Objectives:
Mechanical ventilation can cause ventilator-induced brain injury via afferent vagal signaling and hippocampal neurotransmitter imbalances. The triggering mechanisms for vagal signaling during mechanical ventilation are unknown. The objective of this study was to assess whether pulmonary transient receptor potential vanilloid type-4 (TRPV4) mechanoreceptors and vagal afferent purinergic receptors (P2X) act as triggers of ventilator-induced brain injury.
Design:
Controlled, human in vitro and ex vivo studies, as well as murine in vivo laboratory studies.
Setting:
Research laboratory.
Subjects:
Wild-type, TRPV4-deficient C57BL/6J mice, 8–10 weeks old. Human postmortem lung tissue and human lung epithelial cell line BEAS-2B.
Intervention:
Mice subjected to mechanical ventilation were studied using functional MRI to assess hippocampal activity. The effects of lidocaine (a nonselective ion-channel inhibitor), P2X-purinoceptor antagonist (iso-PPADS), or genetic TRPV4 deficiency on hippocampal dopamine-dependent pro-apoptotic signaling were studied in mechanically ventilated mice. Human lung epithelial cells (BEAS-2B) were used to study the effects of mechanical stretch on TRPV4 and P2X expression and activation. TRPV4 levels were measured in postmortem lung tissue from ventilated and nonventilated patients.
Measurements and Main Results:
Hippocampus functional MRI analysis revealed considerable changes in response to the increase in tidal volume during mechanical ventilation. Intratracheal lidocaine, iso-PPADS, and TRPV4 genetic deficiency protected mice against ventilationinduced hippocampal pro-apoptotic signaling. Mechanical stretch in both, BEAS-2B cells and ventilated wild-type mice, resulted in TRPV4 activation and reduced Trpv4 and P2x expression. Intratracheal replenishment of adenosine triphosphate in Trpv4–/– mice abrogated the protective effect of TRPV4 deficiency. Autopsy lung tissue from ventilated patients showed decreased lung TRPV4 levels compared with nonventilated
Conclusions:
TRPV4 mechanosensors and purinergic receptors are involved in the mechanisms of ventilator-induced brain injury. Inhibition of this neural signaling, either using nonspecific or specific inhibitors targeting the TRPV4/adenosine triphosphate/P2X signaling axis, may represent a novel strategy to prevent or treat ventilator-induced brain injury.
Keywords: lidocaine, mechanical ventilation, purinergic receptors, TRPV4, ventilator-induced
Physical, psychiatric, and/or cognitive impairments are common among survivors of critical illness and are referred to as “postintensive care syndrome” (1). Neuropsychologic disorders ranging from delirium to long-term cognitive impairment can be found in up to 80% of patients admitted to ICUs (2). A precipitating factor for developing delirium in the ICU is the use of ventilatory support (3).
Although various strategies have been designed to minimize ventilator-associated lung injury during the past decades, considerable attention has recently been focused on the potentially adverse effects of mechanical ventilation (MV) on cognitive function. Recent reports suggest that there is significant crosstalk between the brain and the lung during MV (4, 5). We have previously shown that MV activates sensory nerves in the respiratory tract, stimulating vagal afferent signaling and leading to a hyperdopaminergic state within the hippocampus. Activation of these hyperdopaminergic pathways leads to hippocampal cell apoptosis (6). Enhanced release of dopamine preferably activates the dopamine receptor D2, leading to a dephosphorylation cascade of the protein kinase B (AKT)/glycogen synthase kinase-3β (GSK3β) pathway and the subsequent processing of poly(adenosine-diphosphate-ribose) polymerase-1 (PARP-1) (6). So far, the molecular mechanisms linking MV with the development of cognitive disorders have remained elusive, but the link between neurodegeneration and the proteins involved in the hyperdopaminergic pathway (i.e., PARP-1 processing or GSK3β) (7, 8) may help to better understand and facilitate the development of novel therapeutic approaches to prevent ventilator-induced brain injury (VIBI).
Lung mechanosensing may play a role in the crosstalk between the ventilated lung and the brain at risk for cognitive dysfunction. The superfamily of transient receptor potential vanilloid channel (TRPV) comprises a group of cation-selective proteins widely expressed throughout the body. They act as polymodal signal integrators due to their ability to respond to a wide diversity of stimuli. Many activators of these TRPV channels, such as mechanical forces, products of lipid peroxidation, or prostaglandins, are present in mechanically ventilated lungs. Furthermore, TRPV4 channels are functionally linked to pannexin-1–mediated adenosine triphosphate (ATP) release under stretch/strain conditions (9, 10). Once ATP is released into the alveoli, it could activate purinergic receptors present on pulmonary vagal afferent neurons, thereby stimulating vagal signaling (11).
