Supplemental Digital Content is available in the text.
Keywords: acute kidney injury, intensive care, oncology, pediatric, renal replacement therapy, stem cell transplantation
Objective:
Acute kidney injury requiring continuous renal replacement therapy is a serious treatment-related complication in pediatric cancer and hematopoietic stem cell transplant patients. The purpose of this study was to assess epidemiology and outcome of these patients requiring continuous renal replacement therapy in the PICU.
Design:
A nationwide, multicenter, retrospective, observational study.
Setting:
Eight PICUs of a tertiary care hospitals in the Netherlands.
Patients:
Pediatric cancer and hematopoietic stem cell transplant patients (cancer and noncancer) who received continuous renal replacement therapy from January 2006 to July 2017 in the Netherlands.
Interventions:
None.
Measurement and Main Results:
Of 1,927 PICU admissions of pediatric cancer and hematopoietic stem cell transplant patients, 68 of 70 evaluable patients who received continuous renal replacement therapy were included. Raw PICU mortality was 11.2% (216/1,972 admissions). PICU mortality of patients requiring continuous renal replacement therapy was 54.4% (37/68 patients). Fluid overload (odds ratio, 1.08; 95% CI, 1.01–1.17) and need for inotropic support (odds ratio, 6.53; 95% CI, 1.86–23.08) at the start of continuous renal replacement therapy were associated with PICU mortality. Serum creatinine levels increased above 150% of baseline 3 days before the start of continuous renal replacement therapy. Urine production did not reach the critical limit of oliguria. In contrast, body weight (fluid overload) increased already 5 days prior to continuous renal replacement therapy initiation.
Conclusions:
PICU mortality of pediatric cancer and hematopoietic stem cell transplant patients requiring continuous renal replacement therapy is sadly high. Fluid overload at the initiation of continuous renal replacement therapy is the most important and earliest predictor of PICU mortality. Our results suggest that the most commonly used criteria of acute kidney injury, that is, serum creatinine and urine production, are not useful as a trigger to initiate continuous renal replacement therapy. This highlights the urgent need for prospective studies to generate recommendations for effective therapeutic interventions at an early phase in this specific patient population.
Over the last decades, outcome of pediatric cancer and hematopoietic stem cell transplant (HSCT) patients has substantially improved by the introduction of more targeted treatment protocols and advanced supportive therapy. As a consequence, outcomes for these children have evolved from an estimated 20% survival in the late 80s to an 80% survival at this time (1–3). However, these developments also have increased morbidity, that is, disease- or treatment-associated complications, many of which require intensive care treatment (4–7).
Acute kidney injury (AKI) is one of these serious complications, being multifactorial in etiology. The need for nephrotoxic medication, including chemotherapeutics, antibiotics, and immunosuppressants, contributes to renal injury. Furthermore, intervention in these patients during critical illness frequently requires a high volume of IV fluids administration, including the need for blood products, which seriously increases the risk of fluid overload (8–10).
Continuous renal replacement therapy (CRRT) has become the most widely used modality of renal replacement in critically ill children with renal dysfunction, fluid overload, and/or electrolyte imbalances as it allows continuous and programmed removal of fluids as well as nitrous waste products (11–14). However, its use in the complex setting of critically ill pediatric cancer and HSCT patients with various components of multiple organ dysfunction is challenging and sometimes controversial. Studies on outcome of critically ill patients requiring CRRT have been mostly pursued in the general PICU population, and none of these studies assessed outcomes specifically in pediatric cancer and HSCT patients. A better understanding of the impact of the need for CRRT on both short- and long-term outcomes among these children and factors that influence these outcomes is essential for optimal implementation of this therapy in pediatric oncology intensive care settings.
The purpose of the current national study was to assess the epidemiology and outcome of pediatric cancer and HSCT patients who required CRRT in the PICU. In addition, we sought to identify risk factors for mortality in the PICU setting and we have examined the behavior of the determinants of AKI, serum creatinine (SCr), and degree of oliguria in this population.
METHODS
Study Design
This retrospective study included all pediatric cancer and HSCT (oncologic and nononcologic) patients who received CRRT in one of the eight Dutch PICUs from January 2006 to July 2017. The exclusion criteria were a preexisting estimated glomerular filtration rate (eGFR) below 15 mL/min/1.73 m2 of body surface area, maintenance dialysis, or receipt of kidney transplant in the preceding 90 days before the start of CRRT (15).
