Supplemental digital content is available in the text.
Key Words: V˙O2max, ENDURANCE EXERCISE, ENDURANCE PERFORMANCE, MUSCULAR ENDURANCE, STRENGTH, PROTEIN INTAKE
ABSTRACT
Introduction
Recently, it has been speculated that protein supplementation may further augment the adaptations to chronic endurance exercise training. We assessed the effect of protein supplementation during chronic endurance exercise training on whole-body oxidative capacity (V˙O2max) and endurance exercise performance.
Methods
In this double-blind, randomized, parallel placebo-controlled trial, 60 recreationally active males (age, 27 ± 6 yr; body mass index, 23.8 ± 2.6 kg·m−2; V˙O2max, 47 ± 6 mL·min−1·kg−1) were subjected to 12 wk of triweekly endurance exercise training. After each session and each night before sleep, participants ingested either a protein supplement (PRO; 28.7 g casein protein) or an isoenergetic carbohydrate placebo (PLA). Before and after the 12 wk of training, V˙O2max and endurance exercise performance (~10-km time trial) were assessed on a cycle ergometer. Muscular endurance (total workload achieved during 30 reciprocal isokinetic contractions) was assessed by isokinetic dynamometry and body composition by dual-energy x-ray absorptiometry. Mixed-model ANOVA was applied to assess whether training adaptations differed between groups.
Results
Endurance exercise training induced an 11% ± 6% increase in V˙O2max (time effect, P < 0.0001), with no differences between groups (PRO, 48 ± 6 to 53 ± 7 mL·min−1·kg−1; PLA, 46 ± 5 to 51 ± 6 mL·min−1·kg−1; time–treatment interaction, P = 0.50). Time to complete the time trial was reduced by 14% ± 7% (time effect, P < 0.0001), with no differences between groups (time–treatment interaction, P = 0.15). Muscular endurance increased by 6% ± 7% (time effect, P < 0.0001), with no differences between groups (time–treatment interaction, P = 0.84). Leg lean mass showed an increase after training (P < 0.0001), which tended to be greater in PRO compared with PLA (0.5 ± 0.7 vs 0.2 ± 0.6 kg, respectively; time–treatment interaction, P = 0.073).
Conclusion
Protein supplementation after exercise and before sleep does not further augment the gains in whole-body oxidative capacity and endurance exercise performance after chronic endurance exercise training in recreationally active, healthy young males.
Maximum oxygen uptake (V˙O2max) can be defined as the highest rate at which oxygen can be taken up and used by the body during strenuous exercise (1). As the V˙O2max is fundamental to endurance exercise performance, many athletes aim to improve their V˙O2max by endurance exercise training. Indeed, continuous endurance exercise and interval training have been shown effective in improving V˙O2max and endurance performance (2).
Along with endurance exercise training, nutrition plays an important role with regard to endurance exercise performance. Carbohydrate ingestion and endogenous carbohydrate storage have been linked to endurance exercise performance, as carbohydrates are the main fuel source during moderate- to high-intensity endurance exercise (3). Over the past few years, there has been an increasing interest in the role of protein ingestion during and after endurance exercise to support physiological training adaptations (4,5). The proposed increased protein requirements induced by endurance exercise have been attributed to multiple factors, such as increased rates of protein oxidation during (6) and after (7) endurance exercise, and hematological, vascular, and skeletal muscle adaptations induced by endurance exercise training (5). Furthermore, acute protein supplementation has been shown to augment the muscle protein synthetic response to endurance exercise (8–12). Taken together, it can be speculated that chronic protein supplementation enhances recovery and facilitates the adaptive response to endurance exercise training. Although the importance of dietary protein ingestion in relation to muscle mass and strength after chronic resistance exercise training has been well established (13), the role of dietary protein in relation to chronic endurance exercise training remains to be elucidated. To the best of our knowledge, only two studies have investigated the effects of protein supplementation on endurance exercise-induced training adaptations. Robinson et al. (14) reported that healthy older adults achieved greater improvements in V˙O2max after 6 wk of endurance exercise training when protein was supplemented. In line with these findings, Ferguson-Stegall et al. (15) reported that healthy young subjects attained greater improvements in V˙O2max after postexercise supplementation with chocolate milk (carbohydrate–protein mixture) as opposed to placebo during 4.5 wk of endurance exercise training. Although these results suggest that protein supplementation may augment the adaptive response to chronic endurance exercise training, the aforementioned studies were conducted in small cohorts, and performance measures were not included. Clearly, more research is warranted to establish the proposed benefits of protein supplementation for the adaptive response to chronic endurance exercise training.
In the current study, we aimed to assess the effect of protein supplementation on the increase in V˙O2max and endurance exercise performance induced by chronic endurance exercise training. For this purpose, 60 healthy young males were subjected to 12 wk of endurance exercise training, comprising three exercise sessions weekly. After each training session and each night before sleep, participants ingested either a protein supplement (28.7 g casein protein) or an isoenergetic carbohydrate placebo. We hypothesized that supplementation with protein as opposed to a placebo leads to greater improvements in V˙O2max and endurance exercise performance after 12 wk of endurance exercise training.
