Introduction
2009 witnessed the first pandemic of influenza in the 21st Century. This pandemic of influenza A H1N1 (pH1N1) occurred 41 years after the previous pandemic of influenza A H3N2 in 1968. Each of the three pandemics of the 20th Century was different and provided some level of knowledge as to a range of outcomes that might occur. The H1N1 pandemic was unique in that scientists now had the ability to rapidly detect and monitor the spread and impact of the virus and to exchange information around the world. Pre-pandemic planning—while based on the assumption of a more severe level of illness— was effective in guiding preparedness efforts in advance of the H1N1 outbreak unfolding.
In preparation for the possible second wave of H1N1, the Public Health Agency of Canada developed a document in the fall of 2009 identifying two likely scenarios for use by the Agency and other organizations in planning and implementing response efforts. The document included critical considerations relevant to Canada but gleaned from the best information available internationally and was intended as an evergreen tool to be updated as new information became available. It documented both implicit and explicit assumptions upon which plans could be made.
The approach taken to develop this document was not new; it was a compilation of a broad set of data. It was also not the only source of information used for planning, as there were other more technical sources of information required for some planning activities, e.g., vaccination guidelines and surveillance for vaccine-associated adverse events. However, it provided an important framework from which to anticipate and plan for critical elements such as, for example, human resource requirements. The planning considerations presented here are intended to add to our collective knowledge and assist planners preparing for future pandemics of influenza.
Context
The document presented here was compiled by the Public Health Agency of Canada (PHAC) in September 2009, as a public health pandemic response tool, during the time leading up to the fall 2009 wave of pandemic H1N1. It was produced to collate select planning considerations about a potential fall wave relevant to the Canadian context. The ultimate goal was to identify the most likely range of situations that PHAC needed to be prepared to respond to in the fall. Publishing the document at this time provides access to a tool that was in use during the pandemic response, in the fall of 2009.
Background
On 12 April 2009, an outbreak of influenza-like illness in Veracruz, Mexico was reported to the World Health Organization (WHO). By 23 April, cases of a novel influenza strain of swine origin (A/California/07/2009 (H1N1)) had been confirmed in Mexico and southern California, and on 25 April WHO declared a public health event of international concern (1). By 26 April, PHAC had confirmed six cases in Canada. On 27 April, WHO raised the pandemic level to 4, and then to level 5 on 29 April, recognizing the extent of spread in Canada and the United States (2). By 11 June, WHO declared a pandemic (level 6) (3). In July, WHO named the virus “pandemic H1N1/09 virus” (pH1N1/09), to distinguish it from seasonal H1N1 virus strains and the 1918 H1N1 strain.
In Canada, the spring wave peaked in early June, with pH1N1/09 activity declining and reaching its nadir towards the end of August. At PHAC, the Advanced Planning Group (APG) was established early in the spring wave, to support the overall response and management of PHAC’s Incident Command System. The APG’s mandate was to coordinate expertise, knowledge and capacity from across the Health Portfolio, to determine short (3-5 days) and intermediate (5-30 days) essential and high priority anticipated public health response requirements and interventions. The APG focused on parallel issues to PHAC’s Operations Group (e.g. infection control, access to medical treatment, pharmaceutical supplies); however, the initial assessments focused on sustainable public health responses and communication to the public, anticipating a short peak infection curve with moderate-to-severe morbidity and mortality. The APG also focused on accessing human capital resources and financial instruments to sustain a federally coordinated response in the longer term. Throughout the first wave of pH1N1, the APG met daily and reported daily on the APG Incident Command Work Plan. However, there was continued concern for a potentially larger fall wave. To facilitate planning for this potential fall wave, PHAC compiled a working document which outlined planning considerations, as a construct on which to base various planning concepts and review planning processes.
The working document was used for discussion both within PHAC, and between PHAC and its provincial and territorial counterparts in October 2009. Considerations of what might be expected in the Canadian context with regards to a potential fall wave were broken down as: (i) best guess scenarios for plausible fall wave situations, (ii) planning considerations of the potential population impacts of pH1N1/09 in the fall, and (iii) potential resource impacts on PHAC.
The planning considerations from this working document are presented here. Information for the document was gleaned from internal and external sources, including grey and published literature, available developments in pH1N1/09 activity in the southern hemisphere’s winter 2009 wave, and consultation with experts. The review period during which information was searched and compiled was 25 August to 28 September 2009. Information was selected and interpreted by PHAC experts as to its implications in the Canadian context. It is important to note that any qualitative or quantitative values contained within the considerations presented here were not considered predictive; rather they were used for consideration as plausible, and debateable, options for the potential impacts of a fall wave. Additionally, the information provided here contains a mixture of predicted values, plausible information, and actual data.
Planning Considerations
Section 1. “Best Guess” Scenarios
PHAC experts selected the two most likely scenarios from many plausible scenarios for the fall of 2009 (Table 1). These scenarios were selected based on information from historical records of past pandemics, reports of national and international experiences with pH1N1/09, as of 28 September 2009, and consultation with internal and external influenza experts. These two scenarios were:
• ‘Short and intense’, with a high number of predominantly mild and moderate cases occurring in a short period of time, such as a two-month period from October to November, prior to vaccine availability;
• ‘Moderate and longer duration’, with a slightly higher than average number of cases over a longer period of time, such as the four-to-five month period from October to February, with vaccine availability.
The available information guided the choice of these two scenarios, as follows.
Table 1. Two Plausible Scenarios for the Potential pH1N1/09 Fall Wave, from the Public Health Agency of Canada’s Planning Considerations, September 2009. Both Scenarios Assume Cases in the Potential Fall Wave would be Predominantly Mild to Moderate.