The study was approved by the institutional ethical review boards of the participating hospitals (reference number: 17–028/C). Need for informed consent was waived.
Complete details of the study design are provided in the online supplement (Supplemental Digital Content 1, http://links.lww.com/CCM/E888).
Data Collection
Data were collected for each CRRT episode. Baseline patient characteristics and variables regarding PICU admission and treatment were obtained from medical records. Disease severity at PICU admission was assessed using the Pediatric Index of Mortality (PIM) 2 score (16).
Renal function data assessed included SCr levels, urine output, eGFR, AKI stage, and the percentage fluid overload 7 consecutive days before and at the start of CRRT. AKI was defined and classified according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria, which include incremental changes of SCr and decremental urine output (17). In addition, follow-up data on renal function 3 months after PICU discharge, including eGFR and chronic kidney disease (CKD) stage, were collected. SCr was adjusted for fluid balance according to the following formula: corrected creatinine = measured creatinine × (1 + [accumulated net fluid balance/total body water]), where total body water = 0.6 × weight (kg) (18–20). Details on data collection and definitions are described in the online supplement (Supplemental Digital Content 1, http://links.lww.com/CCM/E888).
Outcomes
The primary outcome was PICU mortality. Secondary outcomes were 3-month mortality, renal function status at 3 months after PICU discharge (resolution, new-onset CKD, or worsening of preexisting CKD) (17), resource utilization by measurement of PICU length of stay, and the days of use of PICU technologies such as mechanical ventilation and inotropic/vasopressor support.
Statistical Analysis
Categorical variables were displayed as frequencies (%) and compared using chi-square or Fisher exact test. Normality was tested with the Kolmogorov-Smirnov test. Continuous variables were displayed as medians with interquartile ranges (IQRs) or as means ± sds, based on data distribution, and were compared with the Wilcoxon rank-sum test. With respect to missing data, multiple imputation was used (Supplementary Table S1, Supplemental Digital Content 1, http://links.lww.com/CCM/E888). From seven of the 68 patients, data of SCr, urine production, and fluid overload in the 7 consecutive days before initiation of CRRT were missing. These patients were excluded in the analysis of the course of these parameters.
Variables were evaluated for an association with PICU mortality using multivariate logistic regression analysis after controlling for potential confounders. Odds ratio (OR) and their corresponding 95% CIs were calculated. Variables were entered into the model when the α level of the risk factor was less than 0.15 in univariate analysis.
The continuous variable “degree of fluid overload” was divided into severity strata for univariate analyses: 0% to less than or equal to 3%, 3% to less than or equal to 10%, greater than 10% to less than or equal to 20%, greater than 20%. In addition, percentage of fluid overload was also analyzed as a continuous variable.
P values of less than 0.05 were considered to be significant. Data analysis was generated using SPSS version 23 (IBM, Armonk, NY).
RESULTS
Study Population
During the study period, there were 59,864 PICU admissions in the Netherlands, of which 1,927 admissions had an underlying malignancy or had undergone HSCT. Seventy of these 1,927 patients received CRRT (3.6%). Data on two patients were not available, leaving 68 patients, for inclusion in the analyses (Fig. 1).
Figure 1.
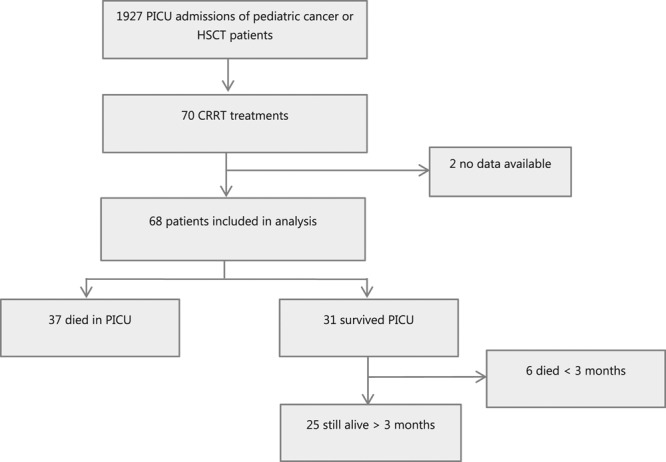
Included subjects from PICU admissions from January 2006 until July 2017. CRRT = continuous renal replacement therapy, HSCT = hematopoietic stem cell transplant.
