Supplemental digital content is available in the text.
Key Words: EXERCISE, CARDIAC MAGNETIC RESONANCE IMAGING, GENOTYPE, HYPERTROPHIC CARDIOMYOPATHY
ABSTRACT
Purpose
Hypertrophic cardiomyopathy (HCM) is characterized by inappropriate left ventricular (LV) wall thickness. Adaptations to exercise can occasionally mimic certain HCM characteristics. However, it is unclear whether physical activity affects HCM genotype expression and disease characteristics. Consequently, we compared lifelong physical activity volumes between HCM gene carriers with and without HCM phenotype, and compared disease characteristics among tertiles of physical activity in phenotypic HCM patients.
Methods
We enrolled n = 22 genotype positive/phenotype negative (G+/P−) HCM gene carriers, n = 44 genotype positive/phenotype positive (G+/P+) HCM patients, and n = 36 genotype negative/phenotype positive (G−/P+) HCM patients. Lifelong physical activity was recorded using a questionnaire and quantified as metabolic equivalent of task hours per week.
Results
We included 102 participants (51 ± 16 yr, 49% male). Lifelong physical activity volumes were not different between G+/P+ and G+/P− subjects (16 [10–29] vs 14 [6–26] metabolic equivalent of task‐hours per week, P = 0.33). Among phenotypic HCM patients, there was no difference in LV wall thickness, mass, and late gadolinium enhancement across physical activity tertiles. Patients with the highest reported physical activity volumes were younger at the time of diagnosis (tertile 1: 52 ± 14 yr, tertile 2: 49 ± 15 yr, tertile 3: 41 ± 18 yr; P = 0.03), and more often had a history of nonsustained ventricular tachycardia (4% vs 30% vs 30%, P = 0.03).
Conclusions
Lifelong physical activity volumes are not associated with genotype-to-phenotype transition in HCM gene carriers. We also found no difference in LV wall thickness across physical activity tertiles. However, the most active HCM patients were younger at the time of diagnosis and had a higher arrhythmic burden. These observations warrant further exploration of the role of exercise in HCM disease development.
Hypertrophic cardiomyopathy (HCM) is the most common inheritable cardiomyopathy and is characterized by inappropriate left ventricular (LV) wall thickness. Exercise training is associated with a reduced incidence of cardiovascular morbidity and mortality (1), but can also increase LV wall thickness dependent on dose and type of exercise (2). These adaptations to exercise can occasionally mimic the HCM phenotype (3).
Recent studies suggested that exercise may accelerate the development of cardiac complications in individuals with genetic mutations. For example, humans with a genetic predisposition for arrhythmogenic right ventricular cardiomyopathy (ARVC) (4–6) and a physically active lifestyle had accelerated appearance (younger age at diagnosis) and progression of disease. Animal models of ARVC confirmed that exercise accelerates ARVC phenotype in mutation carriers (7,8). HCM sarcomere mutations increase the energy cost of contraction and impair resting myocardial energetics (9–12), potentially leading to neuroendocrine activation (13) and subsequent hypertrophy and fibrosis (14). During exercise, this energetic impairment is further exacerbated (9) and may consequently accelerate HCM disease progression.
To explore the relationship between physical activity and HCM phenotype expression, we compared physical activity volumes between HCM gene carriers (G+) with a positive (P+) and negative (P−) HCM phenotype (G+/P+ vs G+/P−). Furthermore, we compared disease characteristics across tertiles of lifelong physical activity among phenotypic HCM patients (G+/P+ and G−/P+).