We, therefore, hypothesized that activation of TRPV4, ATP release, and ATP-gated P2X receptor (P2X) stimulation (TRPV4/ATP/P2X axis) represent a molecular mechanism responsible for the development of VIBI during MV.
MATERIALS AND METHODS
Animals
Eight- to 12-week-old C57BL6 male mice were used in all experiments. Experimental protocols were approved by the Local Animal Research Ethics Committee (Landesamt für Gesundheit und Soziales: G0023/15, Germany) in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria for animal experimentation (12).
Experimental Protocol
Mice deficient in TRPV4 (Trpv4–/–) were randomly assigned to MV or spontaneous breathing (n = 6–8 per group). Spontaneously breathing mice (sham) received only sedation identical to other groups. MV (peak inspiratory pressure, 20 cm H2O; positive end-expiratory pressure, 2 cm H2O; respiratory rate, 50 breaths/min; Fio2, 21%) was applied for 2 hours. In preliminary experiments, these ventilation pressures result in tidal volumes around 20 mL/kg (13). Mice receiving MV were assigned to one out of five treatment groups: intratracheal lidocaine or IV lidocaine hydrochloride (7 mg/kg in 25 µL of saline), intratracheal iso-PPADS-tetrasodium salt (20 mg/kg), intratracheal ATP (100 µg/kg), or saline. At the end of the study protocol, bronchoalveolar lavage fluid (BALF), lung tissue, and hippocampal formation were collected.
Functional MRI
Wild-type mice were positioned in the magnet bore of a 9.4 Tesla small animal functional MRI (fMRI)/Electroencephalogram scanner (USR94/20; Bruker BioSpin MRI, Wissembourg, France) although being mechanically ventilated (tidal volume, 20 mL/kg; positive end-expiratory pressure, 2 cm H2O for 10 min followed by 25 min of 30 mL/kg; Fio2, 0.21) to determine the correlation between hippocampal activity and the increments in tidal volume. fMRI permits to predict changes in neural activity indirectly by detecting changes in blood oxygenation—known as the “blood oxygenation level–dependent” (BOLD) effect (14). We identified robust neuronal networks in the mouse brain by applying independent component analysis (ICA) in the face of tidal volume increases. ICA detects neuronal network activity based on functional connectivity, which is defined as correlations in signal intensity over time (15).
Cell Stretch Experiment and Proximity Ligation Assay
Human lung epithelial cells (BEAS-2B, CRL-9609, ATCC Manassas, VA) were exposed to equibiaxial cyclic stretch of either 5% elongation at 2.5 Hz or 18% at 1.0 Hz for 2 hours in a FX-4000T FLEXCELL Tension Plus System (Flexcell International, Burlington, NC) following a previously established method (16).
Human Postmortem Lung Tissue
Lungs from 10 patients who died in the ICU while receiving MV and from seven nonventilated ICU patients were obtained from the tissue bank at the Hospital Universitario Central de Asturias (Oviedo, Spain) following local research ethics committee approval (reference:2013/094). Clinical data from these patients (age, sex, comorbid conditions, and cause of ICU admission) were collected. Sections of the patients’ lungs were embedded in paraffin, sectioned and immunoprobed with antibodies against TRPV4. The intensity of immunoreaction was quantified by a pathologist who was blinded to the clinical information using a semiquantitative score (0–4).
Statistical Analysis
Data from all experimental groups were tested for normality using Shapiro-Wilk test. In multiple group comparisons, One-way analysis of variance and Dunnett test was used for normally distributed data, otherwise Kurskal-Wallis and Dunn comparisons test was chosen. Comparisons between two groups were performed by Student t test or Mann-Whitney U test for normal and not normal distributed data, respectively. Survival was assessed by Kaplan-Meier curves and log-rank test. A p value of less than 0.05 was considered significant. All calculations were performed with SPSS 24.0 (SPSS/IBM, Chicago, IL).
RESULTS
MV Leads to Changes in Neuronal Activity in the Hippocampus
Previously, we demonstrated the presence of neuronal excitotoxicity in the hippocampal formation in response to vagal signaling during MV (6). In our current study, we have used fMRI to demonstrate that MV results in radiologic evidence of hippocampal activity in a vagus afferent–dependent pathway.
All observed networks were consistent with those described for murine resting-state fMRI (17). Based on our previous results, we focused on the hippocampal brain network that emerged from the group analysis (Fig. 1A). A subsequent inspection of BOLD time courses of correlating hippocampal regions in single subjects revealed considerable changes in response to the increase in tidal volume in all animals (Fig. 1B).
Figure 1.