| Scenario 1: Short and Intense | Scenario 2: Moderate with Longer Duration | |
|---|---|---|
| Historical Basis | • The 1957 pandemic peaked in October in Canada, following reports of unusual deaths over the spring and summer, and the identification of the novel H2N2 strain. The early fall wave was considerably more severe than over the summer in both 1957 and in cities documenting a significant summer 1918 wave. Excess deaths in persons under 50 years of age occurred primarily in the month of October 1957. | • The second wave of the 1957/58 H2N2 pandemic (which occurred during the winter of 1959) was more characteristic of seasonal influenza as the strong shift to younger ages did not persist and the timing of peak activity returned to the seasonal norm. Additionally, the 1968/69 H3N2 pandemic peaked in Canada in January 1969. |
| Potential Timing | • Epidemic peak in October 2009. | • Epidemic peak as is typical for seasonal influenza in Canada (November to April). This wave may occur in the fall or winter of 2009-2010. |
| Potential Confirming Characteristics | • Strong shift to younger ages expected to continue in an early fall 2009 wave. • Relative mortality rates are uncertain, but may be elevated. |
• Age profile observed in the spring and summer of 2009 may not persist into the fall and winter, or may be less pronounced than the spring 2009 wave. • Relative mortality rates are uncertain, but may remain partially elevated. |
| Potential Operational Considerations | • Vaccine would not be available before the epidemic peaks in the fall wave. • Intense activity over several months may overwhelm health system in sporadic intervals. |
• Vaccine would be available before the epidemic peaks in the late fall/winter wave. • Offering vaccine to everyone is consistent with the anticipation that the pandemic age profile observed in the spring may not persist, and that the currently circulating strain may mutate sufficiently that a vaccine offering improved cross-protection would provide better coverage. |
A situation where the pH1N1/09 epidemic would peak prior to the end of November (i.e. prior to the vaccination of the priority groups), was considered plausible, and was characterized by a ‘short and intense’ wave initiated by a strong increase in the transmission rate in early fall. As transmission had continued throughout the summer in most regions across Canada, it was felt that a strong increase in transmission rates in the fall could result in a well-synchronized fall wave across Canada. Such a scenario is similar to both the 1957/58 H2N2 and the 1918 H1N1 pandemics, where reports of a mild spring wave in various jurisdictions preceded a short and intense fall wave. The H2N2 pandemic peaked in October/November of 1957 in Canada. As of September 2009, PHAC anticipated that enough vaccine for everyone who wished to be vaccinated would be available by the end of December 2009, and that priority groups (i.e. people with chronic medical conditions under the age of 65; pregnant women; children six months of age to under five years of age; people living in remote and isolated settings or communities; health-care workers involved in pandemic response or who deliver essential health services; and household contacts and caregivers of individuals who are at high risk, and who cannot be immunized, such as infants under six months of age or people with weakened immune systems) could be vaccinated by the end of November 2009.
It was also considered plausible that the second pH1N1/09 wave could peak over the winter period, as the 1968/69 H3N2 peaked in January of 1969 in Canada. While the second wave, which occurred in the winter of 1970 in Canada, was milder than first wave, various European countries experienced a milder first wave in 1969 followed by a more intense second wave in 1970. These geographic differences were attributed to differences in pre-existing immunity to neuraminidase at the time of emergence of A/H3N2 (remaining from the A/H2N2 era) and the effect of genetic drift in the neuraminidase antigen (4). That the pandemic H3N2 virus was first isolated in Hong Kong in July of 1968 may account for the later timing of peak activity, compared to the 1918 and 1957 pandemics. The first wave of the H3N2 pandemic occurred at a time of year normally associated with peak seasonal influenza activity in Canada. After observing a slow decline in the number of new pH1N1/09 cases and hospital admissions over the summer of 2009, and only moderate increases in activity some jurisdictions in September 2009, the level of preexisting immunity going into the second wave of pH1N1/09 was considered uncertain. A small increase in transmission rates in the fall compared to the summer, together with additional pre-existing immunity could have resulted in a more moderate wave with longer duration than the wave experienced in the southern hemisphere during their winter. Genetic drift could change the characteristics of the second wave. For these reasons, it was considered plausible that the second pH1N1/09 wave could peak over the winter period.
Section 2. Planning Considerations
PHAC’s planning considerations around the potential population impacts of pH1N1/09 are outlined here; estimates and considerations for the Canadian context were collected from both available evidence (Canadian data; data emerging from the southern hemisphere winter 2009 wave experience) and expert opinion. Also presented are considerations of key international bodies: (a) the WHO’s areas for consideration from the 29 May 2009 Weekly Epidemiologic Record (5), (b) the United States’ President’s Council of Advisors on Science and Technology (PCAST) “Report to the President on U.S. Preparations for 2009-H1N1 Influenza”, (6) and (c) the United Kingdom’s “Planning Assumptions for the Current A(H1N1) Influenza Pandemic, 3 September 2009”. (7) The purpose of outlining these considerations was to support the dialogue and planning discussions for the short-term; it was not intended to discuss the meaning between the differences in the reported information.
The planning considerations for the potential pH1N1/09 fall wave are shown by category: possible epidemiologic characteristics are shown in Table 2, possible clinical characteristics and burden are shown in Table 3, and possible vulnerabilities of populations are shown in Table 4. Planning considerations for possible virological characteristics of a potential fall wave were taken solely from the WHO’s areas of consideration, 5) and included sensitivity to antiviral agents, molecular markers of severity, and antigenicity. Also shown are planning considerations for possible response capacity issues in a potential fall wave (Table 5).
Table 2. Possible and Reported Epidemiological Characteristics: Planning Considerations for the Potential pH1N1/09 Fall Wave, from the Public Health Agency of Canada’s Planning Considerations, September 2009.