METHODS
Study population
Subjects were participants of a Dutch multicenter cohort study: the Biomarkers, Exercise Stress Testing, and mRi to Obtain New insiGhts in Hypertrophic CardioMyopathy Study (i.e., the BE STRONG HCM study). Enrollment took place between April 2008 and January 2014 in two outpatient clinics (Radboud University Medical Center and Albert Schweitzer Hospital, the Netherlands) that perform mutation screening, repeated echocardiography cardiac magnetic resonance (CMR) imaging, clinical follow-up, and cascade screening. In short, in this study, biomarkers, exercise stress testing, and magnetic resonance imaging (MRI) characteristics were assessed in phenotypic HCM patients and HCM gene carriers (15). Adult HCM patients and gene carriers were invited to participate. HCM patients had to fulfill the diagnostic criteria for HCM according to prevailing guidelines (16,17). Patients with echocardiographic evidence of LV hypertrophy (maximal wall thickness ≥15 or ≥13 mm in case of an identifying gene mutation and/or compelling factors associated with HCM) without another cardiac or systemic cause at the time of HCM diagnosis were eligible for participation. The diagnosis was verified by a careful case-by-case chart review (D.H.F.G., G.E.C.). In case of a discrepancy between the treating physician and the investigators, the opinion of a third independent reviewer was decisive (M.J.M.K.). As part of routine clinical evaluation, all patients underwent a 12-lead electrocardiogram (ECG), 24-h ECG Holter, and echocardiogram. HCM patients with known coronary disease (previous myocardial infarction, >50% stenosis on coronary angiogram, previous percutaneous coronary intervention, previous coronary artery bypass grafting), stroke, aortic valve stenosis, previous septal reduction therapy, or renal impairment (defined as estimated glomerular filtration rate <30 mL·min−1) were not eligible for study participation. Phenotype-negative HCM gene carriers were detected based on screening according to a cascade strategy and thus represented family members of HCM patients. Participants were not necessarily athletes but represented a “normal” HCM cohort from the general population and were thus not detected by an athletic screening program.
For the present explorative post hoc analysis, we enrolled 1) genotype positive/phenotype negative (G+/P−) HCM gene carriers, 2) genotype positive/phenotype positive (G+/P+) HCM patients, and 3) genotype negative/phenotype positive (G−/P+) HCM patients. The study complies with the Declaration of Helsinki, and the protocol was approved by the local ethical committees and conducted accordingly. Participants provided written informed consent.
Study protocol
At the time of participation in the cohort study, symptoms and medical therapy were recorded, and risk factors for cardiovascular disease (18) and sudden cardiac death (SCD) (16) were scored. A blood sample was drawn for assessment of creatinine and cardiac troponin T concentrations. Eligible patients were invited to the hospital for CMR imaging. CMR imaging was performed, as previously prescribed (15), on a 1.5-T cardiac CMR system Philips Achieva (Philips Healthcare, Best, the Netherlands) or Siemens Avanto (Siemens Healthcare, Erlangen, Germany) according to local protocol. All images were acquired with ECG gating and during repeated breath-holds of 10–15 s. For the assessment of LV morphology and function, cine imaging was performed using a steady-state free precession sequence. T1-weighted inversion-recovery imaging was performed to assess late gadolinium enhancement (LGE) 10 min after the administration of 0.2 mmol·kg−1 contrast medium (Dotarem; Guerbet, Gorinchem, the Netherlands).
Physical activity
Participants reported their lifelong physical activity history per decade including type of exercise, years, days per week, weeks per year, duration of the sessions, and the intensity level at which they performed every exercise or sport. The questionnaire (Supplemental Digital Content 1, Lifelong Physical Activity Questionnaire, http://links.lww.com/MSS/B590) that we used to quantify lifelong physical activity volumes was adapted from the concept described by Friedenreich et al. (19). We have previously used a similar questionnaire in the Nijmegen Exercise Study (20), interviewing individuals with cardiovascular diseases, risk factors, and healthy controls. Both sports-related exercise and physical activity during transportation (walking and cycling) were included. We assigned a metabolic equivalent of task (MET) for all activities (21) and calculated the physical activity volume by multiplying the MET score by the reported physical activity volume (session duration × frequency/week) and weeks of practice per year and years of practice. Average lifetime physical activity exposure was calculated in MET-hours per week, as previously described (22). Physical activity intensity was divided into light (<3 MET, not reported), moderate (3–6 MET), and vigorous (>6 MET) intensity (23), based on the MET scores of the specific activities. We also separately calculated physical activity volumes before and after the HCM phenotype diagnosis to look specifically at the effects of physical activity before diagnosis and to compare prediagnosis and postdiagnosis physical activity volumes. Average physical activity between age 12 yr and the age at study participation was termed lifelong physical activity, whereas physical activity between age 12 yr and the age at HCM diagnosis was termed prediagnosis physical activity.
Data analysis
All parameters were visually inspected for normality, checked for kurtosis and skewness, and tested with Shapiro–Wilk normality tests. Continuous variables are presented as means ± SD or medians (interquartile ranges). Statistical analyses were performed with IBM SPSS Statistics 20.0 (IBM Corp, Armonk, NY). Statistical significance was assumed at P < 0.05.