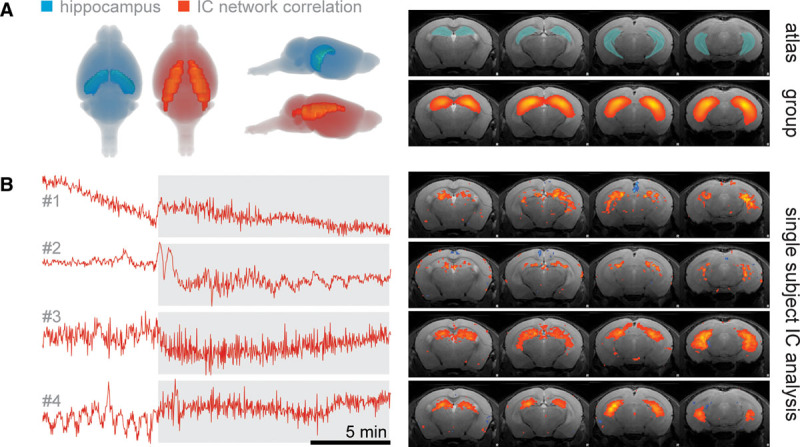
Hippocampal brain network response upon elevation in tidal volume revealed by using independent component analysis (ICA) on functional MRI data. A, Network activity calculated by correlations of signal time courses in ICA group analysis of four mice shows great overlaps with the anatomical structure of the hippocampus. Gray shadings behind mouse brains (left) indicate the range of coronal brain slices (right). B, Signal time courses of independent components (ICs) extracted from the hippocampal network in four single subjects (see right for the spatial distribution of respective IC).
Blockade of Lung Vagus Nerve Afferent Activity by Lidocaine Prevents Experimental VIBI In Vivo
In an effort to test whether vagal nerve stimulation is linked to the generation of VIBI, we treated mice with lidocaine at a dose of 7 mL/kg either intratracheally (it-Lido) or IV (iv-Lido) prior to MV. We hypothesized that pan-blockade of sodium ion (Na+) channels by lidocaine would impair depolarization needed for neuronal triggering, impairing lung-mediated vagal signaling and therefore VIBI. It-Lido but not iv-Lido attenuated the effects of MV on the hippocampal AKT/GSK3β/PARP-1 pathway (Fig. 2A–D). Intratracheal lidocaine also improved survival in mechanical ventilated animals (Fig. 2E). Of note, the levels of proinflammatory cytokines interleukin-1, interleukin-6, and tumor necrosis factor (TNF)-α in the hippocampal formation were elevated in it-Lido and iv-Lido animals compared with sham and ventilated animals (Supplemental Digital Content 1, http://links.lww.com/CCM/E873). In order to assess potential brain cytotoxic side effects of lidocaine, we measured lidocaine levels in brain, lung, and serum and its dependence on the route of administration. Levels of lidocaine within the hippocampus were higher in the iv-Lido compared with it-Lido directly (Supplemental Digital Content 2, http://links.lww.com/CCM/E874). In summary, pan-blockade of neuronal transmission using intratracheal lidocaine protected against VIBI.
Figure 2.
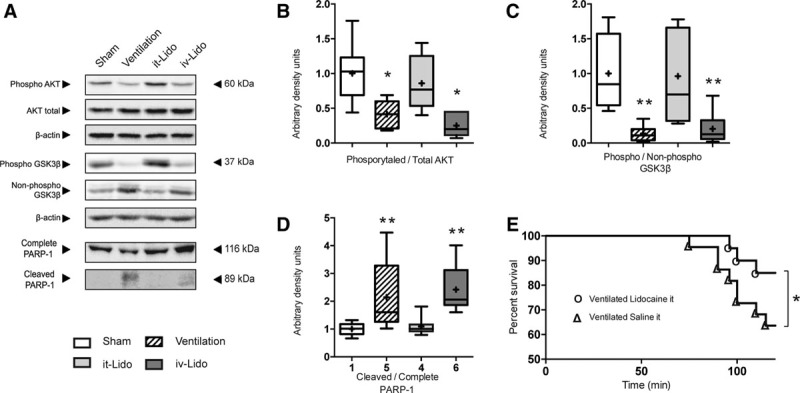
Effects of lidocaine over ventilator-induced brain injury (VIBI). A–D, Representative Western blots and densitometric quantification showing the beneficial effects of intratracheal but not IV lidocaine over the prosurvival protein kinase B (AKT)/glycogen synthase kinase-3β (GSK3β) pathway and the apoptotic pathway (n = 8). The changes triggered by mechanical ventilation in B, phosphorylation levels of AKT at its serine 473; C, phosphorylation levels of GSK3β at its serine 9; and D, cleaved poly(adenosine diphosphate-ribose) polymerase-1 (PARP-1) were significantly mitigated by treatment with intratracheal lidocaine (7 mg/kg) but not IV lidocaine (7 mg/kg). *p < 0.05 in parametric post hoc test versus the sham group. **p < 0.05 in nonparametric post hoc test versus the sham group. E, Survival study (n = 20) shows improved survival to mechanical ventilation in the intratracheal lidocaine group. *p < 0.05 log rank (Mantel-Cox). it-Lido = lidocaine at a dose of 7 mL/kg intratracheally, iv-Lido = lidocaine at a dose of 7 mL/kg IV.
