Serotonin drugs, including clemizole, have recently emerged as potential treatment options for Dravet syndrome. We synthesized a library of clemizole analogues and screened for anti-seizure activity using the zebrafish Dravet syndrome model. Coupled with in vitro binding, we identified 5-HT2B receptors as a mediator in the mechanism of seizure suppression.
Keywords: Chemical biology, epilepsy, serotonin, electrophysiology, drug development
Abstract
Dravet syndrome is a life-threatening early-onset epilepsy not well controlled by antiepileptic drugs. Drugs that modulate serotonin (5-HT) signalling, including clemizole, locaserin, trazodone and fenfluramine, have recently emerged as potential treatment options for Dravet syndrome. To investigate the serotonin receptors that could moderate this antiepileptic activity, we designed and synthesized 28 novel analogues of clemizole, obtained receptor binding affinity profiles, and performed in vivo screening in a scn1lab mutant zebrafish (Danio rerio) model which recapitulates critical clinical features of Dravet syndrome. We discovered three clemizole analogues with 5-HT receptor binding that exert powerful antiepileptic activity. Based on structure–activity relationships and medicinal chemistry-based analysis, we then screened an additional set of known 5-HT receptor specific drug candidates. Integrating our in vitro and in vivo data implicates 5-HT2B receptors as a critical mediator in the mechanism of seizure suppression observed in Dravet syndrome patients treated with 5-HT modulating drugs.
Graphical Abstract
Graphical Abstract.
Introduction
The discovery of antiepileptic drugs (AEDs) has traditionally relied upon acute seizure models in adult rodents. Unfortunately, this approach does not incorporate genetic epilepsies like those associated with de novo gene mutations and classified as catastrophic childhood epileptic encephalopathies (Löscher, 2017a,b). In vivo drug discovery and development using phenotype-based screening in genetically modified larval zebrafish (Danio rerio) offer a promising ‘precision medicine’ focused alternative (Baraban et al., 2013; Dinday and Baraban, 2015; Griffin et al., 2016; Grone et al., 2017). Drug candidates identified using in vivo screens provide valuable biological context and offer a more successful route to clinical implementation. As an example, about 62% of first-in-class small molecules registered by the Food and Drug Administration between 1999 and 2008 had their origin in a phenotype-based approach compared to only 38% in target-based drug discovery (Swinney and Anthony, 2011). Zebrafish are uniquely suited to phenotype-based in vivo drug discovery programs and are highly amenable to the study of structure–activity relationships that represent a critical step in the drug development process (Zon and Peterson, 2005).
Dravet syndrome is a medically refractory catastrophic epileptic encephalopathy. Patients typically exhibit frequent episodes of prolonged seizures before the age of one (Dravet, 2011), and have an increased risk of Sudden Unexplained Death in Epilepsy (Cooper et al., 2016). The majority of Dravet syndrome patients are identified with de novo mutations within the SCN1A gene resulting in haploinsufficiency of a neuronal voltage-gated sodium channel (Catterall et al., 2010; Escayg and Goldin, 2010; Dravet and Oguni, 2013). Current Food and Drug Administration-approved antiepileptic drugs are unable to provide adequate seizure control in this patient population. The lack of effective seizure control using available antiepileptic drugs has resulted in a significant effort to discover and develop new treatment options for these patients (Ziobro et al., 2018). Although genetically modified mouse models and induced pluripotent stem cell derived neurons exist to model features of Dravet syndrome, large-scale drug discovery and development in these systems is lacking. As an alternative, larval zebrafish with a mutation in the SCN1A homologue, scn1lab, display persistent spontaneous electrographic seizures and convulsive swim behaviours, as early as 3 days post-fertilization (Baraban et al., 2013). Importantly, these spontaneous seizures in scn1lab mutant zebrafish exhibit pharmaco-resistance to 13 antiepileptic drugs (Baraban et al., 2013; Ziobro et al., 2018). As such, this zebrafish model faithfully mimics the epileptic phenotype observed in Dravet syndrome patients. Using scn1lab zebrafish and a two-stage in vivo phenotype-based platform, blind screening of more than 3500 compounds identified serotonin (5-HT) receptor agonists (clemizole, trazodone and lorcaserin) and a serotonin reuptake inhibitor (fenfluramine) as potential new Dravet syndrome therapies (Baraban et al., 2013; Dinday and Baraban, 2015; Sourbron et al., 2016; Griffin et al., 2017). Furthermore, limited clinical studies of lorcaserin (Griffin et al., 2017; Tolete et al., 2018), trazodone (Kauppila et al., 2018), and fenfluramine (Ceulemans et al., 2012; Ceulemans et al., 2016; Schoonjans et al., 2017) have demonstrated anti-seizure activity in Dravet syndrome patients.