Patient demographic and clinical characteristics are shown in Table 1. The median age was 8.9 years (IQR, 3.3–8.9 yr). The majority of patients had leukemia/lymphoma (n = 38; 55.9%) or a solid tumor (n = 17; 25.0%) as underlying cancer diagnosis. One third of the patients had undergone HSCT (n = 23; 11 patients with cancer as part of their treatment and 12 noncancer). A detailed description of the HSCT patients is presented in Supplementary Table S2 (Supplemental Digital Content 1, http://links.lww.com/CCM/E888). All patients received continuous venovenous hemofiltration, continuous venovenous hemodialysis, or continuous venovenous hemodiafiltration. Detailed information on CRRT technique is provided in Supplementary Table S3 (Supplemental Digital Content 1, http://links.lww.com/CCM/E888).
TABLE 1.
Pediatric Cancer and Hematopoietic Stem Cell Transplant Patient Characteristics Overall and by PICU Survival Status

PICU Admission and Outcome
Sepsis, renal, and respiratory failure were the three major PICU admission diagnoses (Table 1). Raw PICU mortality was 11.2% (216/1,927 admissions). PICU mortality of patients requiring CRRT was 54.4% (37/68 patients), whereas 3-month mortality was 63.3% (43/68 patients). Univariate analyses showed no significant differences between survivors and nonsurvivors regarding baseline and clinical characteristics. In both groups, around 65% of the patients received CRRT within 24 hours after PICU admission. A combination of oliguria, fluid overload, and/or electrolyte imbalances was the predominant indication for CRRT (Supplementary Table S4, Supplemental Digital Content 1, http://links.lww.com/CCM/E888). In the group with a primary renal reason for admission, fluid overload was the major indication for CRRT among the nonsurvivors (29.7%) versus 9.7% among the survivors (p = 0.02). In the survivor group, tumor lysis syndrome was the major primary renal reason for admission (survivors 16.1% vs nonsurvivors 2.7%; p = 0.08) (Supplementary Table S5, Supplemental Digital Content 1, http://links.lww.com/CCM/E888). Of note, among the patients with fluid overload greater than 10%, fluid overload was the indication for CRRT in 72.7% (24/33 patients).
Percentage fluid overload at the start of CRRT differed significantly between survivors and nonsurvivors (3.5% vs 13.6%, respectively; p ≤ 0.001) (Table 1). The majority of patients who survived their PICU stay (48.4%) had a 0–3% increase of their bodyweight at PICU admission. In contrast, almost 50% of the nonsurvivors showed a 10–20% increase of their bodyweight at PICU admission. In addition, the latter group received significantly more diuretics (p = 0.02) and vasoactive support (p < 0.001) when compared with PICU survivors. Vasoactive-Inotropic Score scores did not differ between both groups. SCr and eGFR at PICU admission were not different between both groups.
Risk Factors of PICU Mortality
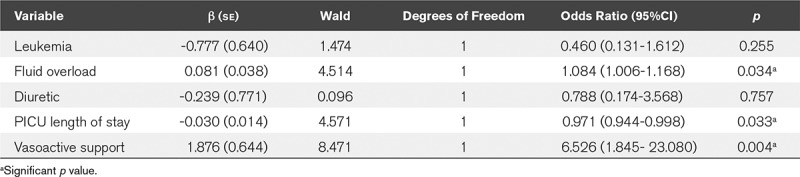
Risk factors of PICU mortality by univariate analyses were fluid overload at admission (% increase body weight), use of diuretics before the start of CRRT, and the need of vasoactive support at the start of CRRT (Table 1). After multivariable adjustment, the important risk factors for PICU mortality were fluid overload (% increase body weight) (OR, 1.08; 95% CI, 1.01–1.17; p = 0.034) and the need of vasoactive support at the start of CRRT (OR, 6.53; 95% CI, 1.85–23.08; p = 0.004) (Table 2). When fluid overload was analyzed dichotomously (<10% or >10%), an OR of 6.16 (95% CI, 1.74–21.8; p = 0.005) was found.
TABLE 2.