MV Activates Lung TRPV4 in Mice and Mechanically Ventilated Patients
In order to study the molecular mechanisms triggering vagus nerve stimulation during MV, we focused on the role of the lung TRPV4-ATP-P2X signaling pathway. We hypothesized that activation of lung mechanosensitive TRPV4 channels, subsequent release of ATP to the alveolar lumen, and the activation of the purinergic P2X channels present on the afferent neurons of the respiratory tract represent a sequence of events involved in vagal nerve stimulation causing VIBI.
MV, with or without intratracheal lidocaine administration, resulted in activation of lung TRPV4, demonstrated by the increased phosphorylation at its serine residue 824 (16) (Fig. 3A). MV also reduced Trpv4 RNA expression (Supplemental Digital Content 3, http://links.lww.com/CCM/E875). To demonstrate the mechanical nature of these results, human lung epithelial cells BEAS-2B were submitted to a biaxial cyclic stretch for 2 hours, also in the presence of absence of lidocaine, confirming in vivo results (Fig. 3B).
Figure 3.
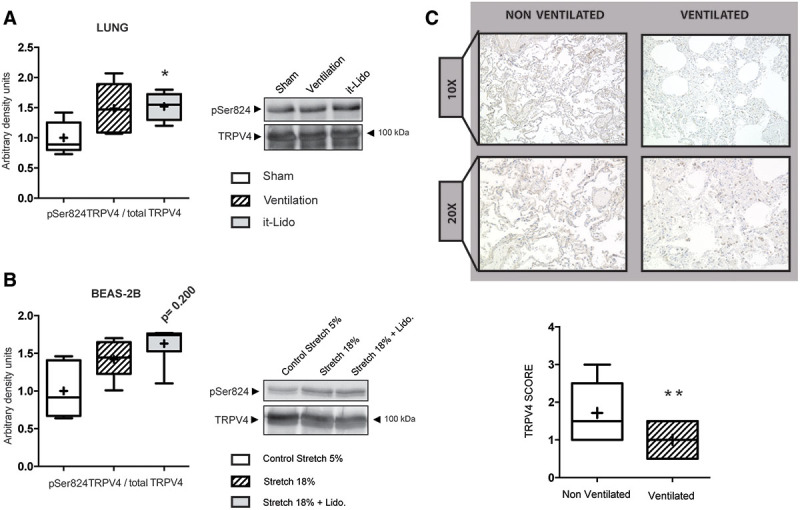
Effects of mechanical ventilation and mechanical cyclic stretch over transient receptor potential vanilloid 4 (TRPV4) phosphorylation. A, Representative Western blots and densitometric quantification showing mechanical ventilation–related increased TRPV4 activation/phosphorylation (serine 824 (serine 824 [pSer824]) in mouse lungs (n = 6). B, Representative Western blots and densitometric quantification showing stretch-induced activation/phosphorylation of TRPV4 at its serine 824 (pSer824) in human epithelial cell line BEAS-2B (n = 5). *p < 0.05 in parametric post hoc test versus the sham group. C, TRPV4 immunohistochemistry of lung sections from lung autopsies obtained from nonventilated (n = 7) and mechanically ventilated patients (n = 10). Immunoreactivity in the ventilated patients is lower compared with the nonventilated patients. The intensity of immunoreaction was semiquantitatively analyzed. **p < 0.05 in Mann-Whitney U test. it-Lido = lidocaine at a dose of 7 mL/kg intratracheally, Lido = lidocaine.
The effects of MV on the TRPV4 activation were confirmed by ATP quantification. Free ATP levels in BALF were elevated after MV and remained elevated in the it-Lido group. In contrast, MV of TRPV4-deficient mice did not resulted in increased ATP levels in BALF (Supplemental Digital Content 4, http://links.lww.com/CCM/E876). Taken together, these data are consistent with the hypothesis that MV activates TRPV4 channels leading to an alveolar release of ATP.
Mechanically ventilated patients usually receive longer ventilation periods than the ones used in our animal model. To overcome this time limitation and to probe for the clinical validity of our findings, we measured for TRPV4 protein content in postmortem lung sections from critically ill, either mechanically ventilated (n = 11) and nonventilated patients (n = 7). Clinical data of these patients are included in Supplemental Digital Content 5 (http://links.lww.com/CCM/E877). Ventilated patients showed a significant reduction in lung TRPV4 immunoreactivity (Fig. 3C).