Serotonin receptors (5-HTR) represent a group of G-protein coupled receptors and ligand-gated ion channels found in the central and peripheral nervous system (Barnes and Sharp, 1999). 5-HTRs can be divided into seven subtypes, with subunits 5-HT1–7 receptors expressed in neurons of the central nervous system, and 5-HT1AR, 5-H2AR and 5-H2BR also present on astrocytes (Carson et al., 1996; Zhang et al., 2010; Miyazaki and Asanuma, 2016). Since the 1970s, there has been a growing body of evidence to implicate 5-HT with seizure susceptibility; however, the precise mechanism remains elusive. Increased brain 5-HT was reported to reduce the severity of audiogenic- and chemoconvulsant-induced seizures (De la Torre et al., 1970; De la Torre and Mullan, 1970), as well as increase the threshold to electroshock-induced seizures (Kilian and Frey, 1973). More recently, 5-HT2CR knockout mice show spontaneous seizures, premature death (Tecott et al., 1995) and are more sensitive to electroshock- or chemoconvulsant-induced seizure protocols (Applegate and Tecott, 1998). Furthermore, studies in DBA/2 mice support a role for 5-HTR modulation in Sudden Unexplained Death in Epilepsy (Tupal and Faingold, 2006; Uteshev et al., 2010).
Here, we report a structure–activity relationship study designed to test the hypothesis that 5-HTRs are a critical site of action for drugs showing anti-seizure activity in Dravet syndrome patients. We synthesized 28 novel clemizole analogues (clemalogues) with varying 5-HT2R binding affinities and identified three clemalogues exerting a powerful suppression of convulsive swim behaviour and electrographic seizure activity in scn1lab zebrafish. Based on these 5-HT2R properties, a medicinal chemistry structure–activity relationship-based analysis led to a second round of screening, and identification of two 5-HT2BR agonists (methylergonovine and 6-(2-aminopropyl)benzofuran (6-APB)) that also suppressed convulsive swim behaviour and electrographic seizure activity. Taken together, these findings have implications for the mechanism of action of 5-HT2R modulating drugs (i.e. clemizole, lorcaserin, trazodone and fenfluramine) currently in clinical development for Dravet syndrome (Knupp and Wirrell, 2018; Ziobro et al., 2018) and for future research into how 5-HT signalling may influence seizure activity.
Materials and methods
Chemical synthesis and compounds
Initially, clemizole analogues were synthesized in house at the UCSF Small Molecule Discovery Center for functional testing. Select compounds were also synthesized independently by Oxygen Healthcare Research Pvt. Ltd. for confirmation purposes using the same UCSF methodology outlined. Chemical reagents and solvents used here are commercially available, unless stated otherwise. For all air and/or moisture sensitive reactions experiments were performed under an argon atmosphere using oven-dried glassware and commercially available anhydrous solvents. All reagents that were deemed air and/or moisture sensitive were transferred via syringe or cannula through rubber septa into the reaction vessel. A rotary evaporator, set to ca. 10–50 Torr, was used for solvent removal. A Varian INOVA-400 400 MHz spectrometer was used to measure 1H nuclear magnetic resonance (NMR) spectra and reported in δ units (ppm). NMR spectra were referenced relative to residual NMR solvent peaks and coupling constants (J) are reported in hertz (Hz). A CEM Discover microwave reactor was used to carry out microwave reactions. An Isolera Four flash chromatography system and SiliaSep silica gel cartridges (Silicycle) were used for column chromatography. A Waters Micromass ZQ mass spectrometer equipped with Waters 2795 Separation Module, Waters 2424 Evaporative Light Scattering Detector, and Waters 2996 Photodiode Array Detector was used to acquire liquid chromatography/mass spectrometry data. Separations were performed on a XTerra® MS C18, 5 µm, 4.6 × 50 mm column, at ambient (unregulated) temperature using a mobile phase of water–methanol containing 0.1% formic acid. Details of synthesis steps for each distinct synthetic route employed are described in the Supplementary material.
Compounds were commercially sourced from Millipore Sigma [Methylergonovine maleate, BW-723C86, 1-(3-chlorophenyl)piperazine hydrochloride (m-CPP), (+)-Norfenfluramine hydrochloride], Cayman Chemicals [Cabergoline, Bromocriptine mesylate, (−)-Apomorphine hydrochloride], ApexBio (Ro 60-0175 fumarate), Tocris Bioscience (CP-809, 101 hydrochloride), AK Scientific, Inc. (Piribedil) and Axon Medchem (TL 99 hydrobromide). Ten millimolars of compound stock solutions were made in dimethyl sulfoxide and then diluted in embryo medium for assays.
Zebrafish maintenance
Zebrafish (D. rerio) was maintained in our zebrafish facility on a 14/10 h light dark cycle. Experimental procedures followed the Guide for the Care and Use of Animals (ebrary Inc. 2011) and were approved by the Institutional Animal Care and Use Committee (protocol # AN108659-03). Embryos were obtained by natural spawning of adult heterozygous scn1lab (didys552) animals maintained on a Tubingen long fin strain background. Starting at 3 days post-fertilization homozygous scn1lab mutants (n = 2500) appear visibly darker than age-matched wild-type larvae.
Seizure monitoring
For locomotion studies, zebrafish larvae (5 days post-fertilization) were placed into a single well of a clear flat-bottomed 96-well microplate containing embryo media. Sex determination is not possible at this stage of development. All drug screening experiments were conducted in an unbiased manner by investigators blinded to the test compounds and all files coded for post hoc analysis. The 96-well plate containing larvae was then placed inside a DanioVision box where they were allowed to acclimate (20 min; room temperature). EthoVision XT software (DanioVision, Noldus Information Technology) was used to obtain locomotion plots (10 min in duration). Seizure scoring was performed, as described (Baraban et al., 2005). All locomotion plots were analysed for distance travelled (in millimetres) and mean velocity (in millimetres per second). After 90 min of drug exposure larvae were examined for toxic side-effects. Compounds that decreased or stopped the larva heartbeat, or reduced or eliminated the escape response when touched, were considered toxic.