HCM gene carriers
Physical activity volumes were compared between G+/P+ (prediagnosis physical activity) and G+/P− individuals (lifelong physical activity) to assess whether habitual physical activity and exercise training relate to HCM phenotype expression. Comparisons between groups were made with use of the Student’s t-test or Mann–Whitney U-test, whichever appropriate. Dichotomous variables were compared using a χ2 or Fisher exact test, whichever appropriate.
Phenotypic HCM patients
To explore the association between lifelong physical activity and disease characteristics, we compared LV function, LV wall thickness, LV mass, the presence of LGE, troponin levels, age at diagnosis, and 24-h ECG recordings across lifelong physical activity tertiles in phenotypic HCM patients (G+/P+ and G−/P+) using one-way ANOVA and Kruskal–Wallis. Within-subject comparison of physical activity volumes before and after diagnosis was done using a related-samples Wilcoxon signed rank test. Finally, comparisons with regard to lifelong physical activity volumes were made according to prespecified HCM subgroups. Phenotypic HCM patients were divided according to LGE presence and LV thickness (<20 and ≥20 mm).
RESULTS
Study population
For this explorative post hoc analysis, we invited 131 possible participants, of whom 102 (49% male; Fig. 1) completed the questionnaire, including 22 G+/P− HCM gene carriers, 44 G+/P+ HCM patients, and 36 G−/P+ HCM patients. The 29 possible participants not included declined participation (n = 14) or did not complete the lifelong physical activity history questionnaire (n = 15). Subject characteristics were not different between included and excluded individuals (data not shown). Mean age ± SD of the studied population was 51 ± 16 yr. Median volumes [and interquartile range] of lifelong physical activity and sports-related exercise were 14 [8–22] MET·h·wk−1 and 7 [2–14] MET·h·wk−1, respectively. Among 102 genetically tested individuals, 66 had a pathogenic HCM mutation, of which n = 49 (74%) had mutations in the cardiac myosin-binding protein (MYBPC3), n = 13 (20%) in the β-myosin heavy chain (MYH7), and n = 4 (6%) in other genes.
FIGURE 1.
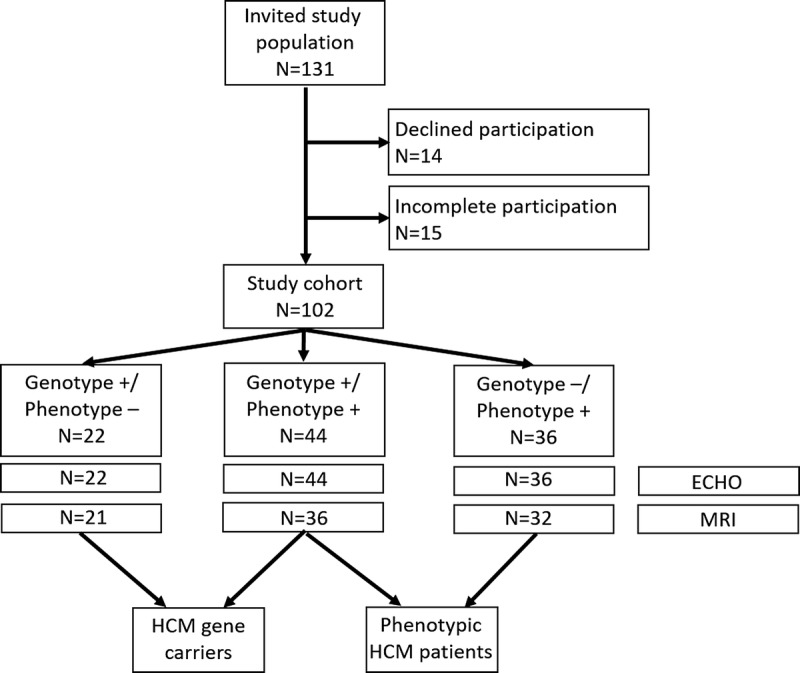
Flowchart of study design. MRI could not be performed in 13 participants because of noncompatible implantable cardiac defibrillators/pacemakers (n = 8), for logistic reasons (n = 3), because of claustrophobia (n = 1), or because of dental prosthesis (n = 1).
HCM gene carriers
Age at diagnosis of G+/P+ individuals (43 ± 15 yr) was similar to age at study participation of G+/P− individuals (40 ± 11 yr, P = 0.37). G+/P+ patients were more often male (52% vs 27%, P = 0.05; Table 1). Echocardiographic parameters, ECG abnormalities, and CMR parameters differed between G+/P+ and G+/P− individuals as expected and according to their disease classification (Table 1).