MV Regulates Lung P2x Channel Expression
Gene expression of the seven P2x subfamilies was studied in lung tissue from wild-type sham, ventilated and it-Lido mice. RNA levels of most of the P2x channels were reduced in mechanically ventilated animals compared with sham (Supplemental Digital Content 6, http://links.lww.com/CCM/E878), showing an effect similar to that observed in Trpv4 expression. Lidocaine treatment maintained expression of most of the P2x subfamilies at levels similar to sham, with the exception of P2x1 and P2x3. Similar results were found in human respiratory epithelial cells (BEAS-2B) submitted to cyclic mechanical stretch (Supplemental Digital Content 3, http://links.lww.com/CCM/E875). Hippocampal expression of P2X channels was not affected by MV (Supplemental Digital Content 7, http://links.lww.com/CCM/E879).
Absence of TRPV4 Protects Against VIBI by Decreasing Free ATP Alveolar Levels
To confirm the role of lung TRPV4 in the development of VIBI, Trpv4–/– mice were randomly assigned to three different groups: sham, MV, and intratracheal instillation of exogenous ATP prior to MV (it-ATP). Hippocampal markers of VIBI (pAKT/AKT, pGSK3β/GSK3β, and cleaved PARP-1/PARP-1 protein ratios) were similar between sham and ventilated Trpv4–/– mice (Fig. 4A–D). Overall, RNA expression of lung P2x subfamilies was less affected by MV in Trpv4–/– mice (Supplemental Digital Content 8, http://links.lww.com/CCM/E880). Furthermore, addition of intratracheal ATP prior MV in Trpv4–/– mice restored the effects previously observed in wild-type ventilated animals and re-established VIBI.
Figure 4.
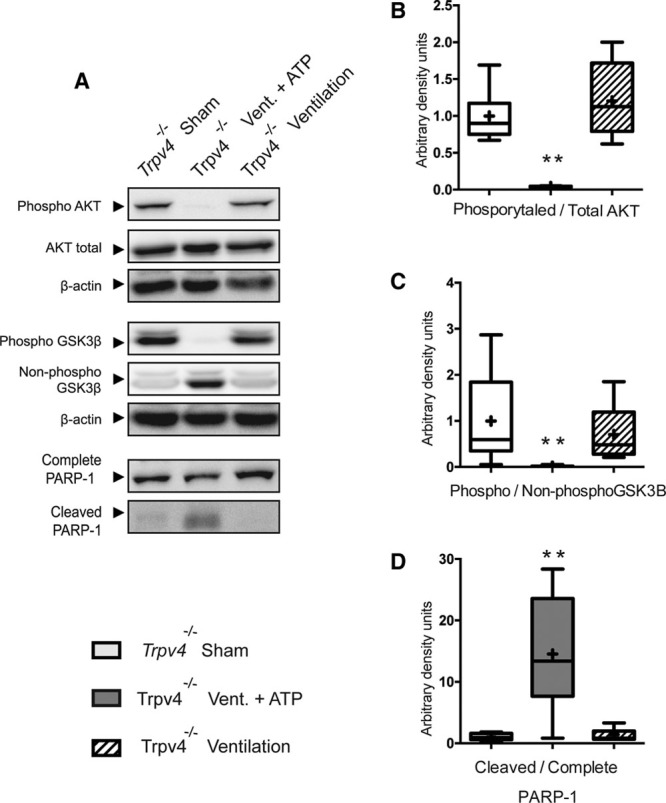
Transient receptor potential vanilloid 4 (TRPV4) deficiency–related protection against ventilator-induced brain injury (VIBI) is lost after adding intratracheal exogenous adenosine triphosphate (ATP). Trpv4 knockout mice were significantly protected against VIBI. Addition of intratracheal ATP (100 μg/kg) prior to ventilation (Vent.) of Trpv4 knockout mice leads to restoration of acute VIBI (n =7). A, Representative Western blots. B, Densitometry analysis of phosphorylation levels of protein kinase B (AKT) at its serine 473. C, Densitometry analysis of phosphorylation levels of glycogen synthase kinase-3β (GSK3β) at its serine 9. D, Densitometry analysis of cleaved poly(adenosine diphosphate-ribose) polymerase-1 (PARP-1). **p < 0.05 in nonparametric post hoc test versus the sham group.
Pan-Inhibition of P2X Channels Protects Against VIBI
As increased lung luminal ATP levels may trigger vagal sensory nerves via P2X receptors, we next tested whether blockade of lung purinergic receptors may abolish VIBI. Administration of iso-PPADS (a nonselective P2X-purinoceptor antagonist) prior to MV resulted in amelioration of VIBI in a similar fashion as the administration of intratracheal lidocaine. Protein ratios of hippocampal pAKT/AKT, pGSK3β/GSK3β, and cleaved PARP-1/PARP-1 showed no significant difference between sham and iso-PPADS ventilated animals (Fig. 5A–D), indicating a protective effect of this drug against VIBI. Regarding lung P2x channels expression, purinoceptor blockade also mimicked the effects observed previously when using intratracheal lidocaine (Supplemental Digital Content 6, http://links.lww.com/CCM/E878).