METHODS
Study Design and Setting
We conducted a 12-wk double-blind, randomized, placebo-controlled trial with a parallel group design. Participants were allocated randomly to either a protein-supplemented exercise intervention group (PRO) or a placebo-supplemented exercise intervention group (PLA). A schematic overview of participant flow is presented as a supplemental figure (Supplemental Digital Content 1, CONSORT Flow Diagram, http://links.lww.com/MSS/B614). The exercise intervention and testing procedures took place at the sport and research center of the HAN University of Applied Sciences in Nijmegen, the Netherlands, between September 2017 and June 2018. The study was approved by the Independent Review Board Nijmegen Medical Ethical Committee, the Netherlands, and conformed to the standards for the use of human participants as outlined in the most recent version of the Declaration of Helsinki. The study was registered at the Netherlands Trial Registry (www.trialregister.nl) as NTR6691.
Participants
Sixty eligible young (18–40 yr), nonobese (body mass index <30 kg·m−2), healthy males were recruited through advertising in local newspapers and social media. Exclusion criteria were participation in structural endurance exercise, performing other structured exercise for more than 6 h·wk−1 and/or a V˙O2max >55 mL·kg−1⋅min−1. After initial screening by telephone, a screening visit was planned. All participants were informed of the nature and possible risks of the experimental procedures before their written informed consent was obtained. Subsequently, medical history was checked and V˙O2max was assessed to verify eligibility.
Randomization and Blinding
Participants were allocated randomly in a 1:1 ratio to either the protein group or the placebo group. A computer-generated randomization list was made by an independent researcher and shared with the supplement manufacturer. Allocation concealment was ensured by the manufacturer of the supplements, who labeled all protein and placebo supplements according to participant number. The researchers responsible for screening allocated each eligible participant to the next available number on entry into the trial. Block randomization was used with block sizes of 30, as the training program was commenced with two consecutive cohorts of 30 participants (cohort 1, September–December 2017; cohort 2, March–June 2018). All study personnel and participants were blinded to treatment assignment for the duration of the study. The randomization list was revealed to the researchers once recruitment, data collection, and data entry were completed and checked, and the data set was locked. Participants’ blinding was confirmed through an exit survey. In this regard, 62% guessed the intervention correctly, whereas 38% guessed the intervention incorrectly.
Intervention
Exercise program
During a 12-wk period, all participants performed three supervised exercise sessions weekly (Mondays, Wednesdays, and Fridays) on a cycle ergometer (Matrix U3X, Matrix Fitness, WI), on a self-selected time between 0800 and 2000 h. Each Monday, participants performed 6 × 4-min work intervals at a target intensity of 85%–90% HRmax interspersed with 4-min active recovery periods. Each Wednesday, participants performed continuous endurance exercise sessions of 60 min at a target intensity of 75%–80% HRmax. Each Friday, participants performed three sets of 6 × 1-min work intervals at a target intensity of 90%–95% HRmax interspersed with 1-min active recovery periods and 5 min of active rest between sets. During the first 4 wk of training, the exercise volume was gradually increased to the full exercise training program described above. All exercise sessions included a 5-min warm-up and cooldown. During all training sessions, verbal encouragement was given by personal trainers, and the heart rate was monitored continuously (Zephyr™ Performance Systems, Medtronic, MD) to verify the prescribed exercise intensity. Training progression was monitored by the registration of the selected workload (W) during each exercise session.
Supplementation
Throughout the 12-wk intervention period, participants consumed 250 mL of either a protein supplement or an isoenergetic carbohydrate placebo immediately after each exercise session and each night before sleep. The protein supplement contained 538 kJ (129 kcal), 28.7 g protein (casein protein), 0.3 g fat, and 2.6 g carbohydrate. The isoenergetic placebo supplement contained 541 kJ (129 kcal), 0.6 g protein, 2.4 g fat, and 25.8 g carbohydrate. The products were provided in opaque white plastic bottles and could not be discerned by taste (vanilla flavored), smell, texture, or color. All products were composed by FrieslandCampina (Amersfoort, the Netherlands) and produced at NIZO (Ede, The Netherlands). Participants received their postexercise supplements from the researchers immediately after each training session, whereas the presleep supplements were distributed in cardboard boxes every 2 wk to take home. Participants were instructed to ingest the presleep supplements each evening within 15 min before bedtime. A supplement log was submitted to the researchers every 2 wk to verify compliance.
In addition to the PRO or PLA supplement, all participants received a carbohydrate-rich snack after each exercise session (439–678 kJ [105–162 kcal], 27.0–36.3 g carbohydrates, 1.3–1.7 g protein, and 0.4–0.9 g fat) to comply with the nutritional recommendations for endurance exercise and to (partly) compensate for the energy deficit induced by the exercise session.