For electrophysiology studies, zebrafish larvae were anaesthetized with cold by placing at 4°C for 5 min until no movement was observed and then immobilized in 1.2% agarose dorsal side up. Using a glass micro-electrode positioned under an upright microscope, local field potential (LFP) recordings were obtained from forebrain or optic tectum structures, as described (Baraban et al., 2005). Agarose-embedded LFP recording sessions (10 min in duration) were obtained using Axoclamp software (Molecular Devices; Sunnyvale, CA, USA) and sampled at 1 kHz. Epileptiform events were identified post hoc. These were classified as multi-spike or poly-spike upward or downward membrane deflections greater than 3× baseline noise level and 150–250 ms in duration (interictal-like) or greater than 5× baseline noise, multi-spike and >500 ms in duration (ictal-like); both events were counted using threshold detection settings in Clampfit (Molecular Devices; Sunnyvale, CA, USA). Agarose-embedded larvae were continuously monitored for blood flow and heart rate using an Axiocam digital camera at video frame rate.
Receptor binding assays
All in vitro binding assay and Ki data studies were performed by the US National Institute of Mental Health Psychoactive Drug Screening Program (NIMH PDSP). For these studies, drugs were screened against recombinant, stably expressed human 5-HT2AR, 5-HT2BR, 5-HT2CR and H1, as described at https://pdspdb.unc.edu/pdspWeb/ (Besnard et al., 2012).
Statistical analysis
For behaviour analysis, the threshold for a change in mean swim velocity ≥40% is considered significant (>1.5× SD of 250 control treated scn1lab). Unless otherwise indicated, all data in this manuscript are presented as the mean ± standard error of the mean. For comparison between more than two groups a one-way analysis of variance test was performed. n the A non-parametric Kruskal–Wallis test was used followed by Dunns multiple comparison test for variance data that did not exhibit a normal distribution. Statistically significant differences are indicated with asterisks (*P < 0.05; **P < 0.01; ***P < 0.001).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and Supplementary material.
Results
Design, synthesis and whole-organism screening of clemizole analogues
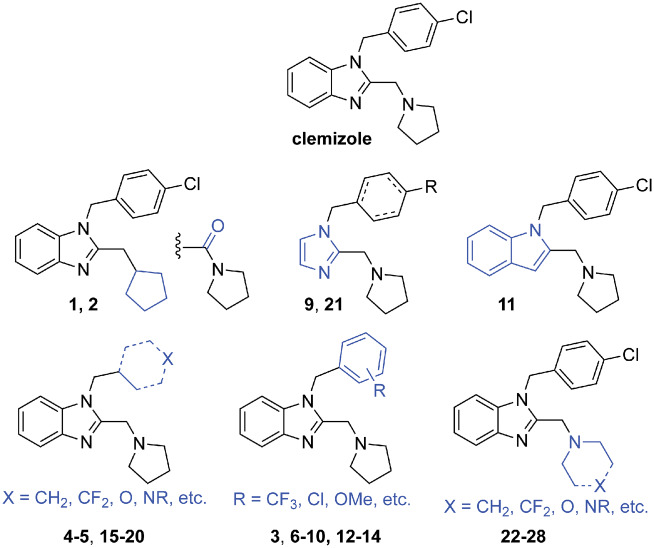
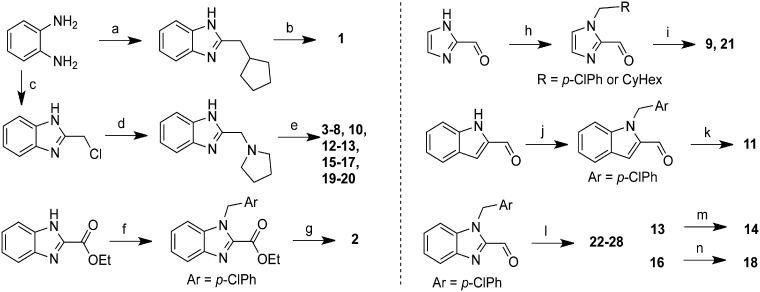
To identify a key pharmacophore responsible for suppressing seizure activity in Dravet syndrome zebrafish, we began with structural optimization of the hit compound clemizole hydrochloride, a first-generation antihistamine shown to inhibit seizures in this model (Baraban et al., 2013). We systematically modified both the benzyl and pyrrolidine side chains in clemizole, as well as the benzimidazole core (Fig. 1). Twenty-eight analogues were prepared, in 1–3 steps, using one of the six synthetic routes shown (Fig. 2 and Supplementary Table S1).
Figure 1.
Structure of clemizole and clemalogues 1–28. Chemical structure of clemizole and 28 clemizole analogues synthesized as part of SAR studies detailed herein. Sites of modification to the clemizole structure are highlighted in blue for each clemalogue subtype shown. Full chemical structures are provided in Supplementary Table S1.
Figure 2.