TABLE 1.
Physical activity and participant characteristics of HCM gene carriers according to phenotype.
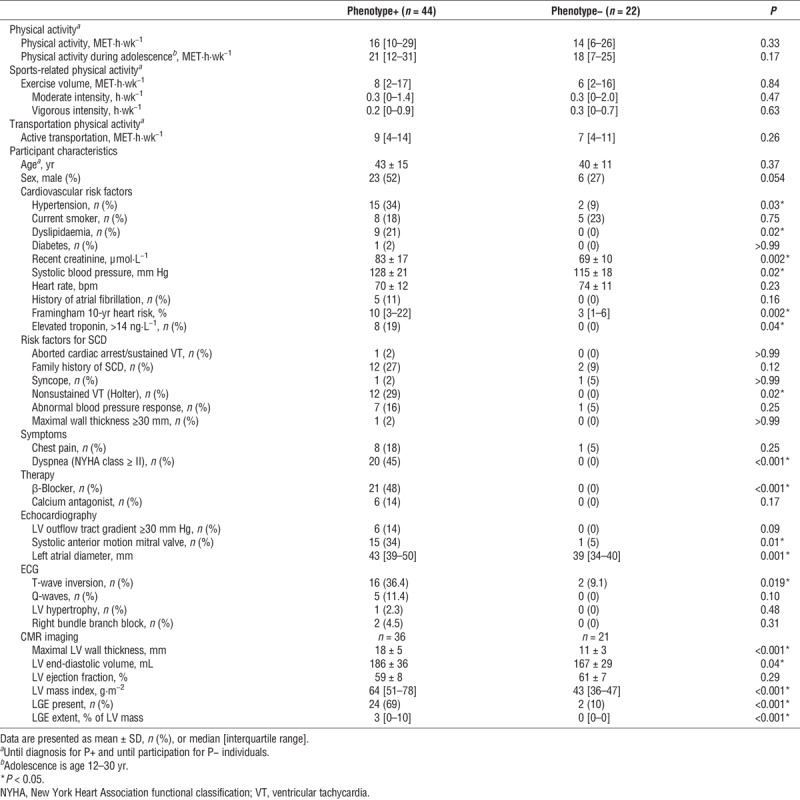
With regard to our study question in HCM gene carriers, there was no difference in physical activity volumes between G+/P+ and G+/P− (16 [10–29] vs 14 [6–26] MET·h·wk−1, P = 0.33; Table 1) or sports-related physical activity volumes (8 [2–17] vs 6 [2–16] MET·h·wk−1, P = 0.84; Table 1). The intensity at which sports were performed did not differ between G+/P+ and G+/P− individuals (Table 1). Distribution of physical activity over time did not differ between G+/P+ and G+/P− individuals (Fig. 2A).
FIGURE 2.
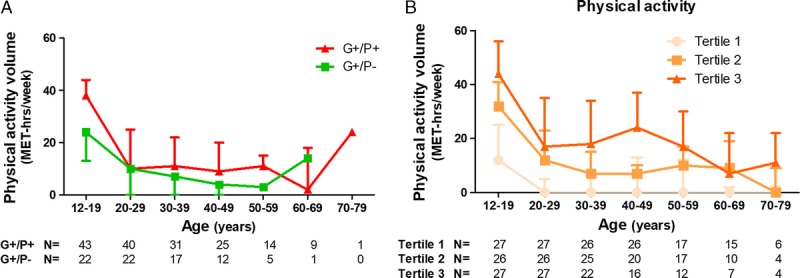
Distribution of physical activity volumes over time of HCM gene carriers according to phenotype status (A) and of phenotypic HCM patients according to tertiles of lifelong physical activity volume (B). Individuals included per decade differ because of the heterogeneity in age of the participants, and numbers are shown below x-axis. Physical activity shown in panel A is prediagnosis physical activity for G+/P+ and lifelong physical activity for G+/P−. Physical activity shown in panel B is lifelong physical activity. Data are shown as median with interquartile range.
Phenotypic HCM patients
G+/P+ HCM patients were younger when diagnosed with HCM and more often had a family history of SCD and LGE on MRI as compared with G−/P+ HCM patients (Table, Supplemental Digital Content 2, Participant characteristics of phenotypic HCM patients according to genotype status, http://links.lww.com/MSS/B591).