Study Procedures
Before assessing baseline data, participants visited the laboratory twice to become familiarized with the exercise testing procedures (V˙O2max, time trial, and muscle function tests). Two weeks before commencing the exercise training program, baseline data were collected during two visits (pretests 1 and 2) separated by at least 48 h. During pretest 1, participants reported to the laboratory in an overnight fasted state. After measuring blood pressure and venous blood sampling, body composition was assessed by dual-energy x-ray absorptiometry. Participants were then provided breakfast, and at least 30 min later, they performed the V˙O2max test. During pretest 2, participants first performed a 10-km time trial. After a 30-min break, muscle function was assessed by isokinetic dynamometry. The measurements of pretest 1 were repeated 3 to 7 d after completing the last exercise session of the 12-wk intervention program (posttest 1), whereas the measurements of pretest 2 were repeated 10–12 d after completing the last exercise session (posttest 2). All testing procedures were conducted on the same time of the day and under the same standardized conditions (i.e., no exercise or alcohol 48 h before and no caffeine 12 h before testing). In addition, measurements of dietary intake and habitual physical activity were assessed at baseline, week 6, and week 12 of the exercise intervention program.
Outcomes
V˙O2max
Participants’ maximal oxygen consumption (V˙O2max), power output (Wmax), and heart rate (HRmax) were determined while performing an incremental cycling test to exhaustion on an electromagnetically braked cycle ergometer (Lode Excalibur Sport, Groningen, the Netherlands). Oxygen consumption and carbon dioxide production were assessed by an automated spiroergometry system with breath-by-breath gas collection technology (Cortex, Metalyser 3B, Leipzig, Germany). The automated spiroergometry system was calibrated to ambient air before each test. A two-point gas calibration and volume calibration was performed daily and after three consecutive tests. After an initial warm-up of 5 min at 75 W, workload was increased progressively with 25 W·min−1 until the participant reached volitional exhaustion. V˙O2max was defined as the mean of the highest consecutive V˙O2 values over 30 s and verified by using the V˙O2max attainment criteria, including the detection of a V˙O2 plateau, the lack of increase in heart rate with continued increase in workload, and an RER >1.10 (16). Wmax was defined as the power reached at exhaustion. HRmax was defined as the highest heart rate achieved during the test.
Time trial
Participants performed a simulated ~10-km cycling time trial on an electromagnetically braked cycle ergometer (Lode Excalibur Sport) to assess endurance performance. The amount of work to be performed was calculated as follows: total amount of work (J) = 0.85 Wmax × 900 (s) (17). The ergometer was set in linear mode in which 85% Wmax was achieved when participants cycled at their preferred pedaling rate as determined during the familiarization sessions. Participants received no verbal encouragement or visible information on elapsed time and pedaling rate throughout the time trial, but they were aware of the absolute (kJ) and relative (%) amount of work completed. The absolute work target (kJ) and linear-mode settings were kept similar between pre- and posttesting to allow for comparison of time to completion.
Muscle function
Isometric testing and isokinetic testing of the upper right leg were performed on a dynamometer (Humac Norm Isokinetic Extremity System; CSMI, Stoughton, MA). Participants were placed in an upright seated position with the back chair seat set to an angle of 85° and fastened to the seat and lever arm of the dynamometer by torso, thigh, and shin straps to reduce contribution of any irrelevant body movement. The dynamometer was adjusted so that the femoral epicondyle was in line with the axis of rotation of the lever arm. Three maximal voluntary isometric knee extensions were performed for a duration of 4 s at a knee angle of 60° with 1-min rest intervals between successive attempts. Subsequently, maximal voluntary isokinetic knee extension and flexion were determined at angular velocities of 90°·s−1 and 180°·s−1. At both velocities, five reciprocal contractions were performed. For each maximal strength test, the single best contraction was used for analyses. Finally, isokinetic muscular endurance was determined by 30 reciprocal contractions at an angular velocity of 180°·s−1. Participants were instructed and verbally encouraged to execute each contraction with maximal force and were given verbal feedback on the number of repetitions. Between each different test, participants rested for 90 s. All isokinetic tests were performed over an 85° range of motion with the knee fully extended being 0°. Analyses were performed over a 10°–75° range of motion to dismiss any end-range deceleration. Data were generated using the Humac Norm system’s software package and MATLAB (Mathworks, Natick, MA).
Body composition
Participants’ body mass and height were determined using a digital scale and a mobile stadiometer, respectively (Seca 770 and Seca 213i, Hamburg, Germany). Whole-body and regional body composition were measured by dual-energy x-ray absorptiometry (Horizon W, Hologic, MA) and determined using the system’s software package (Apex version 5.6.0.5) using the classic calibration algorithm.
Dietary intake and physical activity
Habitual dietary intake was assessed during 3 d at baseline, week 6, and week 12 using a web-based 24-h recall system (Compl-eat; Wageningen University, Wageningen, The Netherlands), as described previously (18). Each 3-d period comprised two weekdays (training and rest day) and one weekend day. The recall days were randomly selected and not announced to the participants. Physical activity was assessed during seven successive days at baseline, week 6, and week 12 using a wrist-worn physical activity monitor (GT9X Link; ActiGraph, Pensacola, FL). The physical activity monitor was worn on the nondominant wrist and measured physical activity for 24 h·d−1 during the 7-d measuring period. The sampling frequency was 30 Hz, and data were stored in 10-s epochs. Physical activity data were used to assess the percentage of time spent sedentary or at light, moderate, and vigorous physical activity according to the classification of Freedson et al. (19).