Multivariate Analysis of Risk Factors for PICU Mortality
There were significantly more HSCT patients in the group with greater than 10% fluid overload compared with the less than or equal to 10% fluid overload group (45.5% vs 22.9%, respectively; p = 0.05) (Supplementary Table S6, Supplemental Digital Content 1, http://links.lww.com/CCM/E888). In addition, patients in the greater than 10% fluid overload group received significantly more antibiotics (p = 0.038) and vasoactive support (p = 0.006) at CRRT initiation when compared with the less than or equal to 10% fluid overload group.
Correction SCr for Fluid Balance
Correction of SCr for fluid balance revealed significantly higher creatinine values compared with uncorrected values in patients with greater than 10% fluid overload from 3 days before CRRT (Supplementary Table S7, Supplemental Digital Content 1, http://links.lww.com/CCM/E888). In addition, adjustment of SCr for fluid balances shifted patients between KDIGO AKI strata, especially in this latter group.
Clinical Characteristics of AKI in Patients
From a total of 61 patients, daily SCr, urine output, and weight during the 7 days before the start of CRRT were available (Fig. 2). Initially, small increments in SCr were observed followed by an exponential increase 3 days before initiation of CRRT. Urine output showed a similar, though inverse, trend; however, urine production did not fall below the critical limit of oliguria, that is, 0.5 mL/kg/hr. In contrast, bodyweight increased daily with an increased bodyweight of greater than 5% already 5 days before to start of CRRT in the PICU nonsurvivors.
Figure 2.
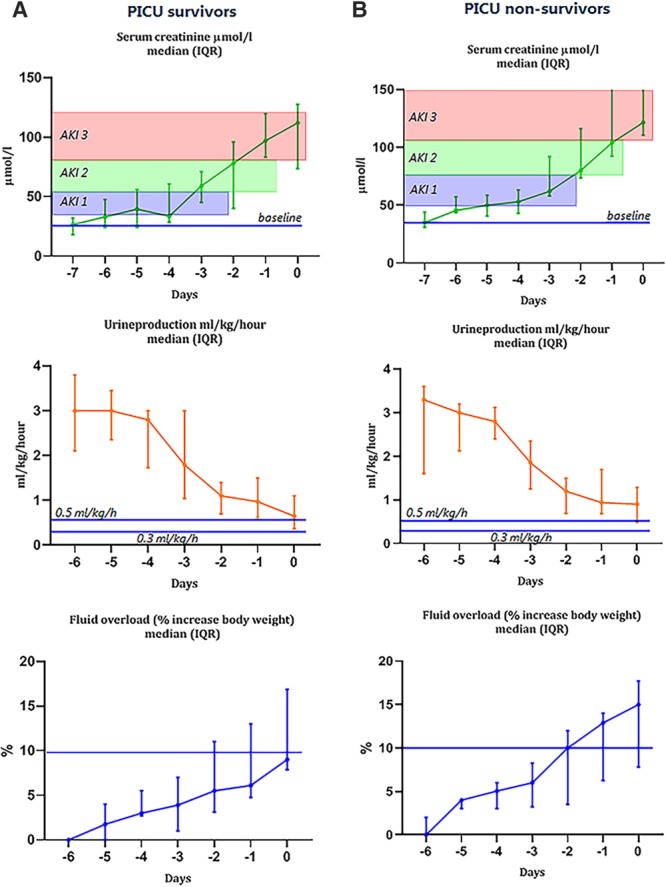
Serum creatinine, urine production, and fluid overload (% increase body weight) to diagnose acute kidney injury (AKI) in pediatric oncology and hematopoietic stem cell transplant patients in days before initiation of continuous renal replacement therapy (= day 0) in PICU survivors (A) and PICU nonsurvivors (B). Serum creatinine values are adjusted for fluid balance. IQR = interquartile range.
Renal Function at 3 Months After PICU Discharge
Of 25 patients who survived greater than 3 months after PICU discharge, follow-up data on renal function were available in 23 patients (Supplementary Fig. S1, Supplemental Digital Content 1, http://links.lww.com/CCM/E888). Renal function after CRRT treatment had fully recovered in 15 patients (65.2%). One patient had an eGFR greater than 90 mL/min/1.73 m2 but showed persistent proteinuria (stage 1 CKD). Seven patients (34.8%) had a persistent eGFR less than 90 mL/min/1.73 m2 more than 3 months after CRRT treatment, thereby fulfilling the criteria of a “new-onset CKD.” Of these seven patients, four patients had stage 2 CKD (mild reduction), one patient stage 3a (mild-moderate reduction), one stage 3b (moderate-severe reduction), and one stage 4 (severe reduction).