Synthesis of clemalogues. Summary of synthetic routes used to prepare clemalogues 1–28. (a) cyclopentylacetic acid, PPA, μw, 80°C, 20%; (b) ClCH2(4-ClPh), K2CO3, DMF, 60°C, 67%; (c) ethyl 4-Cl-3-oxobutanoate, SnCl2, EtOH, 80°C; (d) pyrrolidine, EtOH, 95°C; 83% for two steps (e) Br-CH2R, NaH, THF, 0°C to rt, 15–60% or Br-CH2R, NaH, TBAI, THF, 0°C to rt, 13–54% or propyl iodide, NaH, THF, 0°C to rt, 47%;(f) p-Cl-benzylchloride, NaH, DMF, rt, 54%; (g) (i) LiOH, MeOH/water, rt, (ii) pyrrolidine, HATU, DIEA, DMF, rt, 43% over 2 steps; (h) p-Cl-benzylchloride, K2CO3, CH3CN, 45°C, 85% or cyclohexylmethyl bromide, K2CO3, TBAI, CH3CN, 55°C, 30%; (i) pyrrolidine, Na(OAc)3BH, CH2Cl2; 30–46%; (j) ClCH2Ar, K2CO3, CH3CN, 45°C, 44%; (k) pyrrolidine, Na(OAc)3BH, CH2Cl2, rt, 65%; (l) HNR2, Na(OAc)3BH, CH2Cl2, rt, 47–85%; (m) H2, Pd/C, MeOH, rt, 55%; (n) 4 M HCl in dioxane, rt.
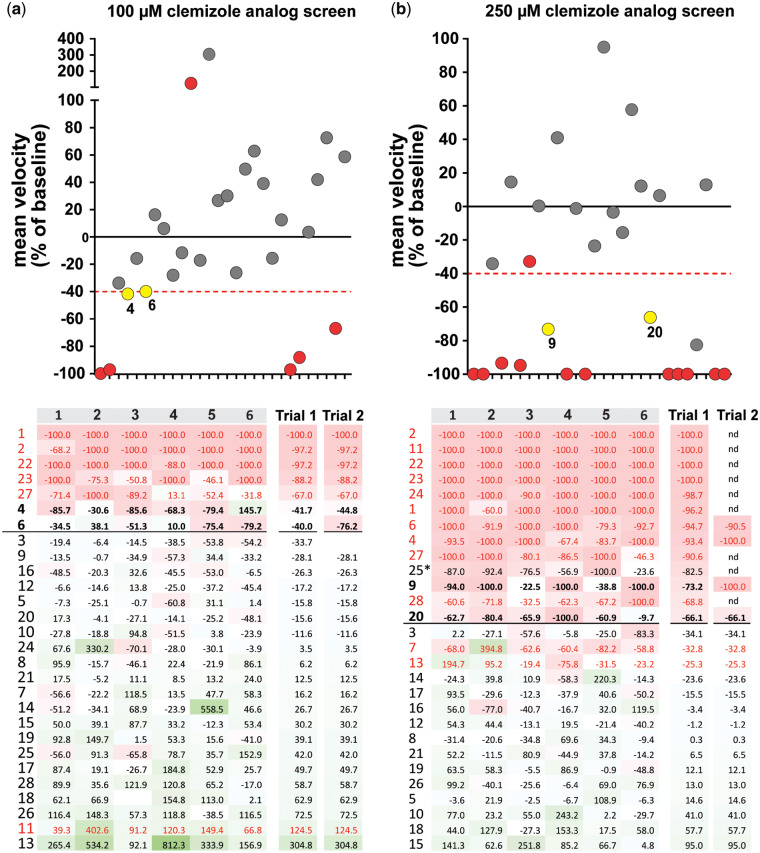
Using the established phenotypic-based screening platform (Baraban et al., 2013), and the previously determined concentration range of the parent compound clemizole (Griffin et al., 2017), we tested the 28 clemizole analogues for their ability to reduce mean swim velocity of 5 days post-fertilization scn1lab mutants at a concentration of 100 µM or 250 µM (six fish per drug treatment). As the initial analogue screening identified non-basic compounds 1 and 2 as toxic, we focused further efforts on analogues that retained the pyrrolidine side chain, or that bore a similar heterocyclic ring with a basic amine. A total of seven such analogues were prepared, among which analogues 22, 23 and 27 bearing six-membered heterocyclic rings as well as acyclic dimethylamino analogue 24 were found to be toxic. In total, five compounds (17.9%) were identified as toxic at 100 µM, which increased to 12 compounds (42.9%) at 250 µM. Subsequent modification of the 4-chlorophenyl ring, or replacement with cycloalkyl ring surrogates resulted in better tolerance in the zebrafish assay, affording several active analogues and few with any notable toxicity, as detailed below.