Sample Size and Statistical Analysis
Sample size was calculated using GPower version 3.1.9.2. (20). On the basis of the effect of protein supplementation on improvements in V˙O2max reported by Ferguson-Stegall et al. (15), we assumed an increase in V˙O2max of 5% in the control group and 10% in the protein-supplemented group. With 0.8 power to detect a significant difference (P < 0.05, two-sided), 25 participants were required for both groups. Taking a potential dropout of 15% into account, we planned to enroll 30 participants per group.
Before hypothesis testing, data were examined for normality. Nonnormally distributed variables were logarithmically transformed before analysis. Baseline characteristics were compared by independent sample t-tests. The primary analyses were conducted on participants who completed the study per protocol. The effect of protein supplementation on training adaptations was assessed by mixed-model design ANOVA with time (pre- vs posttraining) as a within-subject factor and treatment (PRO vs PLA) as a between-subject factor. Statistical significance was set at P < 0.05. Data are presented as mean ± SD and analyzed using SPSS 22.0 (IBM Corp., Armonk, NY). Performance and body composition data were also examined by calculation of Cohen’s d effect size (95% confidence interval).
RESULTS
Participants
A total of 60 participants were randomized to the 12-wk exercise training intervention with supplementation of either placebo (n = 30) or protein (n = 30; Supplemental Digital Content 1, CONSORT Flow Diagram, http://links.lww.com/MSS/B614). Four participants in the placebo group withdrew because of injuries or personal reasons. At baseline, there were no significant differences between the PLA and the PRO groups in participants’ characteristics (Table 1), training workload (Table 2), muscular strength (Table 3), and dietary intake (Table 4).
TABLE 1.
Participants’ characteristics before and after 12 wk of endurance exercise training in healthy young males receiving either placebo or protein supplementation.

TABLE 2.
Selected workload during the interval exercise sessions (Monday, Friday) and continuous endurance exercise sessions (Wednesday) during 12 wk of endurance exercise training in healthy young males receiving either placebo or protein supplementation.
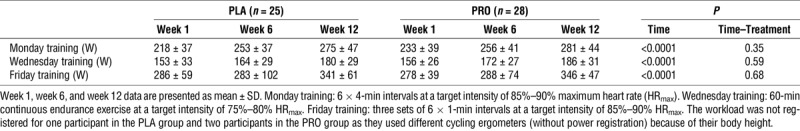
TABLE 3.
Muscular strength before and after 12 wk of endurance exercise training in healthy young males receiving either protein or placebo supplementation.
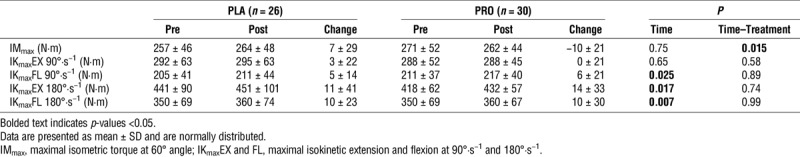
TABLE 4.
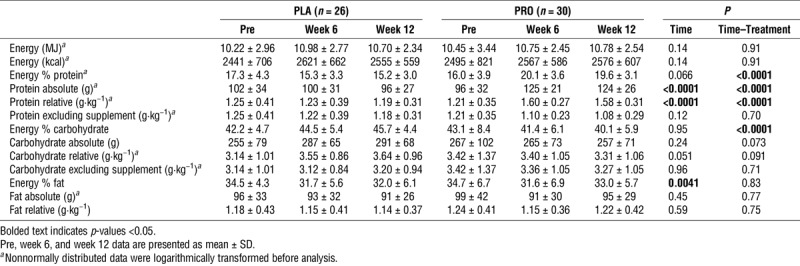
Nutritional intake during 12 wk of endurance exercise training in healthy young males receiving either placebo or protein supplementation.
Compliance
With 97% ± 4% and 96% ± 3%, training attendance was comparable between PLA and PRO (P = 0.77). Both groups achieved comparable progression in selected workload over the 12-wk intervention period (time–treatment interaction, P > 0.35; Table 2). Compliance with the nutritional supplement was 98% ± 2% and 97% ± 2% for PLA and PRO, respectively (P = 0.17).
Whole-body oxidative capacity and performance
The intervention induced an 11% increase in V˙O2max (time effect, P < 0.0001), with no differences between treatments (PLA, 46 ± 5 to 51 ± 6 mL·min−1·kg−1; PRO, 48 ± 6 to 53 ± 7 mL·min−1·kg−1; time–treatment interaction, P = 0.50; Fig. 1). In line, Wmax during the incremental cycling test increased by 11% (time effect, P < 0.0001), with no differences between treatments (PLA, 4.09 ± 0.50 to 4.51 ± 0.56 W·kg−1; PRO, 4.25 ± 0.56 to 4.75 ± 0.64 W·kg−1; time–treatment interaction, P = 0.22). HRmax did not change over time (P = 0.94), nor did it differ between treatments (PLA, 190 ± 11 to 190 ± 10 bpm; PRO, 191 ± 8 to 191 ± 7 bpm; time–treatment interaction, P = 0.94). Although RERmax significantly decreased over time (P = 0.010), no differences were observed between treatments (PLA, 1.18 ± 0.06 to 1.16 ± 0.04; PRO, 1.19 ± 0.05 to 1.17 ± 0.05; time–treatment interaction, P = 0.92).