DISCUSSION
This nationwide, multicenter, retrospective study showed that PICU mortality of pediatric cancer and HSCT patients requiring CRRT at the PICU is high (around 55%). We found that fluid overload and the need of vasoactive support at initiation of CRRT were independent risk factors for PICU mortality. In addition, almost 30% of the survivors had new-onset CKD 3 months after PICU discharge.
AKI in the general pediatric intensive care patient population is common, with a prevalence around 30% (15). Two large studies in a mixed PICU cohort (both studies included general pediatric, surgical, and oncologic patients) found a PICU mortality of patients with AKI requiring CRRT of 33% and 26%, respectively (15, 21). In our cohort of critically ill pediatric cancer and/or HSCT patients requiring CRRT, a PICU mortality rate of 54% was found, which is substantially higher. This high mortality rate is in line with data on outcome of CRRT in HSCT patients found by the prospective pediatric CRRT registry, where a similar (55%) or even higher (94%) PICU mortality was found (22, 23). Data on critically ill pediatric cancer patients with AKI are scarce. AKI in critically ill adult cancer patients has been associated with a mortality rate exceeding 50% when renal replacement therapy is required (8, 24). In addition to the poor outcomes, AKI decreases the chances of achieving a complete remission and adversely affects long-term survival in these patients as cancer therapy reduction or discontinuation is often necessary to avoid treatment-related morbidity (25–27).
We identified two important early risk factors for PICU mortality: fluid overload and the use of vasopressor therapy. Our data on the association of fluid overload with PICU mortality are in line with previous studies suggesting that fluid overload may predict risk of mortality (28–30). The OR for mortality of overall fluid overload of 1.08 in our study implies an 8% increase in mortality for each 1% increase in amount fluid overload at initiation of CRRT. When fluid overload was analyzed dichotomously, patients with fluid overload greater than 10% were 6.16 times more likely to die than those with less than or equal to 10% fluid overload. This OR was higher when compared with several single-center studies using the same strata, in which ORs were found varying from 1.78 to 3.02 (29, 31). The heterogeneity of the cohorts may be an explanation for the observed differences. Here, we studied solely critically ill pediatric cancer and HSCT patients, whereas in other studies, all PICU patients were included. Two studies compared outcome between two groups with fluid overload: less than 20% and greater than 20% (32, 33). They showed that critically ill children receiving CRRT who had developed greater than 20% fluid overload before start of CRRT had an adjusted mortality OR of 6.1 (32) and 8.5 (33), in line with increased risk of mortality with increasing fluid overload.
It is possible that increased fluid overload merely represented patients who were more critically ill or more hemodynamically unstable and required greater fluid administration. This is supported by higher vasopressor use in the greater than 10% fluid overload group and the association of use of vasopressor support and PICU mortality in the present study and which was also found in a number of previous studies (15, 34–36). However, the present study was not able to differentiate between fluids provided for resuscitation purposes and those necessary for the main pathology required treatments that (subtle) kidney (dys)function was not able to cope with. Interestingly, severity of illness as measured by PIM 2 score was not different between survivors and nonsurvivors at PICU admission. However, it has been shown that such prognostic scores fail to accurately predict the outcome of HSCT patients (37, 38).
Taken together, our data suggest that a practice of goal-directed fluid therapy in order to prevent fluid overload without compromising the hemodynamic status of these patients might be beneficial in this patient population. Whether early initiation of CRRT, that is, at a lower fluid overload threshold, might improve outcomes remains a matter of debate. Recently, two randomized controlled trials comparing an early strategy with a delayed strategy for the initiation of CRRT reported conflicting results (39, 40). With the data currently available, it is not possible to determine a definitive fluid overload threshold for CRRT initiation.