The per decade reported physical activity volumes were consistently different across physical activity tertiles as displayed in Figure 2B. Furthermore, physical activity volumes were lower after HCM diagnosis (15 [9–24] MET·h·wk−1 prediagnosis vs 7 [0–19] MET·h·wk−1 postdiagnosis, P < 0.001).
With regard to our study question in phenotypic HCM patients, there was no difference in LV morphology, wall thickness, mass, presence of LGE, and troponin levels across physical activity tertiles (Table 2). The prevalence of T-wave inversion, Q-waves, and LV hypertrophy did not differ on 12-lead ECG recordings across tertiles, whereas the prevalence of bundle branch blocks was lowest in tertile 3. We did observe a difference across tertiles regarding nonsustained ventricular tachycardia (NSVT), with NSVT being significantly more prevalent on 24-h ECG recordings in physically active patients (physical activity: 4% [tertile 1] vs 30% [tertile 2] vs 30% [tertile 3], P = 0.03). We observed a graded decrease in the prevalence of an outflow tract gradient >30 mm Hg across tertiles. The most active patients had a higher prevalence of a positive family history for SCD. Finally, the most active individuals were significantly younger when they received their diagnosis (52 ± 14 vs 49 ± 15 vs 41 ± 18, P = 0.03; Table 2).
TABLE 2.
A comparison of disease characteristics across tertiles of physical activity among phenotypic HCM patients (G+/P+ and G−/P+).
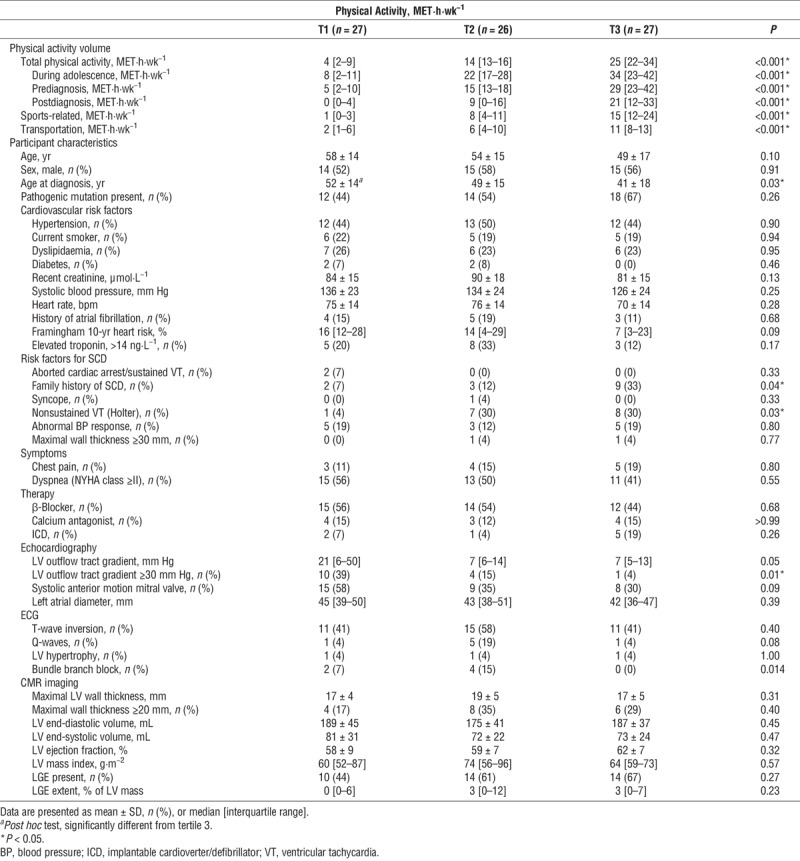
Lastly, lifelong physical activity volumes did not differ between phenotypic HCM patients with LV wall thickness ≥20 and <20 mm (17 [11–23] vs 13 [5–22], P = 0.10), whereas phenotypic HCM patients with LGE had higher lifelong physical activity volumes compared with those without LGE (16 [10–23] vs 11 [5–19] MET·h·wk−1, P = 0.046).