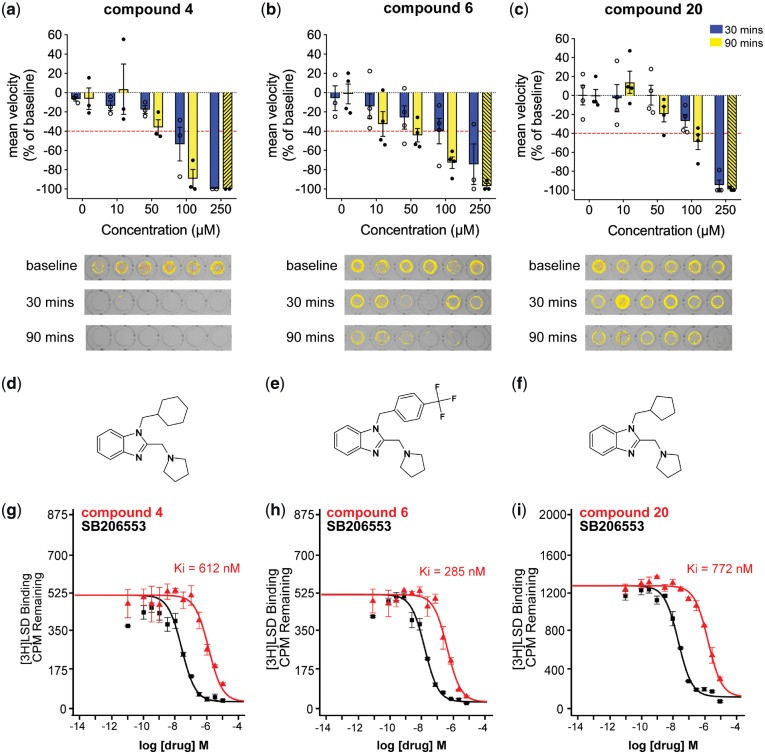
The clemizole analogues 4, 6, 9 and 20 were effective in suppressing the scn1lab mutant seizure-like behaviour at 100 µM or 250 µM, respectively (Fig. 3). To confirm the anti-seizure effect on convulsive behaviours of scn1lab mutants, clemizole analogues 4, 6, 9 and 20 were synthesized independently (by Oxygen Healthcare Research Pvt. Ltd.) using the same methodology as described. Newly synthesized compounds were retested at 10, 50, 100 and 250 µM to confirm a concentration-dependent response. Clemizole analogues 4, 6 and 20 reduced the high-velocity seizure-like swim behaviour observed in the scn1lab mutant zebrafish larvae, confirming the initial screening results with analogues synthesized at UCSF (Fig. 4). The resynthesized compound 9 was toxic at 250 µM. This confirmed the result from the second testing of the original compound and suggests the decrease in swim behaviour observed during trial 1 may be a false positive result. Overall, this preliminary structure–activity relationship study revealed the importance of the pyrrolidine side chain in mitigating toxicity and suggests the side chain at N1 of the benzimidazole (or imidazole) core as a promising avenue for further lead optimization of this chemotype.
Figure 3.
Phenotypic screening of clemizole analogues. Twenty-eight clemizole analogues were screened for efficacy in suppressing the high-velocity seizure-like swim behaviour observed in scn1lab mutant zebrafish. Plots show the change in mean swim velocity of 5 dpf larvae screened at (a) 100 µM, or (b) 250 µM. Threshold for inhibition of seizure activity (positive hits—yellow data points) was determined as a reduction in mean swim velocity of ≥40% (red dashed line). The red data points represent compounds that were classified as toxic after 90-min exposure. The heat map shows the % change in velocity for the six individual larva from the first trial (1–6). Mean velocity change from six individual fish is shown for trial 1 and 2. Clemizole analogue 25 (*) failed to go into solution at 250 µM so it was not considered for further testing.
Figure 4.
Evaluation of clemizole analogues that reduce seizure-like swim behaviour in scn1lab mutant zebrafish. Clemizole analogues identified as positive from the in vivo screen were freshly synthesized and retested for efficacy in suppressing the seizure-like swimming behaviour of 5 dpf scn1Lab mutant zebrafish. Graphs show the change in mean velocity over four concentrations of (a) compound 4, (b) compound 6 and (c) compound 20. Each bar represents the mean change in velocity ± SEM from three independent experiments (six individual larva per experiment). Toxicity is indicated by dashed bars. The threshold for a decrease in velocity is ≥40% (red line). Locomotion of larvae was recorded for 10 min after an exposure of 30 min (blue bars) and 90 min (yellow bars). A representative raw 10 min tracking plot is shown for a single experiment of six individual scn1Lab zebrafish. The chemical structure for each clemizole analogue is shown (d–f). In vitro radioligand binding analyses of (g) compound 4, (h) compound 6 and (i) compound 20 revealed specificity for 5-HT2BR over 5-HT2R subtypes. SB206553 was used as a positive control for 5-HT2BR binding (black). The binding affinity for the other clemizole analogues is given in Supplementary Table S2.
Clemizole analogues with anti-seizure activity selectively bind 5-HT2BR
From our library of clemizole analogues, we identified three compounds which suppress the convulsive high-velocity swim behaviour observed in the scn1lab Dravet syndrome zebrafish model. The parent compound clemizole hydrochloride has previously been reported to have agonist activity for 5-HT2AR and 5-HT2BR (Griffin et al., 2017). Additionally, 5-HT2R modulating compounds exert antiepileptic activity in both preclinical (Sourbron et al., 2016; Griffin et al., 2017) and clinical studies (Griffin et al., 2017; Schoonjans et al., 2017; Kauppila et al., 2018). Therefore, we determined 5-HT2R binding affinities for 21 of the clemizole analogues using the radioligand binding assays performed blinded by the NIMH Psychoactive Drug Screening Program (Besnard et al., 2012). Our three hit compounds, 4, 6 and 20, had significant preference for 5-HT2BR with Ki values of 612 nM, 285 nM and 772 nM, respectively (Fig. 4; Supplementary Table S2). Additionally, these clemizole analogues showed no significant binding to 5-HT2AR or 5-HT2CR (Ki >10 000 nM). Retrospectively, we observed compounds 5, 14 and 23 also show selectivity for 5-HT2BR with Ki values of 219 nM, 606 nM and 515 nM. In our initial library screen compounds 5 and 14 had no significant effect on swim behaviour and compound 23 was identified as toxic. Additional testing of independently synthesized compound confirmed compounds 5 and 14 have no significant effect on the swim behaviour of the scn1lab zebrafish within the constraints of our screening assay (i.e. duration of exposure and compound concentration) (Supplementary Fig. S1). Four of the 21 clemizole analogues (compound 10, 15, 17 and 21) showed no significant binding to any human 5-HT2R.