The international KDIGO definition of AKI is based on SCr increase from baseline and/or urine output (17). Critically ill pediatric cancer patients often have decreased creatinine production secondary to immobilization-related loss of muscular cell mass, low protein intake, cachexia, and inflammation (41). All of these factors affect SCr, independently of renal function, and thus limit the sensitivity of creatinine as an early marker of kidney injury in this particular patient group. In addition, an increase in total body water due to fluid overload might blunt a rise in SCr levels by dilution (18, 19). Indeed, adjusting SCr for fluid balance revealed significantly higher creatinine values compared with uncorrected values in patients with greater than 10% fluid overload. However, in clinical practice, values of SCr are not adjusted for the amount of fluid overload, still implying that unadjusted AKI stage may be difficult to use as a trigger for initiation of CRRT in clinical settings. Tubular damage due to nephrotoxic drugs causes renal sodium loss and interferes with renal concentration capacity, thereby obscuring the development of oliguria as a sign of impeding renal function. Therefore, it may take these patients longer to meet the official KDIGO criteria and consequently to receive appropriate treatment. Our results show that small changes in SCr were already noticed in the days before initiation of CRRT. From our clinical experience, these limited changes in SCr may probably not trigger the clinicians that the risk of AKI is enhanced. Zappitelli et al (42) described that a small postoperative increase of only 25% in SCr predicted the development of AKI in children who underwent cardiac surgery. In addition, after 3- to-5 year follow-up, 40–50% of these patients with AKI showed signs of CKD. These findings are in line with our data and underscore the need for clear and timely identification of patients at high risk for AKI.
We found that renal function 3 months after CRRT treatment had fully recovered in 70% of the patients who were discharged from PICU. Hence, 30% of the patients showed CKD based on eGFR. It is already known that there are late renal effects in childhood cancer treatment (43–45). Whether a history of CRRT treatment is an additional, and perhaps preventable, risk factor for development of CKD in this specific patient population has not been studied yet. Based on results of this present study, close monitoring of kidney function in these patients is warranted.
One of the strengths of the present study is the multicenter national approach. However, we also acknowledge several limitations. An inherent limitation of this study is its retrospective nature, and the fact that we rely on data that were collected from patients’ medical records primarily captured for clinical care, and not for research. Furthermore, limited number of included patients, although relatively large for a pediatric study, and the risk for confounders between groups that remain despite our multivariate adjustment, might have influenced the results. A large international prospective study might overcome these limitations. We have only included pediatric cancer and HSCT patients who received CRRT. A control group of pediatric cancer and HSCT patients with AKI “not” requiring CRRT was not included. This would have been very interesting, especially as such a comparison may have revealed specific risk factors for the need of CRRT. In addition, the study period spans 12 years, which increases the likelihood of changes in clinical practice affecting the outcomes of patients. Finally, although our study is multicenter, generalizability may be limited due to differences in PICU practice patterns as well as standard of care in other countries.
CONCLUSIONS
In this retrospective, multicenter national cohort study, we show that PICU mortality of pediatric cancer and HSCT patients that require CRRT is high. We demonstrated that fluid overload and need for vasopressor support at initiation of CRRT predict PICU mortality.
Our results highlight the urgent need for multicenter prospective studies in pediatric cancer and HSCT patients. These studies may reveal risk factors for AKI and may guide recommendations for effective therapeutic interventions at an early phase. In addition, data from these studies may help guide both intensivists and oncologists in risk stratification of patients in the decision-making process of allocation of PICU resources. It may also identify patients who may benefit from closer monitoring and early interventions. Finally, these studies may advance our understanding of critical illness in the context of pediatric oncology to further refine and reflect on our daily clinical practice.