| World Health Organization’s Areas for Considerationa | Selected Planning Considerations and Estimates from International Sources |
Canadian Areas of Consideration: Selected Planning Considerations, Data and Estimates Relevant to the Canadian Context |
||
|---|---|---|---|---|
|
United States Planning Considerationsb (a plausible scenario for fall resurgence of 2009-H1N1) |
United Kingdom Planning Considerationsc (for the current A(H1N1) influenza pandemic) & U.K. Health Protection Agency Estimates |
Considerations, Data, and Estimates | Source of Information | |
| Total number suspected and confirmed cases | Infection of 30–50% of the U.S. population in the fall and winter (90–150 million infections) Symptoms in 20–40% of the U.S. population (60–120 million) |
4,500 new cases in the UK in the week of 24 August 2009d Weekly number of cases at epidemic peak: 110,000e |
4.5-10.6 million Canadians clinically ill, over possibly three wavesg | Public Health Agency of Canada, Canadian Pandemic Influenza Plan, 2006 |
| British Columbia: project approximately 11,000 courses of antibiotics will be requiredh | Government of British Columbia, British Columbia’s H1N1 Pandemic Influenza Response Plan | |||
| Evidence of concurrent bacterial infection was found in specimens from 22 (29%) of the 77 fatal cases of confirmed 2009 pandemic influenza A (H1N1)i | Northern hemisphere data on pH1N1/09 (Morbidity and Mortality Weekly Report) | |||
| Total number deaths | Estimated deaths: 30,000–90,000 | 70 deaths, cumulative as of 3 September 2009d Estimated number of deaths for the fall wave: 20,000c |
78 deaths, cumulative as of 26 September 2009j | Public Health Agency of Canada, FluWatch data on pH1N1/09 |
| Clinical attack rate | Produce infection of 30–50% of the U.S. population in the fall and winter (90–150 million infections) Symptoms in 20–40% of the U.S. population (60–120 million) |
Up to 30% of populationc Peak clinical attack rate: nationally, up to 6.5% of population per week; locally, 4.5-8.0% of population per weekc |
15-35% over the course of the pandemick | Public Health Agency of Canada, Canadian Pandemic Influenza Plan, 2006 |
| 20-40%l | Other planning considerations on pH1N1/09 (White Paper on Novel H1N1) | |||
| Australia: 20% clinical attack rate; with no intervention, estimated by the end of the winter that 1 in 5 Australians could become infectedm | Southern hemisphere data on pH1N1/09 winter wave | |||
| Case-fatality or mortality rate | - | Estimated case fatality rate reduced from 0.1-0.35% (first major wave)f to 0.1%c | 0.4% in those who are clinically ill (for a pandemic of mild to moderate severity, and in the absence of any interventions, e.g. vaccine, antivirals)k | Public Health Agency of Canada, Canadian Pandemic Influenza Plan, 2006 |
| Comparative health care usage rates between an inter-pandemic and pandemic year: 8.2 times more deaths attributable to influenza during pandemic compared to inter-pandemic yeark | Public Health Agency of Canada, Canadian Pandemic Influenza Plan, 2006 | |||
| Case-fatality or mortality rate | 0.1% case fatality rate for seasonal influenza (4,000 deaths attributed to influenza annually and 10% clinical attack rate), primarily among persons aged 65 years or oldern,o | Canadian data on seasonal influenza | ||
| Highest pH1N1/09 confirmed mortality rate occurred in those over 65 years of age (0.42 per 100,000; spring wave, as of 22 August 2009)p | Public Health Agency of Canada, FluWatch data on pH1N1/09 | |||
| pH1N1/09 confirmed mortality rate 0.23 per 100,000 population in Canada, as of 31 August 2009q | Public Health Agency of Canada data on pH1N1/09 | |||
| Reproduction number (R0) | - | - | 1.4—1.8 (initial wave)k | Public Health Agency of Canada, Canadian Pandemic Influenza Plan, 2006 |
| Other transmission characteristics | Projected peak incidence date (unmitigated): October 15, 2009 At peak incidence, 1–2% of U.S. population infected each day, assuming no change in virus |
Second wave to occur ~ mid-to-late October 2009c | - | - |
a World Health Organization. Considerations for assessing the severity of an influenza pandemic. Weekly epidemiological record (WER). 29 May 2009;84(22):197-202. URL: www.who.int/wer. Date of access: 9 Sept. 2009.
b President’s Council of Advisors on Science and Technology (PCAST). Report to the President on U.S. preparations for 2009-H1N1 influenza. URL: www.whitehouse.gov/assets/documents/PCAST_H1N1_Report.pdf. Date of access: Sept. 2009.
c United Kingdom Department of Health (UKDH). Swine flu: UK planning assumptions (3 September 2009). URL: http://www.businesslink.gov.uk/Horizontal_Services_files/UKplanningassumptions03092009.pdf. Date of access: 3 Sept. 2009.
d Health Protection Agency. Weekly national influenza report. 3 September 2009. URL: http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1251473420520.
e Health Protection Agency. Weekly national influenza report. 30 July 2009. URL: http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1248940851283.
f United Kingdom Department of Health (UKDH). Swine flu: UK planning assumptions—SUPERSEDED (16 July 2009). URL: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_102891.pdf.
g Public Health Agency of Canada. (2006). Canadian Pandemic Influenza Plan for the Health Sector, Annex P. URL: http://www.phac-aspc.gc.ca/cpip-pclcpi/ann-p-eng.php.
h Government of British Columbia. British Columbia’s H1N1 Pandemic Influenza Response Plan (2009): Antibiotics for Secondary Pneumonia in Community and Acute Care Settings. October 2, 2009.
i Bacterial Co-infections in Lung Tissue Specimens from Fatal Cases of 2009 Pandemic Influenza A (H1N1)—United States, May-August 2009. Morbidity and Mortality Weekly Report. October 2, 2009 / 58(38);1071-10.
j Public Health Agency of Canada. FluWatch: September 20, 2009 to September 26, 2009 (Week 38). URL: http://www.phac-aspc.gc.ca/fluwatch/09-10/w38_09/index-eng.php.
k Public Health Agency of Canada. (2006). Canadian Pandemic Influenza Plan for the Health Sector. URL: http://www.phac-aspc.gc.ca/cpip-pclcpi/pdf-e/cpip-eng.pdf.
l Barry, JM. White Paper on Novel H1N1 (Prepared for the MIT Center for Engineering Systems Fundamentals). Massachusetts Institute of Technology Engineering Systems Division, Working Paper Series ESD-WP-2009-07. July 2009. URL: http://esd.mit.edu/WPS/2009/esd-wp-2009-07-072709.pdf.
m Australian Government Department of Health and Ageing. Australian Influenza Surveillance Summary Report No.14, 2009, Reporting period: 8 August 2009—14 August 2009. URL: http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/18D06BAC4644C98DCA25763E00823442/$File/ozflu-no14-2009.pdf.
n Schanzer DL, Langley JM, Tam TWS. Co-morbidities associated with Influenza-Attributed Hospital Admissions and Deaths, 1994-2000, Canada. In: Options for the Control of Influenza VI: June 17-23, 2007, Toronto, Ont., Canada, 2007.
o Schanzer DL, Tam TWS, Langley JM, Winchester BT. Influenza-attributable deaths: Canada 1990-1999. Epidemiol Infect 2007:135;1109-16. (Epub 2007 Feb 19).
p Public Health Agency of Canada. FluWatch: August 23, 2009 to August 29, 2009 (Week 34). URL: http://www.phac-aspc.gc.ca/fluwatch/08-09/w34_09/index-eng.php.
q Public Health Agency of Canada. Deaths associated with H1N1 flu virus in Canada. URL: http://www.phac-aspc.gc.ca/alert-alerte/h1n1/surveillance-archive/20090827-eng.php.