FIGURE 1.
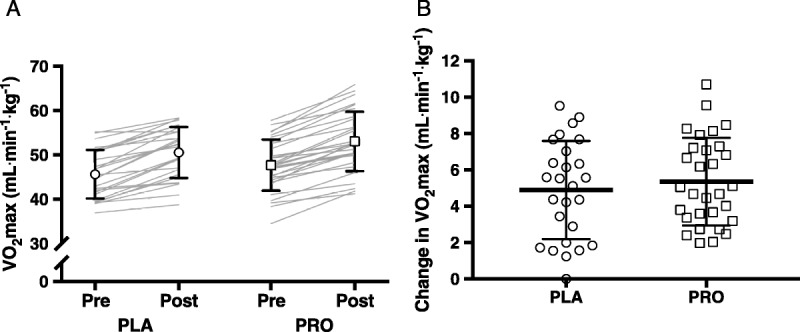
V˙O2max in the PLA (n = 26) and PRO (n = 30) groups. A, V˙O2max values before and after the 12-wk intervention period. The open symbols with error bars represent mean ± SD, and the gray lines represent individual cases. B, Changes in V˙O2max over the 12-wk intervention period. The open symbols represent individual cases, and the black lines represent mean ± SD. Mixed-model design ANOVA indicated an increase in V˙O2max (time effect, P < 0.0001), with no differences between groups (time–treatment interaction, P = 0.50).
The 12-wk intervention successfully improved time trial performance, as evidenced by a 14% reduction in time to completion (time effect, P < 0.0001). No differences were observed between treatments (PLA, 1045 ± 68 to 913 ± 60 s; PRO, 1070 ± 95 to 904 ± 67 s; time–treatment interaction, P = 0.15; Fig. 2A). In line, average power output throughout the time trial increased by 16% (time effect, P < 0.0001) with no differences between treatments (PLA, 3.02 ± 0.47 to 3.47 ± 0.49 W·kg−1; PRO, 3.06 ± 0.49 to 3.61 ± 0.53 W·kg−1; time–treatment interaction, P = 0.28).
FIGURE 2.

Time trial performance, muscular endurance, and leg LM in the PLA (n = 26) and PRO (n = 30) groups. The open symbols represent individual cases and the black lines represent mean ± SD. A, Changes in time trial performance over the 12-wk intervention period in the PLA (n = 26) and PRO (n = 30) groups. Mixed-model design ANOVA indicated a significant reduction in time to complete the time trial (time effect, P < 0.0001), with no differences between groups (time–treatment interaction, P = 0.22). B, Changes in muscular endurance over the 12-wk intervention period in the PLA (n = 26) and PRO (n = 30) groups. Mixed-model design ANOVA indicated a significant increase in work completed during 30 reciprocal contractions (time effect, P < 0.0001), with no differences between groups (time–treatment interaction, P = 0.84). C, Changes in leg LM over the 12-wk intervention period in the PLA (n = 26) and PRO (n = 30) groups. Mixed-model design ANOVA indicated a significant increase in leg LM (time effect, P < 0.0001), which tended to be greater in the PRO group (time–treatment interaction, P = 0.073).
Estimated mean differences between interventions and effect sizes for V˙O2max and time trial performance are presented as a supplemental table (Supplemental Digital Content 2, Estimated Mean Differences and Effect Sizes for Performance Outcomes and Body Mass, http://links.lww.com/MSS/B615).
Muscular strength and endurance
The effects of the 12-wk intervention on muscular strength are presented in Table 3. A significant time–treatment interaction (P = 0.015) was detected for maximal isometric torque, which can be explained by marginal increase over time in PLA group (7 ± 29 N·m) along with a marginal decrease in the PRO group (−10 ± 21 N·m). Maximal voluntary isokinetic contraction of the knee extensors showed an increase over time at a velocity of 180°·s−1 (P = 0.017), but not at a velocity of 90°·s−1 (P = 0.65), without any differences between treatment (time–treatment interactions, P > 0.58 for all).
Muscular endurance, defined as the total work performed during 30 reciprocal isokinetic contractions, increased by 6% (time effect, P < 0.0001), with no difference between treatments (PLA, 3268 ± 609 to 3438 ± 666 kJ; PRO, 3203 ± 451 to 3384 ± 462 kJ; time–treatment interaction, P = 0.84; Fig. 2B).
Estimated mean differences between interventions and effect sizes for muscular strength and endurance are presented (Table, Supplemental Digital Content 2, Estimated Mean Differences and Effect Sizes for Performance Outcomes and Body Mass, http://links.lww.com/MSS/B615).