5-HT2BR agonists suppress seizure activity in the scn1lab zebrafish model
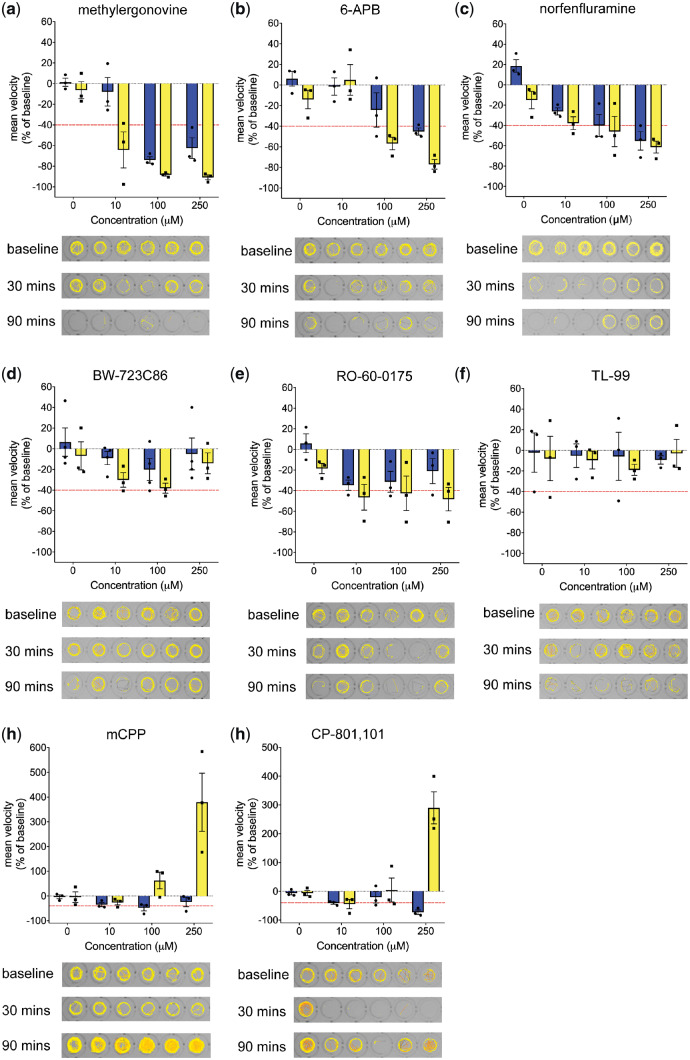
Structure–activity relationship analysis using clemizole analogues suggests 5-HT2BR may contribute to the observed anti-seizure activity in scn1lab zebrafish. Next, we tested a series of commercially available compounds known to bind 5-HT2BR (Roth et al., 2000) for their ability to reduce the seizure-like swim behaviour of the scn1lab zebrafish larvae (Supplementary Table S3). Three 5-HT2BR agonists, methylergonovine, 6-APB and norfenfluramine suppressed convulsive swim behaviours in a concentration-dependent manner (Fig. 5). Additionally, scn1lab mutant larvae treated with 5-HT2BR agonist BW-723C86, showed a decrease in seizure-like swim behaviour, but also failed to reach our significance threshold to warrant further testing. This confirms previous observations for the 5-HT2BR agonist BW-723C86 (Sourbron et al., 2016). Similarly, the 5-HT2BR/5-HT2CR agonist Ro60-0175 consistently decreased mean velocity and was borderline effective after 90 min of drug exposure. Treatment with CP-809, 101 gave a biphasic response, as did m-CPP, the active metabolite of trazodone, confirming behavioural responses observed in other model systems (Yonezawa et al., 2008). TL-99, which had the lowest affinity for 5-HT2Rs, failed to elicit any behavioural effect in scn1lab mutant larvae in our assay (Fig. 5f).
Figure 5.
Dose response evaluation of 5HT2BR agonists in scn1lab mutant zebrafish. 5HT2BR agonists were tested for efficacy in reducing the high-speed seizure-like behaviour in 5 dpf scn1lab mutant zebrafish. Graphs show the change in mean velocity over three concentrations of (a) methylergonovine, (b) 6-APB, (c) norfenfluamine, (d) BW-723C86, (e) RO-60-0175, (f) TL-99, (g) m-CPP and (h) CP-809, 101. Larvae locomotion was recorded for 10 min after an exposure of 30 min (blue bars) and 90 min (yellow bars). Each bar represents the mean change in velocity ± SEM from three independent experiments (six individual larva per experiment). The threshold for a decrease in velocity is ≥40% (red line). Representative tracking plots of a 10 min recording are shown for six individual 5 dpf scn1lab zebrafish at baseline, and following 30 min and 90 min exposure of 100 µM of each compound.