Table 3. Possible and Reported Clinical Characteristics and Burden: Planning Considerations for the Potential pH1N1/09 Fall Wave, from the Public Health Agency of Canada’s Planning Considerations, September 2009.
| World Health Organization’s Areas for Considerationa | Selected Planning Considerations and Estimates from International Sources |
Canadian Areas of Consideration: Selected Planning Considerations, Data and Estimates Relevant to the Canadian Context |
||
|---|---|---|---|---|
|
United States Planning Considerationsb (a plausible scenario for fall resurgence of 2009-H1N1) |
United Kingdom Planning Considerationsc (for the current A(H1N1) influenza pandemic) |
Considerations, Data, and Estimates | Source of Information | |
| Signs and symptoms | - | - | Cases will rise but rates of lab testing to decline, thus identification of cases to decline; we will identify a lower proportion of infections in the fall, than in April to August | Assumption |
| Clinical course and outcome | Needing medical attention: 15–30% (45–90 million) | - | 2.1-5 million (50% of cases) will need outpatient care, i.e. 3.7 times more outpatient visits per population versus non-pandemic yearsd | Public Health Agency of Canada, Canadian Pandemic Influenza Plan, 2006 |
| Number hospitalised cases | Needing hospital care: 0.3–0.6% of US population (0.9–1.8 million) Peak occupancy of hospital beds due to H1N1: 50–150 hospital beds/100,000 population Bed availability: 211 hospital beds/100,000 population |
Case hospitalization ratio: up to 1% of clinical cases would be hospitalized Majority of hospitalisations occur in children <5yrs of agek |
1,441 H1N1 confirmed hospitalized cases, or 5 H1N1 confirmed admissions per 100,000 population reported as of 22 August 2009e | Public Health Agency of Canada, FluWatch data on pH1N1/09 |
| 1% of cases hospitalised; i.e. 3.9 times as many hospitalisations per population versus inter-pandemic yearsf | Public Health Agency of Canada, Canadian Pandemic Influenza Plan, 2006 | |||
| Before 25 June 2009 3% of confirmed H1N1 cases in Ontario were hospitalizedg | Ontario data on pH1N1/09 | |||
| As per August 15, 2009: 20% of cases in Canada were hospitalized: those under 15 years have the highest rates of hospitalizationh | Public Health Agency of Canada, FluWatch data on pH1N1/09 | |||
| Length of stay in hospital: 89% of discharged cases in Ontario had a length of stay of ≥2 daysi | Ontario data on pH1N1/09 | |||
| Australia: highest hospitalisation rate occurs in children under 5yearsj | Southern hemisphere data on pH1N1/09 winter wave | |||
| Number cases in intensive care | Needing Intensive Care facilities: 0.05–0.1% of the US population (150,000–300,000) Peak occupancy of Intensive Care beds due to H1N1: 10–25 ICU beds/100,000 population Bed availability: 20 ICU beds/100,000 population. The number of ICU beds available for paediatric patients is especially limited |
Up to 25% of hospitalized cases could require intensive care | 20% of H1N1 confirmed admissions during the spring wave were also admitted to an intensive care unit (278 admissions; 22 August 2009)e | Public Health Agency of Canada, FluWatch data on pH1N1/09 |
| For infection rates greater than 25%, expected intensive care unit demand will exceed capacity; for rates less than 25% hospitals may be able to handle demand if majority of intensive care unit beds are available for H1N1.l | Canadian-based study on pH1N1/09, unpublished data | |||
| Australia: 20% of hospitalised cases transferred to ICUm | Southern hemisphere data on pH1N1/09 winter wave | |||
| Number cases requiring mechanical ventilation | - | - | 8.7 mechanically ventilated beds per 100,000 population, running at ~90% capacityl | Canadian-based study on pH1N1/09, unpublished data |
| Proportion of cases with severe illness | - | - | Chile: 48% of hospitalised cases had underlying chronic disease.n | Southern hemisphere data on pH1N1/09 winter wave |
a World Health Organization. Considerations for assessing the severity of an influenza pandemic. Weekly epidemiological record (WER). 29 May 2009;84(22):197-202. URL: www.who.int/wer. Date of access: 9 Sept. 2009.
b President’s Council of Advisors on Science and Technology (PCAST). Report to the President on U.S. preparations for 2009-H1N1 influenza. URL: www.whitehouse.gov/assets/documents/PCAST_H1N1_Report.pdf. Date of access: Sept. 2009.
c United Kingdom Department of Health (UKDH). Swine flu: UK planning assumptions (3 September 2009). URL: http://www.businesslink.gov.uk/Horizontal_Services_files/UKplanningassumptions03092009.pdf. Date of access: 3 Sept. 2009.
d Public Health Agency of Canada. (2006). Canadian Pandemic Influenza Plan for the Health Sector. URL: http://www.phac-aspc.gc.ca/cpip-pclcpi/pdf-e/cpip-eng.pdf.
e Public Health Agency of Canada. FluWatch: August 16, 2009 to August 22, 2009 (Week 33). URL: http://www.phac-aspc.gc.ca/fluwatch/08-09/w33_09/index-eng.php.
f Public Health Agency of Canada. (2006). Canadian Pandemic Influenza Plan for the Health Sector, Annex P. URL: http://www.phac-aspc.gc.ca/cpip-pclcpi/ann-p-eng.php.
g Ontario Agency for Health Protection and Promotion. Weekly synthesis of surveillance, information, literature, and government updates (Week 24—ending June 19, 2009). URL: http://www.oahpp.ca/resources/documents/reports/h1n1weeklysynthesis/Weekly%20Synthesis%20-%20June%2019,%202009.pdf.
h Public Health Agency of Canada. FluWatch: August 9, 2009 to August 15, 2009 (Week 32). URL: http://www.phac-aspc.gc.ca/fluwatch/08-09/w32_09/index-eng.php.
i Ontario Agency for Health Protection and Promotion. Weekly synthesis of surveillance, information, literature, and government updates (Week ending August 21, 2009). URL: http://www.oahpp.ca/resources/documents/reports/h1n1weeklysynthesis/H1N1%20Weekly%20Synthesis%20August%2021,%2009.pdf.