Body composition
Body composition data are presented in Table 1. Although whole-body lean mass (LM) did not change over time (P = 0.097), a significant increase in leg LM was observed (time effect, P < 0.0001; Fig. 2C). The increase in leg LM in the PRO group (0.5 ± 0.7 kg) tended to be greater when compared with the PLA group (0.2 ± 0.6 kg), although this finding did not reach statistical significance (time–treatment interaction, P = 0.073). In line, the increase in leg LM showed a small to moderate effect size (ES 0.49) in favor of protein supplementation (Supplemental Digital Content 2, Estimated Mean Differences and Effect Sizes for Performance Outcomes and Body Mass, http://links.lww.com/MSS/B615).
There was a significant decrease in total fat mass over time (P = 0.002), with no differences between PLA and PRO (−0.7 ± 1.4 and − 0.4 ± 1.2 kg, respectively; time–treatment interaction, P = 0.43).
Dietary intake and physical activity
Table 4 presents the dietary intake at baseline, week 6, and week 12 of the intervention. In the PRO group, the daily protein intake increased from 1.21 ± 0.35 at baseline to 1.58 ± 0.31 g·kg−1 body mass at week 12, whereas no changes were observed in the PLA group (1.25 ± 0.41 to 1.19 ± 0.31 g·kg−1 body mass; time–treatment interaction, P < 0.0001). By contrast, the increase in carbohydrate intake tended to be greater in PLA when compared with PRO, although this interaction did not reach statistical significance (time–treatment interaction, P = 0.091). There were no changes in energy intake over time (P = 0.14) or between treatments (time–treatment interaction, P = 0.91). There were no changes in habitual activity over time or differences between PLA and PRO groups (Supplemental Digital Content 3, Habitual Physical Activity Level, http://links.lww.com/MSS/B616).
DISCUSSION
The current study was designed to assess the effect of protein supplementation on adaptations to chronic endurance exercise training in recreationally active, healthy young males. Although the study showed robust improvements in V˙O2max, 10-km time trial performance, and muscular endurance after 12 wk of endurance exercise training, these adaptations were not further augmented by supplementing ~30 g of protein after exercise and before sleep.
The current study confirms the effect of chronic endurance exercise training on V˙O2max. The observed improvements in V˙O2max (~5 mL·min−1·kg−1; ~11%) are in line with the improvements in V˙O2max reported in the meta-analysis of Milanovic et al. (2). Noteworthy is the fact that each individual participant in the current study showed an increase in V˙O2max (Fig. 1). The robust improvements in V˙O2max may be attributed to the tightly controlled exercise training program, comprising a combination of continuous endurance exercise and interval training. Indeed, the inclusion of interval training at higher intensities has been shown superior to strict continuous endurance exercise training to increase V˙O2max (2). Along with the improvements in V˙O2max, the efficacy of the current exercise training program is further evidenced by the ~14% improvement in 10-km time trial performance. Despite the effective training program, no significant differences in V˙O2max and endurance performance were observed between the placebo and the protein groups. These findings are in contrast with two previous studies that reported on the effect of protein supplementation on the gains in V˙O2max after endurance exercise training (14,15). Those studies reported a substantial increase in V˙O2max in the protein-supplemented participants, whereas the increase in V˙O2max appeared to be marginal or even absent in the control groups. Some other differences should be noted as well. When compared with both previous studies, our participants had a higher training level according to their V˙O2max, the duration of the training intervention was longer, and protein was supplemented more frequently and in higher doses. By contrast, the duration of exercise sessions was higher in the study of Ferguson-Stegall et al. (15); ~5 h weekly) compared with the current study (~3 h weekly). Regardless of the differences in study designs, the results of the current study indicate that supplementation with protein does not further augment improvements in whole-body oxidative capacity and endurance performance when an intense training stimulus is being provided in recreationally active, healthy young males.
Besides V˙O2max and time trial performance, the current study assessed the effect of endurance exercise training on muscle strength. Maximal isokinetic strength of the knee flexors and extensors was significantly increased. This finding seems somewhat surprising, as endurance exercise is often associated with blunted gains in hypertrophy and strength when combined with resistance exercise training (21,22). However, it should be noted that the increase in strength observed in our study was paralleled by an increase in leg LM. It is possible that the increase in leg strength and leg LM are attributed to the interval component of our exercise training. This type of endurance exercise was included in the exercise training program, as this is a standard component of contemporary endurance exercise training programs. Recently, such an endurance exercise program has been shown to increase leg LM after 12 wk of training (23). In agreement with the benefits of protein supplementation during resistance exercise training (13), our results suggest that protein supplementation has the potential to further increase the gains in LM induced by endurance exercise training (small to moderate effect size in favor of protein).
Along with the effect on maximal isokinetic strength, we also observed a profound effect of endurance exercise training on muscular endurance, as evidenced by an increase in total work conducted during 30 maximal reciprocal isokinetic contractions (Fig. 2B). To the best of our knowledge, the effect of strictly endurance exercise training on muscular endurance has not been investigated previously. Resistance exercise training with high repetitions and minimal rest between sets is normally seen as the most effective way to improve muscular endurance (24). We speculate that robust improvements in muscular endurance as observed in the current study result from the high-intensity interval exercise sessions where high workloads were sustained during 1-min periods. As such, this type of endurance exercise training might be an attractive alternative to resistance exercise training to improve muscular endurance. Protein supplementation, however, does not seem to further augment the effect of endurance exercise training on muscular endurance.