ACKNOWLEDGMENTS
Research consortium SKIC members (Dutch Collaborative PICU Research Network): Amsterdam University Medical Centers, Amsterdam, The Netherlands: Job van Woensel, Reinout Bem, Marc van Heerden, and Maaike Riedijk; Sophia Children’s Hospital/Erasmus Medical Center Rotterdam, Rotterdam, The Netherlands: Matthijs de Hoog and Sascha Verbruggen; Wilhelmina Children’s Hospital/University Medical Center Utrecht, Utrecht, The Netherlands: Roelie Wösten-van Asperen; Beatrix Children’s Hospital/University Medical Center Groningen, Groningen, The Netherlands: Martin Kneyber; University Medical Center Nijmegen, Nijmegen, The Netherlands: Joris Lemson; Maastricht University Medical Center, Maastricht, The Netherlands: Dick van Waardenburg; and Leiden University Medical Center, Leiden, The Netherlands: P. P. Roeleveld.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Bleyer WA. The U.S. pediatric cancer clinical trials programmes: International implications and the way forward. Eur J Cancer 1997; 33:1439–1447 [DOI] [PubMed] [Google Scholar]
- 2.Petridou ET, Dimitrova N, Eser S, et al. Childhood leukemia and lymphoma: Time trends and factors affecting survival in five southern and eastern European cancer registries. Cancer Causes Control 2013; 24:1111–1118 [DOI] [PubMed] [Google Scholar]
- 3.Pritchard-Jones K, Kaatsch P, Steliarova-Foucher E, et al. Cancer in children and adolescents in Europe: Developments over 20 years and future challenges. Eur J Cancer 2006; 42:2183–2190 [DOI] [PubMed] [Google Scholar]
- 4.Dalton HJ, Slonim AD, Pollack MM. MultiCenter outcome of pediatric oncology patients requiring intensive care. Pediatr Hematol Oncol 2003; 20:643–649 [PubMed] [Google Scholar]
- 5.Kress JP, Christenson J, Pohlman AS, et al. Outcomes of critically ill cancer patients in a university hospital setting. Am J Respir Crit Care Med 1999; 160:1957–1961 [DOI] [PubMed] [Google Scholar]
- 6.Staudinger T, Stoiser B, Müllner M, et al. Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit Care Med 2000; 28:1322–1328 [DOI] [PubMed] [Google Scholar]
- 7.Tamburro RF, Barfield RC, Shaffer ML, et al. Changes in outcomes (1996-2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med 2008; 9:270–277 [DOI] [PubMed] [Google Scholar]
- 8.Benoit DD, Hoste EA. Acute kidney injury in critically ill patients with cancer. Crit Care Clin 2010; 26:151–179 [DOI] [PubMed] [Google Scholar]
- 9.Lameire NH, Flombaum CD, Moreau D, et al. Acute renal failure in cancer patients. Ann Med 2005; 37:13–25 [DOI] [PubMed] [Google Scholar]
- 10.Rajasekaran S, Jones DP, Avent Y, et al. Outcomes of hematopoietic stem cell transplant patients who received continuous renal replacement therapy in a pediatric oncology intensive care unit. Pediatr Crit Care Med 2010; 11:699–706 [DOI] [PubMed] [Google Scholar]
- 11.RENAL Study investigators: Renal replacement therapy for acute kidney injury in Australian and New Zealand intensive care units: A practice survey. Crit Care Resusc 2008; 10:225–230 [PubMed] [Google Scholar]
- 12.Goldstein SL. Overview of pediatric renal replacement therapy in acute renal failure. Artif Organs 2003; 27:781–785 [DOI] [PubMed] [Google Scholar]
- 13.Strazdins V, Watson AR, Harvey B; European Pediatric Peritoneal Dialysis Working Group: Renal replacement therapy for acute renal failure in children: European guidelines. Pediatr Nephrol 2004; 19:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walters S, Porter C, Brophy PD. Dialysis and pediatric acute kidney injury: Choice of renal support modality. Pediatr Nephrol 2009; 24:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaddourah A, Basu RK, Bagshaw SM, et al. ; AWARE Investigators: Epidemiology of acute kidney injury in critically Ill children and young adults. N Engl J Med 2017; 376:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slater A, Shann F, Pearson G; Paediatric Index of Mortality (PIM) Study Group: PIM2: A revised version of the paediatric index of mortality. Intensive Care Med 2003; 29:278–285 [DOI] [PubMed] [Google Scholar]
- 17.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120:c179–c184 [DOI] [PubMed] [Google Scholar]
- 18.Basu RK, Andrews A, Krawczeski C, et al. Acute kidney injury based on corrected serum creatinine is associated with increased morbidity in children following the arterial switch operation. Pediatr Crit Care Med 2013; 14:e218–e224 [DOI] [PubMed] [Google Scholar]
- 19.Liu KD, Thompson BT, Ancukiewicz M, et al. ; National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network: Acute kidney injury in patients with acute lung injury: Impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med 2011; 39:2665–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macedo E, Bouchard J, Soroko SH, et al. ; Program to Improve Care in Acute Renal Disease Study: Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care 2010; 14:R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westrope CA, Fleming S, Kapetanstrataki M, et al. Renal replacement therapy in the critically Ill child. Pediatr Crit Care Med 2018; 19:210–217 [DOI] [PubMed] [Google Scholar]