j United States Department of Health and Human Services. Assessment of the 2009 Influenza A (H1N1) Outbreak on Selected Countries in the Southern Hemisphere: Annex I—Assessment of the 2009 H1N1 Pandemic on Individual Countries: Argentina, Australia, Chile, New Zealand and Uruguay. 2009. URL: http://www.flu.gov/professional/global/annex1.pdf.
k Health Protection Agency. Weekly national influenza report. 10 September 2009. URL: http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1252514887004.
l Smetanin P, Stiff D. Potential ICU and ventilator demand due to novel soH1N1: modelling of severe disease in Canada. (2009). URL: http://www.riskanalytica.com/Solutions/Pandemic.aspx. Date of access: 9 Sept. 2009.
m Lum ME, McMillan AJ, Brook CW, Lester R, Piers LS. Impact of pandemic (H1N1) 2009 influenza on critical care capacity in Victoria. Med J Aust 2009; 191 (9): 502-506.
n Department of Health and Human Services in collaboration with other U.S. Government (USG) Departments for the White House National Security Council. Assessment of the 2009 Influenza A (H1N1) Pandemic on Selected Countries in the Southern Hemisphere: Argentina, Australia, Chile, New Zealand and Uruguay. August 26, 2009.
Table 4. Possible and Reported Vulnerability of Populations: Planning Considerations for the Potential pH1N1/09 Fall Wave, from the Public Health Agency of Canada’s Planning Considerations, September 2009.
| World Health Organization’s Areas for Considerationa |
United States Planning Considerationsb (a plausible scenario for fall resurgence of 2009-H1N1) |
Canadian Areas of Consideration: Selected Planning Considerations, Data and Estimates Relevant to the Canadian Context |
|
|---|---|---|---|
| Considerations, Data, and Estimates | Source of Information | ||
| People who may be considered at increased risk | High-risk groups for death or hospitalization: Pregnant women; children (0–4 years old); patients with neuromuscular / neurocognitive disorders, asthma, chronic obstructive pulmonary disease, cardiovascular disease, diabetes, severe obesity, or immuno-compromising conditions | Persons at higher risk of complications from pH1N1: <65 years with chronic conditions, e.g. asthma; pregnant women; children 6-59 months; Aboriginals; persons in remote or isolated communitiesc | Public Health Agency of Canada pH1N1/09 Guidance Document |
| 5% of H1N1 confirmed deaths occurred in pregnant women, as of 22 August 2009d | Public Health Agency of Canada, FluWatch data on pH1N1/09 | ||
| 11% of H1N1 confirmed deaths occurred in Aboriginals (who make up 3% of population; as of 22 August 2009)d | Public Health Agency of Canada, FluWatch data on pH1N1/09 | ||
| On-reserve First Nations and remote communities; also vulnerabilities related to overcrowding.e | Public Health Agency of Canada, FluWatch data on pH1N1/09 | ||
| Europe: Compared to those 0-9 and 20-29 years old, those 10-19 years have an attack rate 1.5 times and those 40-49 have an attack rate 0.25 timesf | Northern hemisphere data on pH1N1/09 | ||
| Australia: Median age of confirmed cases who died was 54 years versus 83 years for seasonal influenzag | Southern hemisphere data on pH1N1/09 winter wave | ||
| Brazil: In cases of influenza-like illness with severe acute respiratory illness in women aged 15-49, 23% (525 of 2256) were pregnanth | Southern hemisphere data on pH1N1/09 winter wave | ||
| Other confounding factors to consider: laboratory testing based on severity; unidentified age distributions of subpopulations (e.g. international travellers); starting time, initial growth rate, and speed of disease spread by age group depends on social environment; social mixing patterns by age (e.g. school aged or not). | Assumption | ||
a World Health Organization. Considerations for assessing the severity of an influenza pandemic. Weekly epidemiological record (WER). 29 May 2009;84(22):197-202. URL: www.who.int/wer. Date of access: 9 Sept. 2009.
b President’s Council of Advisors on Science and Technology (PCAST). Report to the President on U.S. preparations for 2009-H1N1 influenza. URL: www.whitehouse.gov/assets/documents/PCAST_H1N1_Report.pdf. Date of access: Sept. 2009.
c Public Health Agency of Canada. Guidance Document on the Use of Pandemic Influenza A (H1N1) 2009 Inactivated Monovalent Vaccine. URL: http://www.phac-aspc.gc.ca/alert-alerte/h1n1/vacc/monovacc/recom-eng.php.
d Public Health Agency of Canada. FluWatch: August 30, 2009 to September 5, 2009 (Week 35). URL: http://www.phac-aspc.gc.ca/fluwatch/09-10/w35_09/pdf/fw2009-35-eng.pdf.
e Public Health Agency of Canada. H1N1 in Aboriginal, First Nation and Inuit Communities. 2009. URL: http://www.phac-aspc.gc.ca/alert-alerte/h1n1/faq/faq_rg_h1n1-anic-eng.php.
f Vaillant L, La Ruche G, Tarantola A, Barboza P. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Eurosurveillance 2009;14(33):1.
g Australian Government Attorney-General’s Department: Emergency Management Australia. URL: http://www.ema.gov.au/ema/emadisasters.nsf/6a1bf6b4b60f6f05ca256d1200179a5b/70e3a7c1e0e7cd87ca257625001a60d4?OpenDocument.
h Oliveira WK, Carmo EH, Penna GO, Kuchenbecker RS, Santos HB, Araujo WN, Malaguti R, Duncan BB, Schmidt MI, on behalf of the Surveillance Team for the pandemic influenza A(H1N1) 2009 in the Ministry of Health. Pandemic H1N1 influenza in Brazil: Analysis of the first 34,506 notified cases of influenza-like illness with severe acute respiratory infection (SARI). Euro Surveill. 2009;14(42):pii=19362. URL: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19362.