DISCUSSION
We explored the relationship between physical activity and HCM phenotype expression. We found no differences in physical activity characteristics between HCM gene carriers with and without HCM phenotype. We also found no evidence of increased LV wall thickness, LV mass, or elevated cardiac troponin levels among the most active phenotypic HCM patients. However, we did find a younger age at diagnosis and higher prevalence of a history of NSVT in the most active phenotypic HCM patients. Furthermore, lifelong physical activity levels were higher in phenotypic HCM patients with LGE. These findings suggest that lifelong physical activity volumes are not associated with genotype-to-phenotype transition in HCM gene carriers or LV wall thickness in HCM patients. However, given our findings of a younger age at diagnosis and a higher prevalence of ventricular arrhythmias among the most active phenotypic HCM patients, a potential role of physical activity in accelerating disease presentation and severity cannot be ruled out.
Transition from genotype to phenotype
To date, the reasons why gene carriers of sarcomeric mutations show very heterogeneous expression of disease remain ill-defined (24). In the present analysis, average physical activity volumes were comparable among HCM gene carriers with and without HCM phenotype, suggesting that exercise does not increase genotype-to-phenotype transition in genetically susceptible individuals. On the other hand, few of our subjects were athletes and the majority engaged primarily in aerobic physical activities. Hence, the dose and type of physical activity may have been too low to induce detrimental morphological changes in our cohort. Also, aerobic exercise training produces only small increases in LV wall thickness, whereas strength training produces considerably greater increases in LV wall thickness (2).
Consequently, although we did not find a relationship between primarily aerobic physical activity and HCM genotype-to-phenotype transition, we cannot exclude the possibility that more intense aerobic or strength training could provoke onset of the HCM phenotype in genetically susceptible individuals.
Physical activity and disease characteristics
Adaptations to exercise can occasionally mimic HCM LV hypertrophy (3). However, we did not find a difference in LV wall thickness across physical activity tertiles. This is in line with previous studies showing that LV wall thickness did not change in HCM patients after 16 wk of moderate-intensity exercise training (25) and was not different between phenotypic HCM athletes and nonathletes (26).
Higher physical activity volumes were related to a younger age at diagnosis, which could indicate that physical activity produced symptoms that prompted the diagnosis or that physical activity accelerated disease progression. Similar observations were reported in exercising individuals with ARVC (4,6), in which exercise is known to enhance disease severity.
We also found increased NSVT prevalence in the most active phenotypic HCM patients, which could indicate that activity might adversely impact disease progression. Exercise might lead to a temporary supply-demand mismatch due to the inappropriate LV hypertrophy and might subsequently induce myocardial ischemia and development of myocardial fibrosis. The observation that phenotypic HCM patients with LGE had higher lifelong physical activity volumes compared with those without LGE supports the hypothesis that high volumes of exercise may be harmful to some HCM patients. Alternatively, more activity may increase the frequency of NSVT without altering the underlying disease. Exercise-induced ischemia may provide the trigger for potentially life-threatening arrhythmias, as has been suggested by the observation that HCM is a common cause of SCD among athletes during sports participation (27). Future studies including larger populations of HCM patients are needed to better define the association between exercise characteristics and disease severity.
Individuals in the more active tertiles less often had LV outflow tract pressure gradients ≥30 mm Hg. It is possible that subjects with lower LV outflow tract gradients were better able to perform higher volumes of exercise. Alternatively, a physically active lifestyle could improve LV outflow tract pressure gradients among phenotypic HCM patients because 2 and 6 months of exercise training reduced markers of HCM disease severity (i.e., hypertrophy, fibrosis, myocyte disarray) in a murine HCM model (28). However, these explanations do not align with a landmark study showing an increase in LV outflow tract pressure gradients among HCM patients completing 16 wk of moderate-intensity exercise training (25). Future studies should further explore the effects of controlled exercise training on HCM disease development in humans.
Clinical implications
In the general population, exercise drastically reduces the risk of cardiovascular diseases and events (1). Individuals with HCM are often advised not to participate in most competitive sports, in line with current guidelines (29). A recent study randomized individuals with HCM to an exercise (moderate-intensity exercise training) or usual activity group for 16 wk (25). HCM patients in the exercise group successfully increased their V̇O2 peak (from 21.3 to 22.7 mL·min−1·kg−1), which aligns with a previous observation that patients with HCM improved their exercise test time and New York Heart Association functional class with cardiac rehabilitation (30). However, 32% of the HCM patients in the exercise group versus 23% of the HCM patients in the usual activity group experienced NSVT during the study protocol (25). Although exercise training was not associated with an increased occurrence of arrhythmias (no P value given), this trend is in line with our finding of increased NSVT in the most active individuals.