Dopamine receptor agonists with reported 5-HT2R were also tested for their ability to reduce seizure-like swim behaviour (Supplementary Fig. S2). Cabergoline, a dopamine agonist with recognized high affinity for activating 5-HT2BR (Ki = 1.2 nM) significantly reduced convulsive swim behaviour at 250 µM; however, due to the lack of a concentration-response it did not undergo further testing. Bromocriptine significantly reduced seizure-like swim behaviour at 10 µM; however, toxicity was observed at higher concentrations. Piribedil, a dopamine 2 receptor agonist (Ki = 1.3 nM), also showed toxicity at 100 and 250 µM and the non-selective dopamine agonist, apomorphine, significantly increased mean swim velocity of scn1lab mutant larvae, an effect which is also seen in wild-type zebrafish larvae (Ek et al., 2016).
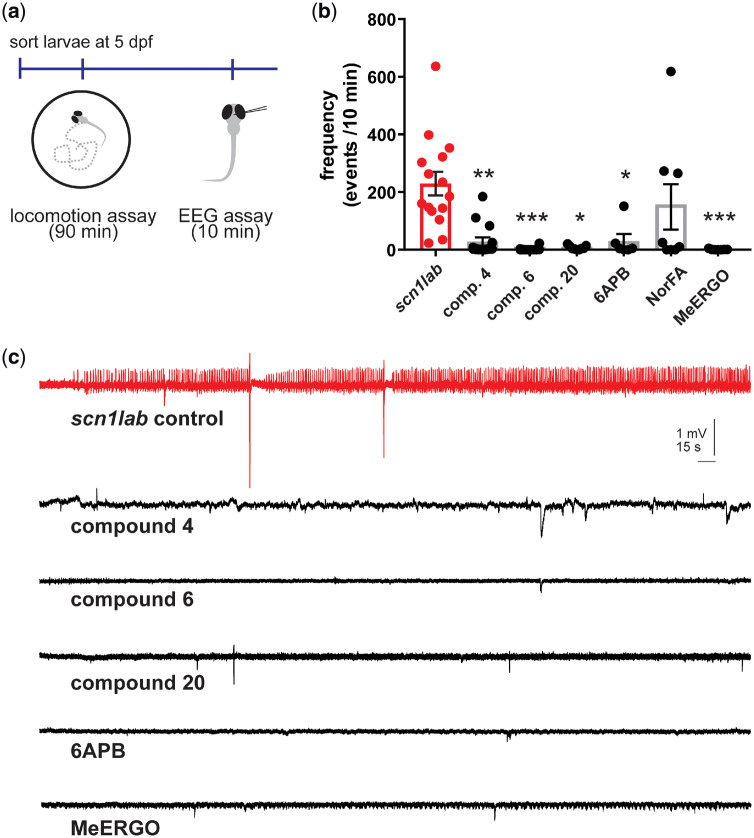
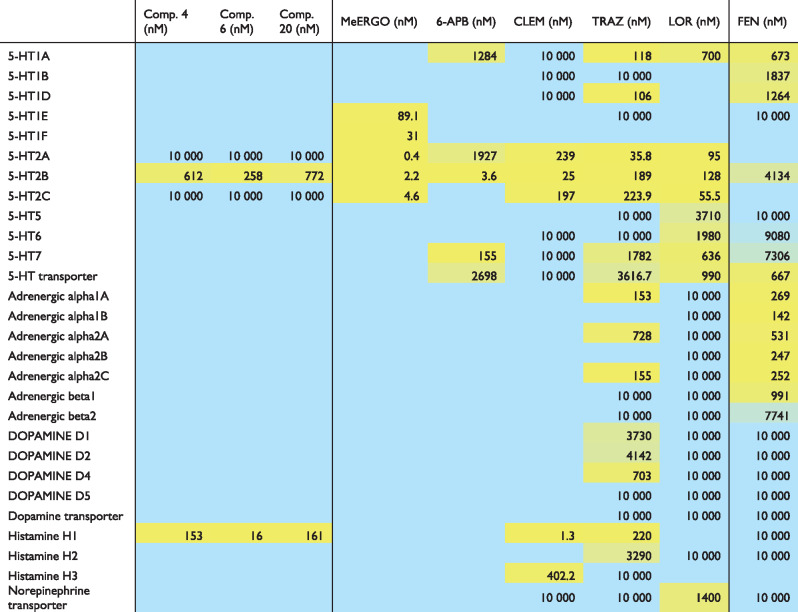
Monitoring electrographic brain activity to confirm seizure suppression is an essential assay to eliminate false positives from behavioural testing (Griffin et al., 2018). By placing a micro-electrode into a visually identified brain region of an agar-immobilized zebrafish larval, stable LFP recordings can be monitored for several hours (Baraban, 2013). At 5 days post-fertilization, LFP recordings of scn1Lab zebrafish larvae show an average of 250 abnormal electrographic seizure events during a 10 min recording epoch. LFP recordings of scn1lab mutants confirmed significant suppression of electrographic seizure activity after exposure to clemizole analogues 4, 6 and 20 at 100 µM. Representative LFP recording epochs with only the occasional abnormal electrographic event are shown in Fig. 6. Similarly, 250 µM methylergonovine (n = 7; P < 0.001) or 250 µM 6-APB (n = 6; P = 0.0238) significantly suppressed the frequency of electrographic seizure events in a manner similar to the 5-HT2BR selective clemizole analogues 4, 6 and 20. Radioligand binding data for methylergonovine, 6-APB and the positively identified clemizole analogues 4, 6 and 20, suggest that all five compounds share a binding affinity for 5-HT2BR (Table 1).