- 22.Flores FX, Brophy PD, Symons JM, et al. Continuous renal replacement therapy (CRRT) after stem cell transplantation. A report from the prospective pediatric CRRT Registry Group. Pediatr Nephrol 2008; 23:625–630 [DOI] [PubMed] [Google Scholar]
- 23.Al-Ayed T, Rahman NU, Alturki A, et al. Outcome of continuous renal replacement therapy in critically ill children: A retrospective cohort study. Ann Saudi Med 2018; 38:260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darmon M, Vincent F, Canet E, et al. Acute kidney injury in critically ill patients with haematological malignancies: Results of a multicentre cohort study from the Groupe de Recherche en réanimation respiratoire en onco-hématologie. Nephrol Dial Transplant 2015; 30:2006–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canet E, Zafrani L, Lambert J, et al. Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: Impact on remission and survival. PLoS One 2013; 8:e55870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahoti A, Kantarjian H, Salahudeen AK, et al. Predictors and outcome of acute kidney injury in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Cancer 2010; 116:4063–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munker R, Hill U, Jehn U, et al. Renal complications in acute leukemias. Haematologica 1998; 83:416–421 [PubMed] [Google Scholar]
- 28.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int 2005; 67:653–658 [DOI] [PubMed] [Google Scholar]
- 29.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: A retrospective analysis. Crit Care Med 2004; 32:1771–1776 [DOI] [PubMed] [Google Scholar]
- 30.Hoste EA, De Corte W. Epidemiology of AKI in the ICU. Acta Clin Belg 2007; 62(Suppl 2):314–317 [DOI] [PubMed] [Google Scholar]
- 31.Gillespie RS, Seidel K, Symons JM. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol 2004; 19:1394–1399 [DOI] [PubMed] [Google Scholar]
- 32.Hayes LW, Oster RA, Tofil NM, et al. Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care 2009; 24:394–400 [DOI] [PubMed] [Google Scholar]
- 33.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: The prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 2010; 55:316–325 [DOI] [PubMed] [Google Scholar]
- 34.Payen D, de Pont AC, Sakr Y, et al. ; Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators: A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 2008; 12:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang JW, Jeng MJ, Yang LY, et al. The epidemiology and prognostic factors of mortality in critically ill children with acute kidney injury in Taiwan. Kidney Int 2015; 87:632–639 [DOI] [PubMed] [Google Scholar]
- 36.de Galasso L, Emma F, Picca S, et al. Continuous renal replacement therapy in children: Fluid overload does not always predict mortality. Pediatr Nephrol 2016; 31:651–659 [DOI] [PubMed] [Google Scholar]
- 37.van Veen A, Karstens A, van der Hoek AC, et al. The prognosis of oncologic patients in the pediatric intensive care unit. Intensive Care Med 1996; 22:237–241 [DOI] [PubMed] [Google Scholar]
- 38.Schneider DT, Lemburg P, Sprock I, et al. Introduction of the oncological pediatric risk of mortality score (O-PRISM) for ICU support following stem cell transplantation in children. Bone Marrow Transplant 2000; 25:1079–1086 [DOI] [PubMed] [Google Scholar]
- 39.Gaudry S, Hajage D, Schortgen F, et al. ; AKIKI Study Group: Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016; 375:122–133 [DOI] [PubMed] [Google Scholar]
- 40.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically Ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA 2016; 315:2190–2199 [DOI] [PubMed] [Google Scholar]
- 41.Lameire N, Vanholder R, Van Biesen W, et al. Acute kidney injury in critically ill cancer patients: An update. Crit Care 2016; 20:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zappitelli M, Parikh CR, Akcan-Arikan A, et al. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 2008; 3:948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dekkers IA, Blijdorp K, Pieters R, et al. [Chronic renal insufficiency following childhood cancer]. Ned Tijdschr Geneeskd 2014; 158:A6692. [PubMed] [Google Scholar]
- 44.Dekkers IA, Blijdorp K, Cransberg K, et al. Long-term nephrotoxicity in adult survivors of childhood cancer. Clin J Am Soc Nephrol 2013; 8:922–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knijnenburg SL, Jaspers MW, van der Pal HJ, et al. Renal dysfunction and elevated blood pressure in long-term childhood cancer survivors. Clin J Am Soc Nephrol 2012; 7:1416–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]