Figure 5.
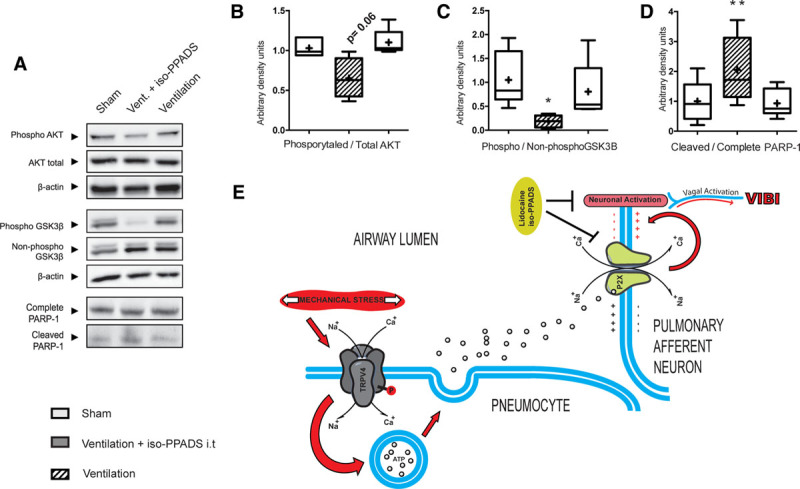
triggering mechanisms within the lung. The mechanical stretch–caused ventilation (Vent.) cause phosphorylation/activation of transient receptor potential vanilloid 4 (TRPV4) channels. Increased activation of TRPV4 leads to release of adenosine triphosphate (ATP) to the alveoli that in turn activates the P2X purinergic channels present in the lung tissue including the sensory nerves. The increased influx of sodium ion (Na+) and calcium ion (Ca+) would trigger lung sensory neurons that would carry on the signal to the brain through the afferent vagus nerve triggering VIBI. *p < 0.05 in parametric post hoc test versus the sham group. **p < 0.05 in nonparametric post hoc test versus the sham group.
Interestingly, ventilated animals treated with iso-PPADS presented lower levels of interleukin-6, interleukin-1β, and TNF-α in the hippocampus when compared with it-Lido or mechanically ventilated animals, reaching levels similar to the sham group in most cases (Supplemental Digital Content 1, http://links.lww.com/CCM/E873). Furthermore, unlike the use of intratracheal lidocaine in ventilated animals, iso-PPADS did not increase lung edema (ventilation-induced lung injury [VILI] parameters can be found in Supplemental Digital Content 9, http://links.lww.com/CCM/E881). Taken together, these data show a protective effect of both iso-PPADS and lidocaine against VIBI.
DISCUSSION
There is strong evidence that MV may cause brain damage by a variety of mechanisms. This study provides evidence that the deleterious effect of MV on the hippocampus is directly related to the activation of a TRPV4-ATP-P2X signaling cascade within the lung tissue (Fig. 5E), which in turn may represent a precipitating factor to the development of the cognitive disorders observed in mechanically ventilated patients.
The vagus nerve represents a major conduit of information between lung and brain. Vagal nerve fibers protrude between epithelial cells of the intrapulmonary airways forming arborized intraepithelial terminals with distinct molecular signatures and receptor characteristics (18, 19). Recently, Zanos et al (20) were able to identify a unique signaling pattern from lung-innervating sensory neurons responding to specific cytokine mediators. The vagus nerve is, therefore, able to detect changes in the lung environment, encode the information based on the triggering factors, and lead the physiologic response in order to reinstate homeostasis. In this context, the TRPV4-ATP-P2X axis might be a part of an encoding network aimed to react to the nonphysiologic stimulus originated by the MV.
Translation of mechanical forces into neuronal activity during MV lays on a close interplay between mechanosensing and the release of mediators. In our study, stretched BEAS-2B epithelial cells and ventilated wild-type animals showed increased activation and decreased expression of TRPV4 channels. Similarly, mechanically ventilated patients showed reduced lung TRPV4 immunostaining compared with nonventilated patients. These data support the notion of a tachyphylaxis-related down-regulation of TRPV4 channels during MV. This phenomenon has been previously observed in experimental models studying vanilloid receptors (21, 22).
ATP release following TRPV4 activation is a key step to activate neural signaling. Our results showed that TRPV4-deficient mice were protected against VIBI. In line with our results, previous studies have shown that fibroblast, alveolar epithelial, and airway smooth muscle cells are able to release ATP in response to mechanical stimuli (23–26) and have documented the role of TRPV4 in ATP release (27). Altered nucleotide presence in airways is a common effect of MV (28) because ATP acts not only as an energy token but also as an autocrine and paracrine damage-associated molecular pattern. Recently, Hasan et al (29) reported that excessive extracellular ATP is also associated with surfactant impairment, thereby promoting VILI.