The protein supplement used in the current study contained 28.7 g of high-quality casein protein and was ingested after each exercise session and each day before sleep. Hence, the weekly protein dose was approximately three- to fourfold greater than the weekly doses previously applied in the studies of Fergusson-Stegall et al. (15) and Robinson et al. (14). Ingestion of the protein supplement resulted in an increase in daily protein intake from ~1.2 g·kg−1 body mass at baseline to ~1.6 g·kg−1 body mass during the 12-wk intervention period, whereas the daily protein intake remained at ~1.2 g·kg−1 body mass in the placebo group. Current recommendations for endurance athletes suggest a daily protein intake of 1.2 to maximally 1.7 g·kg−1 body mass (25). As such, the protein intake in the placebo group was in the lower range of the protein intake recommendations for endurance athletes, whereas the protein intake recommendations were covered adequately by participants in the protein group. Along with the increase in daily protein intake, the supplementation of protein was timed strategically, with 28.7 g of protein being ingested after each exercise session and each day before sleep. Such an approach with protein supplementation before sleep has been shown to augment the gains in muscle strength and muscle mass after 12 wk of resistance exercise training (26). This effect might be attributed to the stimulation of muscle protein synthesis during overnight recovery from resistance exercise (27). Despite the substantial increase in daily protein intake and strategic timing, we found no additional benefits of protein supplementation on whole-body oxidative capacity and endurance performance after chronic endurance exercise. Hence, a habitual daily protein intake of 1.2 g·kg−1 body mass, as reported by the placebo group, seems sufficient to support adaptations to endurance exercise training in moderately trained individuals performing ~3 h of endurance exercise weekly. It can be speculated that the lack of effect of protein supplementation is related to the relatively low volume and frequency of endurance exercise training applied in the current study. Recent evidence suggests that the protein requirements of well-trained endurance athletes, performing higher volumes of endurance exercise training, may be higher than previously assumed (7,28). In this regard, protein oxidation rates increase when the duration and intensity of an endurance exercise bout increase (5). Furthermore, a higher frequency of exercise sessions allows for less recovery time between successive exercise bouts. Nevertheless, the effect of protein supplementation on endurance capacity and exercise performance in well-trained and elite endurance athletes remains an issue for further investigation.
The current study was designed specifically to assess the effect of protein supplementation during chronic endurance exercise training on whole-body oxidative capacity and endurance performance. Strengths of the current study include the tightly controlled endurance exercise training program, high adherence to the exercise program, high compliance with the intake of supplemental beverages, and extensive assessment of whole-body oxidative capacity and endurance performance. Nevertheless, we should also acknowledge some limitations. First, the study was powered to detect a difference in V˙O2max between the placebo and the protein groups. This does not exclude the possibility for a statistical type 2 error with respect to time trial performance and muscular strength and endurance. In this regard, it should be noted that most outcomes displayed small to moderate effect sizes in favor of protein supplementation. Second, we included a relatively homogenous population of healthy young males. Hence, it is difficult to generalize our findings to other populations aiming to maximize the adaptive response to endurance exercise, such as females and well-trained or elite athletes. Third, the endurance exercise training program comprised cycling exercise only, involving mainly concentric exercise of the leg muscles. Other exercise modes may involve more active muscle mass (e.g., rowing or cross-country skiing) or may cause more muscle damage (e.g., running), thereby potentially increasing the protein requirements. Therefore, it remains to be established whether the results obtained in the current study are valid for other exercise modes. Finally, many athletes use a combination of endurance and resistance exercise, which may hamper maximal adaptations to either type of exercise (21,22). Therefore, it would be relevant to assess whether protein supplementation may further augment gains in endurance performance and strength during chronic concurrent exercise training.
In conclusion, protein supplementation after exercise and before sleep does not further augment the gains in whole-body oxidative capacity and endurance exercise performance after 12 wk of endurance exercise training in recreationally active healthy young males.
Supplementary Material
Acknowledgments
This project was funded by the Dutch Ministry of Economic Affairs (Topsector Agri&Food), award number AF16501 (PPS Allowance). K. L. J., K. J. M. P., S. L. D., and I. J. M. C. declare that they have no conflict of interest. F. C. W., L. J. C. v. L., and J.-W. v. D. have received research grants, consulting fees, and/or speaking honoraria from FrieslandCampina. L. J. C. v. L. has received research grants, consulting fees, and speaking honoraria from Pepsico/Gatorade. AMHH is an employee at FrieslandCampina.
The authors thank Nigel Ooms, Celeste Pijnenborg, Niek van Stralen, David Zegers, and Irith van Boven for their assistance during the training sessions. They also thank Nicole van Wijk, Mirna Klaiber, and Birna Vardardóttir for their help during the nutritional and activity assessments and analyses and Cindy van der Avoort for her assistance during the test days.