Short-term intervention studies primarily demonstrated beneficial exercise-induced adaptations in HCM patients, but we found a younger age at diagnosis, a higher prevalence of NSVT, and higher lifelong physical activity volumes in individuals with LGE, which questions the long-term health effects of exercise training in this patient population. A recent study found that vigorous exercise was not related to pathological LV hypertrophy and ventricular arrhythmias in HCM (26). However, athletic HCM patients were distinctly younger (51 ± 14 yr vs 57 ± 13 yr, P = 0.01) and more frequently demonstrated LGE (73% vs 53%, P = 0.09) compared with nonathlete HCM patients, which is consistent with our findings. Italian athletes with HCM had a similar risk of symptoms and events during 9 ± 6-yr follow-up whether they continued performing exercise or interrupted their exercise training (31). However, it is unclear how these groups differed based on baseline characteristics, risk due to disease severity and follow-up time.
In conclusion, although exercise is a potent medicine for the general population, it remains unclear whether individuals with HCM may miss the health benefits of a physically active lifestyle due to exercise restriction policies in current guidelines or whether they are protected from potential detrimental effects of exercise training on disease progression.
Future studies should focus on long-term outcomes for individuals with HCM and physical activity characteristics, with specific attention for a potential earlier onset of disease, and increase in LGE and arrhythmic burden. For example, the Lifestyle and Exercise in HCM study is an ongoing prospective observational study (32), enrolling patients with HCM across a range of activity levels, which is expected to provide novel findings in the near future.
Limitations
This study is observational and retrospective, making it impossible to separate the cause versus effect relationships. However, it is extremely difficult, if not impossible, to randomize HCM gene carriers to exercise training or a control condition to study the long-term effects of physical activity on HCM progression and severity. Our study may also suffer from selection bias due to our study design and inclusion. Furthermore, our results depended on subjects recalling their lifelong physical activity volumes, but potential bias should have affected all participants similarly. Finally, our study (and other studies on this topic (26,31)) suffers from a limited sample size but adds valuable information to the currently limited data available on the association between exercise and HCM.
CONCLUSIONS
We found no association between lifelong physical activity volumes and HCM phenotype among HCM gene carriers. We also found no difference in LV wall thickness across physical activity tertiles. These findings suggest that lifelong physical activity volumes are not associated with genotype-to-phenotype transition in HCM gene carriers or LV wall thickness in HCM patients. However, the most active HCM patients were younger when diagnosed and more often had a history of NSVT, and HCM patients with LGE had higher physical activity volumes. These observations warrant further exploration of the role of exercise in HCM disease development.
Supplementary Material
Acknowledgments
V. L. A. is financially supported by a grant from the Radboud Institute for Health Sciences. T. M. H. E. is supported by a Horizon 2020 grant from the European Commission (Marie Sklodowska-Curie Fellowship 655502).
The authors have no relationships or conflicts to disclose. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
G. E. C. and T. M. H. E. are equal author contributions.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
REFERENCES
- 1.Eijsvogels TM, Molossi S, Lee DC, Emery MS, Thompson PD. Exercise at the extremes: the amount of exercise to reduce cardiovascular events. J Am Coll Cardiol. 2016;67(3):316–29. [DOI] [PubMed] [Google Scholar]
- 2.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101(3):336–44. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, Merghani A, Mont L. Exercise and the heart: the good, the bad, and the ugly. Eur Heart J. 2015;36(23):1445–53. [DOI] [PubMed] [Google Scholar]
- 4.James CA, Bhonsale A, Tichnell C, et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62(14):1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzanti A, Ng K, Faragli A, et al. Arrhythmogenic right ventricular cardiomyopathy: clinical course and predictors of arrhythmic risk. J Am Coll Cardiol. 2016;68(23):2540–50. [DOI] [PubMed] [Google Scholar]
- 6.Ruwald AC, Marcus F, Estes NA, 3rd, et al. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2015;36(27):1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz FM, Sanz-Rosa D, Roche-Molina M, et al. Exercise triggers ARVC phenotype in mice expressing a disease-causing mutated version of human plakophilin-2. J Am Coll Cardiol. 2015;65(14):1438–50. [DOI] [PubMed] [Google Scholar]