MV also alters the expression of the P2x purinergic receptor family. Purinergic receptors have been shown to have an important role in several pulmonary diseases (27, 30–33) also participating in mechanosensory functions of the pain- and stretch-sensing neurons (34–36). In the present study, The P2X receptor antagonists lidocaine and iso-PPADS significantly ameliorated VIBI in wild-type ventilated mice.
Although intratracheal instillation of lidocaine proved successful, IV administration did not. The lack of IV effect could be related to a dose-response effect, and it should be taking in consideration that IV dosages needed to obtain effects over VIBI might trigger neurotoxicity side effects. Furthermore, the protective effects observed in the it-Lido group might not limited to a pan-blockade of Na+ channels. Recent reports also describe that lidocaine has a direct inhibitory effect on the purinergic P2X channels (37). Another important result of our study was that P2X channel antagonists iso-PPADS reduced VIBI in a similar way as lidocaine although not being associated with secondary effects of lidocaine such as neurotoxicity or lung edema formation (38). Finally, it is important to mention the role of vagal nerve signaling in the anti-inflammatory cholinergic reflex. Bilateral vagotomy prior to MV has been shown to protect against VIBI in mice (6) but also to have deleterious effects on the lung in VILI models (4). The timing of MV in our model was relatively short because the model aimed at deciphering the neurologic pathways of VIBI, with no intention to induce VILI. We cannot discard differences in hemodynamics that could play a role in the development of VIBI. However, the differences in wild-type and knockout animals subjected to the same ventilator protocol and the abolition of VIBI using drugs with no known vasoactive effects support our conclusions.
In conclusion, we have identified a novel mechanism that explains how VIBI is triggered within the lung in response to mechanical stretch. The interplay among mechanical stretch, mechanogated TRPV4 channels, ATP release, and purinergic P2X channels activation during MV supports the existence of a lung neuronal signaling axis. Presence and activation of such a TRPV4-ATP-P2X signaling axis are also supported by TRPV4 immunoreactivity of lung autopsies from ventilated patients. A supplementary extended version of the methods can be viewed in Supplemental Digital Content 11 (http://links.lww.com/CCM/E883).
REFERENCES
- 1.Marra A, Pandharipande PP, Girard TD, et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med 2018; 46:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandharipande PP, Girard TD, Jackson JC, et al. ; BRAIN-ICU Study Investigators: Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369:1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavallazzi R, Saad M, Marik PE. Delirium in the ICU: An overview. Ann Intensive Care 2012; 2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.dos Santos CC, Shan Y, Akram A, et al. Neuroimmune regulation of ventilator-induced lung injury. Am J Respir Crit Care Med 2011; 183:471–482 [DOI] [PubMed] [Google Scholar]
- 5.Quilez ME, Fuster G, Villar J, et al. Injurious mechanical ventilation affects neuronal activation in ventilated rats. Crit Care 2011; 15:R124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-López A, López-Alonso I, Aguirre A, et al. Mechanical ventilation triggers hippocampal apoptosis by vagal and dopaminergic pathways. Am J Respir Crit Care Med 2013; 188:693–702 [DOI] [PubMed] [Google Scholar]
- 7.Martire S, Mosca L, d’Erme M. PARP-1 involvement in neurodegeneration: A focus on Alzheimer’s and Parkinson’s diseases. Mech Ageing Dev 2015; 146-148:53–64 [DOI] [PubMed] [Google Scholar]
- 8.Giese KP. GSK-3: A key player in neurodegeneration and memory. IUBMB Life 2009; 61:516–521 [DOI] [PubMed] [Google Scholar]
- 9.Baxter M, Eltom S, Dekkak B, et al. Role of transient receptor potential and pannexin channels in cigarette smoke-triggered ATP release in the lung. Thorax 2014; 69:1080–1089 [DOI] [PubMed] [Google Scholar]
- 10.Mihara H, Suzuki N, Boudaka AA, et al. Transient receptor potential vanilloid 4-dependent calcium influx and ATP release in mouse and rat gastric epithelia. World J Gastroenterol 2016; 22:5512–5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman M, Sun R, Mukherjee S, et al. TRPV4 stimulation releases ATP via pannexin channels in human pulmonary fibroblasts. Am J Respir Cell Mol Biol 2018; 59:87–95 [DOI] [PubMed] [Google Scholar]
- 12.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blázquez-Prieto J, López-Alonso I, Amado-Rodríguez L, et al. Exposure to mechanical ventilation promotes tolerance to ventilator-induced lung injury by Ccl3 downregulation. Am J Physiol Lung Cell Mol Physiol 2015; 309:L847–L856 [DOI] [PubMed] [Google Scholar]