Table 5. Possible Response Capacity Implications: Planning Considerations for the Potential pH1N1/09 Fall Wave, from the Public Health Agency of Canada’s Planning Considerations, September 2009.
| World Health Organization’s Areas for Considerationa | Selected Planning Considerations and Estimates from International Sources |
Canadian Areas of Consideration: Selected Planning Considerations, Data and Estimates Relevant to the Canadian Context |
||
|---|---|---|---|---|
|
United States Planning Considerationsb (a plausible scenario for fall resurgence of 2009-H1N1) |
United Kingdom Planning Considerationsc (for the current A(H1N1) influenza pandemic) |
Considerations, Data, and Estimates | Source of Information | |
| Access to Health Care | Bed availability: 20 ICU beds/100,000 population. The number of ICU beds available for paediatric patients is especially limited |
- | More results on effectiveness of single dose vaccination are still emerging | Assumption |
| During severe influenza seasons, the number of influenza-like illness-related physician visits increased to 35%-40%.d | Public Health Agency of Canada, Canadian Pandemic Influenza Plan, 2006 | |||
| China & Novartis (UK) Vaccine Trial: Pilot clinical trial, 100 subjects, indicates strong, potentially protective response in 80% of subjects after 1 dose, and more than 90% of subjects after 2 doses.e | International data on pH1N1/09 | |||
| Communication and Social Mobilisation | Improving Communications | - | Social Voices, e.g. supporting/opposing viewpoints or (mis) information from individuals with ‘clout’f | Canadian experience with pH1N1/09 |
| Community mitigation measures, including effects on sub-populations and tourismd | Public Health Agency of Canada, Canadian Pandemic Influenza Plan, 2006 | |||
| Advance Preparedness and Planning | Ensuring Adequate Data for Decision Making: Surveillance Systems Responding to the Pandemic Lowering Financial and Regulatory Barriers to Effective Response Planning for More Effective Future Strategies Against Influenza |
Large corporations must gear pandemic planning towards local environment (“one size does not fit all”) Small and medium sized businesses are disproportionately affected by pandemics; in most cases, insurance will not cover losses Workplace absenteeism: 50% absenteeism rates for private sector 57% of UK employers have no pandemic plan in place |
4.6% to 21.2% of PHAC workforce absent on a given dayg | Public Health Agency of Canada, internal data |
| Non-pandemic related daily absenteeism for PHAC is 3.66% (sick leave + family leave; January 2009)g | Public Health Agency of Canada, internal data | |||
| Pandemic-related daily absenteeism for PHAC may range from 0.9% (long, protracted outbreak; sick leave only) to 17.5% (short, intense outbreak; sick + family leave)g | Public Health Agency of Canada, internal data | |||
| Mexico: reported valuable public health measures included: 1/rapid notification of the public;2/rapid diagnosis, treatment, and quarantine; 3/hand washing. Ineffective steps included: 1/travel bans; 2/school closures; 3/widespread use of surgical masks; 4/screening at border; 5/border closures.h | Northern hemisphere data on pH1N1/09 | |||
a World Health Organization. Considerations for assessing the severity of an influenza pandemic. Weekly epidemiological record (WER). 29 May 2009;84(22):197-202. URL: www.who.int/wer. Date of access: 9 Sept. 2009.
b President’s Council of Advisors on Science and Technology (PCAST). Report to the President on U.S. preparations for 2009-H1N1 influenza. URL: www.whitehouse.gov/assets/documents/PCAST_H1N1_Report.pdf. Date of access: Sept. 2009.
c Business Continuity Institute and the Chartered Institute of Personnel and Development. (2009). Risk and business continuity management. URL: http://www.bcipartnership.com/businesscontinuitymanagementguide0809.pdf.
d Public Health Agency of Canada. (2006). Canadian Pandemic Influenza Plan for the Health Sector. URL: http://www.phac-aspc.gc.ca/cpip-pclcpi/pdf-e/cpip-eng.pdf.
e The Medical News, 4 September 2009. University of Leicester: Clinical trial of Novartis MF59 swine-flu vaccine elicits a strong immune response. URL: http://www.news-medical.net/news/20090904/Clinical-trial-of-Novartis-MF59-swine-flu-vaccine-elicits-a-strong-immune-response.aspx.
f Schabas R and Rau N. Canada’s H1N1 decision: policy or politics?: Waiting for that ‘second wave’ of influenza. The Globe and Mail, Thursday, Aug. 13, 2009 4:18PM EDT. URL: http://www.theglobeandmail.com/news/opinions/canadas-h1n1-decision-policy-or-politics/article1251003/.
g Health Canada & the Public Health Agency of Canada. (2009). Report on absenteeism since April 1, 2007 by department, fiscal year and leave type. Internal report.
h University of Minnesota, Center for Infectious Disease Research and Policy. (2009). Mexico: Health officials tout openness as most effective tactic in fighting pandemic H1N1. URL: http://depts.washington.edu/einet/newsbrief380.html.
Other information from sources additional to those above was used for comparison and discussion, and to provide additional context to PHAC’s planning deliberations. This information is shown below under two contexts: (a) the spring 2009 wave, as had been observed in the northern hemisphere, and (b) the winter 2009 wave, as had been observed in the southern hemisphere.
Information from the northern hemisphere was:
• School outbreaks in the UK report clinical attack rates of 30% (8);
• The reproduction number (Ro), as observed in Mexico, was 1.44 (9);
• The number of pH1N1/09 deaths increased in the United States, from early May (8 deaths) (10) to mid-June (87 deaths) (11);
• The estimated case fatality ratio in Mexico was 0.4% (range: 0.3 to 1.8%), based on confirmed and suspected deaths reported to late April 2009; upper 95% bound of 0.6% (12).
• pH1N1/09 confirmed mortality rates per 100,000 population, as of 31 August 2009, were: 0.23 (Canada), 0.19 (United States), and 0.19 (Mexico); and the pH1N1/09 confirmed mortality rates per 100,000 population, as of 18 September 2009, were: 0.23 (Canada), 0.22 (United States), and 0.20 (Mexico) (13).
Information from the southern hemisphere was:
• 132 pH1N1/09 confirmed cases per 100,000 population by 11 August 2009, observed in Australia (14);
• The fatality rate reported from Australia (calculated as the number of pH1N1/09 confirmed deaths divided by the number of pH1N1/09 confirmed cases) was 0.34% (14);
• pH1N1/09 confirmed mortality rates per 100,000 population, as of 31 August 2009, were: 1.2 (Argentina), 0.9 (Uruguay), 0.8 (Australia), 0.8 (Chile), 0.4 (New Zealand), and 0.37 (Brazil); and the pH1N1/09 confirmed mortality rates per 100,000 population, as of 18 September 2009, were: 1.3 (Argentina), 0.81 (Australia), and 0.5 (Brazil) (13).
