019), Provincial Subject Research Institutes Foundation (201608), Heilongjiang Academy of Medical Sciences Research and Transformation Special Fund (CR 201809).
Appendix 1. These are IL-33 and ST2 expressions in conjunctival impressive cytology of all subjects.
To access the data, click or select the words “Appendix 1.”
References
- 1.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson D, Nichols JJ, Tsubota K, Stapleton F. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, Uchino Y, Yokoi N, Zoukhri D, Sullivan DA. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510. doi: 10.1016/j.jtos.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Tsubota K, Yokoi N, Shimazaki J, Watanabe H, Dogru M, Yamada M, Kinoshita S, Kim HM, Tchah HW, Hyon JY, Yoon KC, Seo KY, Sun X, Chen W, Liang L, Li M, Liu Z. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul Surf. 2017;15:65–76. doi: 10.1016/j.jtos.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Baudouin C, Aragona P, Van Setten G, Rolando M, Irkeç M, del Castillo JB, Geerling G, Labetoulle M, Bonini S. Diagnosing the severity of dry eye: a clear and practical algorithm. Br J Ophthalmol. 2014;98:1168–76. doi: 10.1136/bjophthalmol-2013-304619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baudouin C. The pathology of dry eye. Surv Ophthalmol. 2001;45:S211–20. doi: 10.1016/s0039-6257(00)00200-9. [DOI] [PubMed] [Google Scholar]
- 6.Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003;44:124–9. doi: 10.1167/iovs.02-0581. [DOI] [PubMed] [Google Scholar]
- 7.Enríquez-de-Salamanca A, Castellanos E, Stern ME, Fernández I, Carreño E, García-Vázquez C, Herreras JM, Calonge M. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862. [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 10.Martin MU. Special aspects of interleukin-33 and the IL-33 receptor complex. Semin Immunol. 2013;25:449–57. doi: 10.1016/j.smim.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 11.M Martin NT Martin M U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17:122. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 12.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 13.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 14.Junttila IS, Watson C, Kummola L, Chen X, Hu-Li J, Guo L, Yagi R, Paul WE. Efficient cytokine-induced IL-13 production by mast cells requires both IL-33 and IL-3. J Allergy Clin Immunol. 2013;132:704–12. doi: 10.1016/j.jaci.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cayrol C, Girard JP. Interleukin‐33 (IL‐33): A nuclear cytokine from the IL‐1 family. Immunol Rev. 2018;281:154–68. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Zhang L, Chen X, Chen D, Hua X, Bian F, Deng R, Lu F, Li Z, Pflugfelder SC, Li DQ. Pollen/TLR4 innate immunity signaling initiates IL-33/ST2/Th2 pathways in allergic inflammation. Sci Rep. 2016;6:36150. doi: 10.1038/srep36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S, Jiang S, Wang Y, Tu S, Wang Z, Chen Z. Interleukin 34 Upregulation Contributes to the Increment of MicroRNA 21 Expression through STAT3 Activation Associated with Disease Activity in Rheumatoid Arthritis. J Rheumatol. 2016;43:1312–9. doi: 10.3899/jrheum.151253. [DOI] [PubMed] [Google Scholar]
- 18.Niu L, Zhang S, Wu J, Chen L, Wang Y. Upregulation of NLRP3 inflammasome in the tears and ocular surface of dry eye patients. PLoS One. 2015;10:e0126277. doi: 10.1371/journal.pone.0126277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Q, Ren Y, Reinach PS, Xiao B, Lu H, Zhu Y, Qu J, Chen W. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp Eye Res. 2015;134:133–40. doi: 10.1016/j.exer.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Caffery B, Joyce E, Heynen ML, Jones L, Ritter R, III, Gamache DA, Senchyna M. MUC16 expression in Sjogren’s syndrome, KCS, and control subjects. Mol Vis. 2008;14:2547. J. [PMC free article] [PubMed] [Google Scholar]
- 21.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H. Innate production of T(H)2 cytokines by adipose tissue‐associated c‐kit(+)Sca‐1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 22.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H. Innate production of T(H)2 cytokines by adipose tissue‐associated c‐kit(+)Sca‐1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 24.Margiotta DP, Navarini L, Vadacca M, Lo Vullo M, Pignataro F, Basta F, Afeltra A. The IL33/ST2 axis in Sjogren syndrome in relation to disease activity. Eur Rev Med Pharmacol Sci. 2016;20:1295–9. [PubMed] [Google Scholar]
- 25.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cayrol C, Girard JP. Interleukin‐33 (IL‐33): A nuclear cytokine from the IL‐1 family. Immunol Rev. 2018;281:154–68. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- 27.Gao Y, Min K, Zhang Y, Su J, Greenwood M, Gronert K. Female-specific downregulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye disease. J Immunol. 2015;195:3086–99. doi: 10.4049/jimmunol.1500610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kari O, Saari KM. Diagnostics and new developments in the treatment of ocular allergies. Curr Allergy Asthma Rep. 2012;12:232–9. doi: 10.1007/s11882-012-0252-9. [DOI] [PubMed] [Google Scholar]
- 29.Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109:1673–8. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou W, Zhang J, Toki S, Goleniewska K, Johnson MO, Bloodworth MH, Newcomb DC, Peebles RS., Jr The PGI2 Analog Cicaprost Inhibits IL-33-Induced Th2 Responses, IL-2 Production, and CD25 Expression in Mouse CD4+ T Cells. J Immunol. 2018;201:1936–45. doi: 10.4049/jimmunol.1700605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takatori H, Makita S, Ito T, Matsuki A, Nakajima H. Regulatory mechanisms of IL-33–ST2-mediated allergic inflammation. Front Immunol. 2018;9:2004. doi: 10.3389/fimmu.2018.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kari O, Saari KM. Diagnostics and new developments in the treatment of ocular allergies. Curr Allergy Asthma Rep. 2012;12:232–9. doi: 10.1007/s11882-012-0252-9. [DOI] [PubMed] [Google Scholar]
- 33.Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109:1673–8. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coursey TG, Henriksson JT, Barbosa FL, de Paiva CS, Pflugfelder SC. Interferon-γ–induced unfolded protein response in conjunctival goblet cells as a cause of mucin deficiency in Sjögren syndrome. Am J Pathol. 2016;186:1547–58. doi: 10.1016/j.ajpath.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo G, Xin Y, Qin D, Yan A, Zhou Z, Liu Z. Correlation of interleukin-33 with Th cytokines and clinical severity of dry eye disease. Indian J Ophthalmol. 2018;66:39. doi: 10.4103/ijo.IJO_405_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