- 8.Martherus R, Jain R, Takagi K, et al. Accelerated cardiac remodeling in desmoplakin transgenic mice in response to endurance exercise is associated with perturbed Wnt/β-catenin signaling. Am J Physiol Heart Circ Physiol. 2016;310(2):H174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dass S, Cochlin LE, Suttie JJ, et al. Exacerbation of cardiac energetic impairment during exercise in hypertrophic cardiomyopathy: a potential mechanism for diastolic dysfunction. Eur Heart J. 2015;36(24):1547–54. [DOI] [PubMed] [Google Scholar]
- 10.Crilley JG, Boehm EA, Blair E, et al. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol. 2003;41(10):1776–82. [DOI] [PubMed] [Google Scholar]
- 11.Güçlü A, Knaapen P, Harms HJ, et al. Disease stage–dependent changes in cardiac contractile performance and oxygen utilization underlie reduced myocardial efficiency in human inherited hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2017;10(5):e005604. [DOI] [PubMed] [Google Scholar]
- 12.Witjas-Paalberends ER, Guclu A, Germans T, et al. Gene-specific increase in the energetic cost of contraction in hypertrophic cardiomyopathy caused by thick filament mutations. Cardiovasc Res. 2014;103(2):248–57. [DOI] [PubMed] [Google Scholar]
- 13.Huke S, Knollmann BC. Familial hypertrophic cardiomyopathy: is the Frank–Starling law kaput? Circ Res. 2013;112(11):1409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito A, De Cobelli F, Perseghin G, et al. Impaired left ventricular energy metabolism in patients with hypertrophic cardiomyopathy is related to the extension of fibrosis at delayed gadolinium-enhanced magnetic resonance imaging. Heart. 2009;95(3):228–33. [DOI] [PubMed] [Google Scholar]
- 15.Gommans DF, Cramer GE, Bakker J, et al. High T2-weighted signal intensity is associated with elevated troponin T in hypertrophic cardiomyopathy. Heart. 2017;103(4):293–9. [DOI] [PubMed] [Google Scholar]
- 16.Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42(9):1687–713. [DOI] [PubMed] [Google Scholar]
- 17.Gersh BJ, Maron BJ, Bonow RO, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124(24):e783–831. [DOI] [PubMed] [Google Scholar]
- 18.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 19.Friedenreich CM, Courneya KS, Bryant HE. The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc. 1998;30(2):266–74. [DOI] [PubMed] [Google Scholar]
- 20.Maessen MF, Verbeek AL, Bakker EA, Thompson PD, Hopman MT, Eijsvogels TM. Lifelong exercise patterns and cardiovascular health. Mayo Clin Proc. 2016;91(6):745–54. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81. [DOI] [PubMed] [Google Scholar]
- 22.Aengevaeren VL, Mosterd A, Braber TL, et al. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation. 2017;136(2):138–48. [DOI] [PubMed] [Google Scholar]
- 23.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–93. [DOI] [PubMed] [Google Scholar]
- 24.Finocchiaro G, Magavern E, Sinagra G, et al. Impact of demographic features, lifestyle, and comorbidities on the clinical expression of hypertrophic cardiomyopathy. J Am Heart Assoc. 2017;6(12):e007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saberi S, Wheeler M, Bragg-Gresham J, et al. Effect of moderate-intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy: a randomized clinical trial. JAMA. 2017;317(13):1349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dejgaard LA, Haland TF, Lie OH, et al. Vigorous exercise in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2018;250:157–63. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Malhotra R, Chiampas G, et al. Cardiac arrest during long-distance running races. N Engl J Med. 2012;366(2):130–40. [DOI] [PubMed] [Google Scholar]
- 28.Konhilas JP, Watson PA, Maass A, et al. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res. 2006;98(4):540–8. [DOI] [PubMed] [Google Scholar]
- 29.Maron BJ, Udelson JE, Bonow RO, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a Scientific Statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132(22):e273–80. [DOI] [PubMed] [Google Scholar]
- 30.Klempfner R, Kamerman T, Schwammenthal E, et al. Efficacy of exercise training in symptomatic patients with hypertrophic cardiomyopathy: results of a structured exercise training program in a cardiac rehabilitation center. Eur J Prev Cardiol. 2015;22(1):13–9. [DOI] [PubMed] [Google Scholar]
- 31.Pelliccia A, Lemme E, Maestrini V, et al. Does sport participation worsen the clinical course of hypertrophic cardiomyopathy? Clinical outcome of hypertrophic cardiomyopathy in athletes. Circulation. 2018;137(5):531–3. [DOI] [PubMed] [Google Scholar]
- 32.Saberi S, Day SM. Exercise and hypertrophic cardiomyopathy: time for a change of heart. Circulation. 2018;137(5):419–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