Section 3. Impact on PHAC Resources
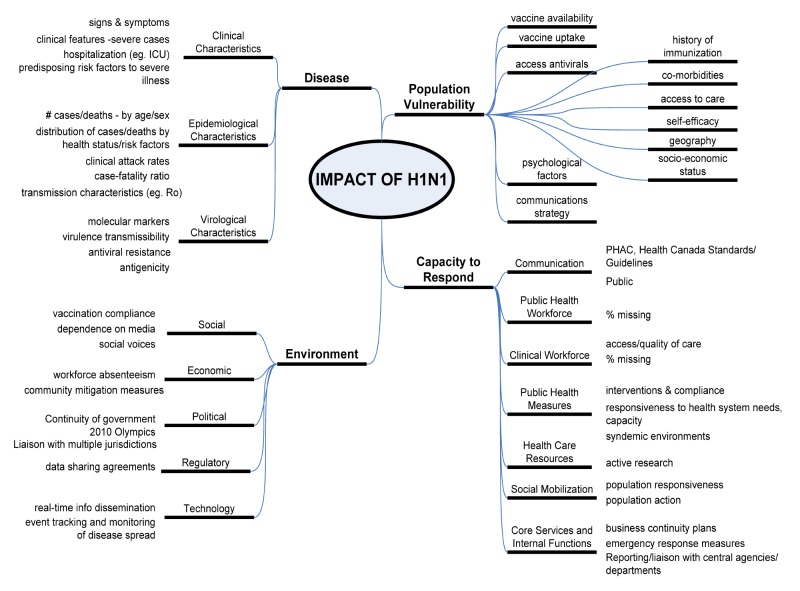
Heading into the fall 2009 wave, it was thought that the impact of pH1N1/09 on PHAC’s human and financial resources, and infrastructure would continue to be far reaching, with impacts on the population, the capacity to respond and our social, political, technological, economic and regulatory environments. Adapted from the WHO’s 29 May 2009 Weekly Epidemiological Record (5), to the Canadian context, an overview of the categories of factors that could have potential impact on PHAC’s resources is presented (Figure 1); this Figure provided a tool for assessing the potential severity of impact, in order to plan for the appropriate targeting and scaling of use of limited resources and interventions. The categories included were intended to provide the broadest capture of all major elements that could act to impact PHAC’s resources, heading into a potential second wave.
Figure 1.
Potential Factors which may have Impacted Public Health Agency of Canada Human and Financial Resources, heading into the Potential pH1N1/09 Fall Wave, from the Public Health Agency of Canada’s Planning Considerations, September 2009 (adapted from the World Health Organization’s 29 May 2009 Weekly Epidemiological Record)
Summary
In September 2009, PHAC compiled a working document of planning considerations for the potential fall wave of pH1N1/09. This document was intended as a construct on which to base various planning concepts and review planning processes, and as a basis for discussion and debate. The working document described what might be expected in the Canadian context with regards to a potential fall wave, specifically, (i) plausible scenarios for a fall wave, (ii) planning considerations of the potential population impacts of pH1N1/09 in the fall, and (iii) potential impacts on PHAC resources. Information for the document was gleaned from internal and external sources, including grey and published literature, available developments in pH1N1/09 activity in the southern hemisphere’s winter 2009 wave, and consultation with experts. The review period during which information was searched and compiled was 25 August to 28 September 2009.
The information contained within this report is subject to several limitations. First, any qualitative or quantitative values contained within the considerations presented here were not treated as predictive; rather they were used for consideration as plausible, and debateable, options for the potential impacts of a fall wave. Additionally, the considerations presented here contain a mixture of predicted values, plausible information, and actual data.
Despite these limitations, working documents such as this one, which summarize an Agency’s planning considerations, are useful in that they provide (a) a single focal point documenting both implicit and explicit considerations on which plans are made, and (b) a vehicle for debate and deliberation about possible futures as unknown circumstances unfold. This document was used to support PHAC’s Advanced Planning Group’s discussions and planning for the second wave. It provided a cohesive and simplified summary of what was available, extracting select information from the plethora of available material. The result was a single, evidence-based reference document that facilitated consistency across PHAC, and allowed policy and program areas to plan for how these situations might impact their responsibilities.
Epilogue
The planning considerations document summarized above was produced in September 2009 using knowledge available at that time. Since its production, the H1N1 pandemic proceeded as follows. On 23 October 2009, the start of Canada’s second wave was declared; based on indicators of influenza activity from FluWatch surveillance data. Notably, a significant increase in the number of laboratory confirmed cases was identified for the reporting week of 4–10 October 2009, where the percent positive increased to 11%, from 3.5% in late summer and 5% in the previous week. The start of the fall wave was formally considered to be the week of 30 August 2009, since the week of 23–29 August 2009 was observed retrospectively to be the nadir point between the spring and fall wave.
Influenza admissions continued to increase exponentially, peaking in some regions of Canada at the end of October. During periods of peak activity, many jurisdictions opened flu clinics to reduce the demands on emergency rooms and family physicians, and many hospitals had to cancel surgeries as demand for intensive care units reached capacity. Nationally, the fall wave peaked in the first week of November 2009, a week after the vaccination campaign started. On 2 December 2009, PHAC cautiously announced that the fall wave had peaked; at that time, data for the week ending 28 November 2009 indicated that the number of laboratory confirmed cases had dropped to less than 25% of the peak number of cases observed 1–7 November 2009.
Since some jurisdictions had experienced significant back-to-back waves during earlier pandemics, PHAC continued to watch for an additional winter wave. All indicators of influenza activity continued to decline through the rest of December 2009 and into January 2010. On 27 January 2010, PHAC formally announced that the fall wave had tapered off (15).
As of 1 March 2010, the level of influenza activity in Canada was below the seasonal norm for this time of year, and only a few cases of seasonal strains of influenza were detected this season. It is important to remember, however, that the pH1N1/09 influenza virus is still circulating in some communities. Thus, as of the writing of this document (March 2010), PHAC continues to remain vigilant, paying close attention to the situation in the southern hemisphere as it heads into its coming influenza season, and maintaining preparations in case the next wave of the pandemic H1N1 2009 strain should be more severe than our experience with the fall wave of 2009.
It is important to note that during the pH1N1/09 pandemic, in Canada and worldwide, public health made a significant effort to use laboratory testing to identify cases. In the absence of laboratory testing to identify cases, our understanding of the waves of historic pandemics and epidemics has relied on statistical methods which establish seasonal baselines and attribute all cases in excess of these thresholds to influenza (16). The use of threshold methods means that, traditionally, the start and end of a given wave often remain hidden, resulting in an apparently shorter wave. However, with the extensive use of laboratory testing during the pandemic, we were able to observe the development of the waves over a much longer period of time.