The authors’ responsibilities were as follows: J.-W. v. D., F. C. W., and L. J. C. v. L. designed the research; K. L. J., K. J. M. P., S. L. D., I. J. M. C., and A. M. H. H. conducted the research; K. L. J. and K. J. M. P. analyzed the data and performed the statistical analyses; K. L. J., K. J. M. P., and J.-W. v. D. wrote the manuscript and hold primary responsibility for the final content; all authors contributed to the interpretation of data and discussion of the manuscript for important intellectual content and approved the final manuscript. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
K. L. J. and K. J. M. P. contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
REFERENCES
- 1.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32(1):70–84. [DOI] [PubMed] [Google Scholar]
- 2.Milanović Z, Sporiš G, Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for V˙O2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45(10):1469–81. [DOI] [PubMed] [Google Scholar]
- 3.Jeukendrup AE. Nutrition for endurance sports: marathon, triathlon, and road cycling. J Sports Sci. 2011;29(1 Suppl):S91–9. [DOI] [PubMed] [Google Scholar]
- 4.Knuiman P, Hopman MTE, Verbruggen C, Mensink M. Protein and the adaptive response with endurance training: wishful thinking or a competitive edge? Front Physiol. 2018;9:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore DR, Camera DM, Areta JL, Hawley JA. Beyond muscle hypertrophy: why dietary protein is important for endurance athletes. Appl Physiol Nutr Metab. 2014;39(9):987–97. [DOI] [PubMed] [Google Scholar]
- 6.Mazzulla M, Parel JT, Beals JW, et al. Endurance exercise attenuates postprandial whole-body leucine balance in trained men. Med Sci Sports Exerc. 2017;49(12):2585–92. [DOI] [PubMed] [Google Scholar]
- 7.Kato H, Suzuki K, Bannai M, Moore DR. Protein requirements are elevated in endurance athletes after exercise as determined by the indicator amino acid oxidation method. PLoS One. 2016;11(6):e0157406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levenhagen DK, Gresham JD, Carlson MG, Maron DJ, Borel MJ, Flakoll PJ. Postexercise nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis. Am J Physiol Endocrinol Metab. 2001;280(6):E982–93. [DOI] [PubMed] [Google Scholar]
- 9.Howarth KR, Moreau NA, Phillips SM, Gibala MJ. Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J Appl Physiol. 2009;106(4):1394–402. [DOI] [PubMed] [Google Scholar]
- 10.Pasiakos SM, McClung HL, McClung JP, et al. Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr. 2011;94(3):809–18. [DOI] [PubMed] [Google Scholar]
- 11.Bohé J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552(Pt 1):315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson SB, Phillips SM, Atherton PJ, et al. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586(15):3701–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–64. [DOI] [PubMed] [Google Scholar]
- 14.Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J. 2011;25(9):3240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson-Stegall L, McCleave E, Ding Z, et al. Aerobic exercise training adaptations are increased by postexercise carbohydrate-protein supplementation. J Nutr Metab. 2011;2011:623182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltz NM, Gibson AL, Janot JM, Kravitz L, Mermier CM, Dalleck LC. Graded exercise testing protocols for the determination of V˙O2max: historical perspectives, progress, and future considerations. J Sports Med (Hindawi Publ Corp). 2016;2016:3968393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeukendrup A, Saris WH, Brouns F, Kester AD. A new validated endurance performance test. Med Sci Sports Exerc. 1996;28(2):266–70. [DOI] [PubMed] [Google Scholar]
- 18.Wardenaar FC, Steennis J, Ceelen IJ, Mensink M, Witkamp R, de Vries JH. Validation of web-based, multiple 24-h recalls combined with nutritional supplement intake questionnaires against nitrogen excretions to determine protein intake in Dutch elite athletes. Br J Nutr. 2015;114(12):2083–92. [DOI] [PubMed] [Google Scholar]
- 19.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–81. [DOI] [PubMed] [Google Scholar]
- 20.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JM, Marin PJ, Rhea MR, Wilson SM, Loenneke JP, Anderson JC. Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Cond Res. 2012;26(8):2293–307. [DOI] [PubMed] [Google Scholar]
- 22.Coffey VG, Hawley JA. Concurrent exercise training: do opposites distract? J Physiol. 2017;595(9):2883–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamim B, Devlin BL, Timmins RG, et al. Adaptations to concurrent training in combination with high protein availability: a comparative trial in healthy, recreationally active men. Sports Med. 2018;48(12):2869–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American College of Sports Medicine. American College of Sports Medicine Position Stand: progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. [DOI] [PubMed] [Google Scholar]
- 25.Tarnopolsky M. Protein requirements for endurance athletes. Nutrition. 2004;20(7–8):662–8. [DOI] [PubMed] [Google Scholar]
- 26.Snijders T, Res PT, Smeets JS, et al. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J Nutr. 2015;145(6):1178–84. [DOI] [PubMed] [Google Scholar]
- 27.Res PT, Groen B, Pennings B, et al. Protein ingestion before sleep improves postexercise overnight recovery. Med Sci Sports Exerc. 2012;44(8):1560–9. [DOI] [PubMed] [Google Scholar]
- 28.Bandegan A, Courtney-Martin G, Rafii M, Pencharz PB, Lemon PWR. Indicator amino acid oxidation protein requirement estimate in endurance-trained men 24 h postexercise exceeds both the EAR and current athlete guidelines. Am J Physiol Endocrinol Metab. 2019;316(5):E741–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