- 14.Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990; 87:9868–9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 2010; 20:519–534 [DOI] [PubMed] [Google Scholar]
- 16.Michalick L, Erfinanda L, Weichelt U, et al. Transient receptor potential vanilloid 4 and serum glucocorticoid-regulated kinase 1 are critical mediators of lung injury in overventilated mice in vivo. Anesthesiology 2017; 126:300–311 [DOI] [PubMed] [Google Scholar]
- 17.Grandjean J, Zerbi V, Balsters JH, et al. Structural basis of large-scale functional connectivity in the mouse. J Neurosci 2017; 37:8092–8101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brouns I, Adriaensen D, Burnstock G, et al. Intraepithelial vagal sensory nerve terminals in rat pulmonary neuroepithelial bodies express P2X(3) receptors. Am J Respir Cell Mol Biol 2000; 23:52–61 [DOI] [PubMed] [Google Scholar]
- 19.Chang RB, Strochlic DE, Williams EK, et al. Vagal sensory neuron subtypes that differentially control breathing. Cell 2015; 161:622–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanos TP, Silverman HA, Levy T, et al. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc Natl Acad Sci U S A 2018; 115:E4843–E4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci 2005; 25:8924–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alessandri-Haber N, Yeh JJ, Boyd AE, et al. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 2003; 39:497–511 [DOI] [PubMed] [Google Scholar]
- 23.Grygorczyk R, Furuya K, Sokabe M. Imaging and characterization of stretch-induced ATP release from alveolar A549 cells. J Physiol 2013; 591:1195–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol 1997; 272:C1058–C1066 [DOI] [PubMed] [Google Scholar]
- 25.Takahara N, Ito S, Furuya K, et al. Real-time imaging of ATP release induced by mechanical stretch in human airway smooth muscle cells. Am J Respir Cell Mol Biol 2014; 51:772–782 [DOI] [PubMed] [Google Scholar]
- 26.Murata N, Ito S, Furuya K, et al. Ca2+ influx and ATP release mediated by mechanical stretch in human lung fibroblasts. Biochem Biophys Res Commun 2014; 453:101–105 [DOI] [PubMed] [Google Scholar]
- 27.Bonvini SJ, Birrell MA, Grace MS, et al. Transient receptor potential cation channel, subfamily V, member 4 and airway sensory afferent activation: Role of adenosine triphosphate. J Allergy Clin Immunol 2016; 138:249–261.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douillet CD, Robinson WP, III, Zarzaur BL, et al. Mechanical ventilation alters airway nucleotides and purinoceptors in lung and extrapulmonary organs. Am J Respir Cell Mol Biol 2005; 32:52–58 [DOI] [PubMed] [Google Scholar]
- 29.Hasan D, Satalin J, van der Zee P, et al. Excessive extracellular ATP desensitizes P2Y2 and P2X4 ATP receptors provoking surfactant impairment ending in ventilation-induced lung injury. Int J Mol Sci 2018; 19:pii: E1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders AP, Baylin GJ. A common denominator in the etiology of adult respiratory distress syndrome. Med Hypotheses 1980; 6:951–965 [DOI] [PubMed] [Google Scholar]
- 31.Riteau N, Gasse P, Fauconnier L, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 2010; 182:774–783 [DOI] [PubMed] [Google Scholar]
- 32.Vieira RP, Müller T, Grimm M, et al. Purinergic receptor type 6 contributes to airway inflammation and remodeling in experimental allergic airway inflammation. Am J Respir Crit Care Med 2011; 184:215–223 [DOI] [PubMed] [Google Scholar]
- 33.Matsuyama H, Amaya F, Hashimoto S, et al. Acute lung inflammation and ventilator-induced lung injury caused by ATP via the P2Y receptors: An experimental study. Respir Res 2008; 9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook SP, McCleskey EW. Desensitization, recovery and Ca(2+)-dependent modulation of ATP-gated P2X receptors in nociceptors. Neuropharmacology 1997; 36:1303–1308 [DOI] [PubMed] [Google Scholar]
- 35.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes–a possible sensory mechanism? J Physiol 1997; 505(Pt 2):503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight GE, Bodin P, De Groat WC, et al. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol 2002; 282:F281–F288 [DOI] [PubMed] [Google Scholar]
- 37.Okura D, Horishita T, Ueno S, et al. Lidocaine preferentially inhibits the function of purinergic P2X7 receptors expressed in xenopus oocytes. Anesth Analg 2015; 120:597–605 [DOI] [PubMed] [Google Scholar]
- 38.Rooke NT, Milne B. Acute pulmonary edema after regional anesthesia with lidocaine and epinephrine in a patient with chronic renal failure. Anesth Analg 1984; 63:363–364 [PubMed] [Google Scholar]