The early cases in Canada were identified by testing persons with influenza symptoms who had recently returned from Mexico. We observed sustained transmission of the first wave from May to August and into September 2009, over a period of almost five months. Compared to the typical September levels of seasonal influenza activity, levels of pH1N1/09 activity were still high when transmission rates started to increase again in September 2009. As well, the fall wave continued from the end of September 2009 into January 2010, with some transmission still occurring at the end of February 2010. A comparison of the epidemic curve of the fall wave of pH1N1/09 with that of seasonal influenza indicates that the rate of increase in the number of cases during the epidemic growth phase of the fall wave was significantly elevated compared to seasonal influenza A waves (17). As well, consultation rates for influenza-like illnesses surpassed the usual seasonal peak levels of 50 per 1000 physician visits for a period of five weeks (18 October—21 November, 2009), resulting in a short period of intense activity, accentuated by the relatively high degree of synchronization of peak activity across Canada.
Acknowledgements
The compilation of the planning considerations and this resulting report represented a cross-Agency collaboration between multiple individuals and teams, with particular effort from Jan Trumble Waddell, Victoria L. Edge, and Shannon E. Majowicz (Population Health Assessment and Scenarios Team, Office of Public Health Practice), Dena L. Schanzer (Surveillance and Risk Assessment Division, Centre for Communicable Diseases and Infection Control), Louise Pelletier (Influenza Surveillance Section, Centre for Immunization and Respiratory Infectious Diseases), Rachel Rodin (Centre for Immunization and Respiratory Infectious Diseases), Mark Raizenne (Centre for Food-borne, Environmental, and Zoonotic Infectious Diseases), Gregory Taylor (Office of Public Health Practice), John Lynch (Policy Integration, Planning, Reporting and International Directorate), and John Spika (Centre for Immunization and Respiratory Infectious Diseases).
In addition, the informal Planning Considerations Review Group were solicited for feedback during the fall of 2009, as the planning considerations were compiled. This group consisted of: Larry Bredesen, Daniel Gillis, Luc Ladouceur, Michel Martineau, Debra O’Donnell, Claire Sevenhuysen, Andrew Stevermer, Ping Yan, and the Regional Offices (Public Health Agency of Canada); David Fisman (University of Toronto); Babak Pourbohloul (University of British Columbia Centre for Disease Control).
References
- 1.World Health Organization. Statement by WHO Director-General Dr. Margaret Chan, “Swine Influenza”. 25 April 2009. URL: http://www.who.int/mediacentre/news/statements/2009/h1n1_20090425/en/index.html
- 2.World Health Organization. Statement by WHO Director-General Dr. Margaret Chan, “Influenza A(H1N1)”. 29 April 2009. URL: http://www.who.int/mediacentre/news/statements/2009/h1n1_20090429/en/index.html
- 3.World Health Organization. Statement by WHO Director-General Dr. Margaret Chan, “World now at the start of 2009 influenza pandemic”. 11 June 2009. URL: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html
- 4.Viboud C, Grais RF, Lafont BA, Miller MA, Simonsen L; Multinational Influenza Seasonal Mortality Study Group. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis 2005. Jul;192(2):233–48. 10.1086/431150 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Considerations for assessing the severity of an influenza pandemic. Weekly epidemiological record (WER). 29 May 2009; 84(22):197-202. URL: www.who.int/wer. Date of access: 9 Sept. 2009. [PubMed]
- 6.President’s Council of Advisors on Science and Technology (PCAST). Report to the President on U.S. preparations for 2009-H1N1 influenza. URL: www.whitehouse.gov/assets/documents/PCAST_H1N1_Report.pdf. Date of access: Sept. 2009.
- 7.United Kingdom Department of Health (UKDH). Swine flu: UK planning assumptions (2009). URL: http://www.businesslink.gov.uk/Horizontal_Services_files/UKplanningassumptions03092009.pdf. Date of access: 3 Sept. 2009.
- 8.European Centre for Disease Prevention and Control (ECDC). ECDC interim risk assessment: pandemic (H1N1)2009 influenza. 21 August 2009. URL: http://ecdc.europa.eu/en/healthtopics/H1N1/Documents/1001_RA_090821.pdf. Date of access: 8 Sept. 2009.
- 9.Pourbohloul B, Ahued A, Davoudi B, Meza R, Meyers LA, Skowronski DM et al. Initial human transmission dynamics of the pandemic (H1N1) 2009 virus in North America. Influenza Other Respir Viruses 2009. Sep;3(5):215–22. 10.1111/j.1750-2659.2009.00100.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Novel H1N1 Flu Situation Update, May 20, 2009. URL: http://www.cdc.gov/h1n1flu/updates/052009.htm. Date of access: 7 Sept. 2009.
- 11.Centers for Disease Control and Prevention. Novel H1N1 Flu Situation Update, June 19, 2009. URL: http://www.cdc.gov/h1n1flu/updates/061909.htm
- 12.Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD et al. ; WHO Rapid Pandemic Assessment Collaboration. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 2009. Jun;324(5934):1557–61. 10.1126/science.1176062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wikipedia. HIN1 confirmed deaths by country. URL: http://en.wikipedia.org/wiki/2009_flu_pandemic_by_country. Date of access: 25 Sept. 2009.
- 14.Sweet M. Pandemic lessons from Australia. BMJ 2009. Aug;339 b3317:b3317. 10.1136/bmj.b3317 [DOI] [PubMed] [Google Scholar]
- 15.Public Health Agency of Canada Alert. 27 January 2010. URL: http://www.phac-aspc.gc.ca/alert-alerte/h1n1/wave-vague2-eng.php
- 16.Schanzer DL, Tam TW, Langley JM, Winchester BT. Influenza-attributable deaths, Canada 1990-1999. Epidemiol Infect 2007. Oct;135(7):1109–16. 10.1017/S0950268807007923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schanzer DL, Langley JM, Dummer T, Viboud C, Tam TW. A composite epidemic curve for seasonal influenza in Canada with an international comparison. Influenza Other Respir Viruses 2010. Sep;4(5):295–306. 10.1111/j.1750-2659.2010.00154.x [DOI] [PMC free article] [PubMed] [Google Scholar]