Figure 6.
Electrophysiological assay to identify drugs that rescue the scn1lab mutant epilepsy phenotype. (a) Electrophysiology recording were obtained with an electrode placed in the forebrain of 5 dpf agar-immobilized scn1lab larvae that had previously showed suppressed seizure-like behaviour in the locomotion assay. (b) Bar graphs show the frequency of epileptiform events in a 10 min recording epoch for scn1lab larvae exposed to clemizole analogues 4 (n = 15), 6 (n = 12), 20 (n = 9), 6-APB (n = 6), norfenfluramine (NorFA) (n = 8), methylergonovine (MeERGO) (n = 7) or scn1lab mutants (n = 15). The graph represents mean ± SEM and individual data points are shown. Kruskal–Wallis one-way analysis of variance was used to test for significance. *P < 0.05; **P < 0.01; ***P < 0.001. (c) Representative field electrode recording epochs (10 min) are shown for clemizole analogues 4, 6, 20, methylergonovine (MeERGO) and 6-APB. These compounds showed significant changes in the frequency of events compared to untreated scn1lab mutant zebrafish (red).
Table 1.
Receptor specificity and binding affinity (Ki) for compounds effective in suppressing spontaneous seizure activity in scn1lab mutant zebrafish
|
MeERGO, methylergonovine; CLEM, clemizole; TRAZ, trazodone; LOR, lorcaserin; FEN, fenfluramine. Compounds in yellow represent significant binding to the receptor target.
Discussion
The 5-HT modulating compounds, clemizole, lorcaserin, trazodone and fenfluramine are currently in clinical development for Dravet syndrome. Focusing on clemizole as a promising screening hit, we performed structural–activity–relationship studies to identify targets required for antiepileptic activity. By generating novel clemalogues, we determined that compounds capable of suppressing spontaneous seizures in vivo had a binding preference to 5-HT2BR. Additionally, we present pharmacological data which suggest that activation of 5-HT2BR may be the common antiepileptic mechanism of action shared by the 5-HT modulating compounds showing efficacy in clinical trials and validated preclinical models of Dravet syndrome.
Our approach used an iterative medicinal chemistry process involving the analysis of the chemical structure of clemizole, generating novel analogues of this compound, and using target-engagement assessment through a series of in vivo and in vitro assays. As a low-cost and efficient alternative to typical experimental animals, this zebrafish-based strategy can be considered a novel disruptive technology in the field of antiepileptic drug discovery. While we are not suggesting that zebrafish replace mammalian models, these findings can be taken as validation of our zebrafish-based approach, as lorcaserin (Belviq; EPX-200), trazodone (EPX-300) and fenfluramine (ZX-008) are already showing clinical anti-seizure efficacy in Dravet syndrome patients (Ceulemans et al., 2012; Ceulemans et al., 2016; Griffin et al., 2017; Schoonjans et al., 2017; Kauppila et al., 2018; Tolete et al., 2018).
Our laboratory has completed blinded phenotype-based screening of over 3500 compounds in a Dravet syndrome zebrafish model (Griffin et al., 2018). This screening platform has spanned multiple drug classes targeting several suggested therapeutic mechanisms. Importantly, a common feature of the small number of compounds identified as capable of suppressing spontaneous seizure activity in this assay (<0.2% of all compounds screened; i.e. clemizole, lorcaserin and trazodone), appears to be a binding affinity for 5HT2R subtypes. Here, we identified three novel clemizole analogues and two additional commercially available compounds that mimic the suppression of seizure activity seen previously. These findings support a working hypothesis that targeting a combination of, or a single, 5HT2R is therapeutic for Dravet syndrome. Similar to how most of the present antiepileptic drugs have been discovered and studied, these findings are limited to the pharmacological tools available and will, ultimately, require molecular and/or functional strategies to precisely confirm a mechanism of action. Nonetheless, our conclusion is consistent with pharmacological and knockout mouse studies implicating 5-HT2 receptors in anti-seizure and anti- Sudden Unexplained Death in Epilepsy actions (Tupal and Faingold, 2006).
The structural similarity for 5-HT2 receptors makes developing therapeutic compounds with high receptor subtype specificity and affinity challenging. In comparison to the parent compound clemizole, our clemizole analogues gained 5-HT2 receptor specificity but decreased receptor affinity. Therefore, these analogues provide a useful tool for understanding the mechanism of the anti-seizure action of clemizole. In addition to receptor subtype similarity, 5-HT2R agonists may have functional selectivity, whereby, a ligand can preferentially activate one receptor-linked intracellular signalling pathway (i.e., Gq-linked calcium flux or β-arrestin recruitment). While the signalling bias of each clemizole analogue remains to be determined, methylergonovine is known to have a strong preference for activating the β-arrestin pathway (Wacker et al., 2013). As methylergonovine is capable of reducing seizures in the scn1lab larvae, functional signalling may be an additional consideration when determining the mechanism of antiepileptic action shared between these serotonin modulating compounds.
Within the field of drug development, 5-HT2BR agonist activity has sometimes been perceived as negative due to the putative involvement of this receptor subtype in heart valve pathogenesis (Roth, 2007; Papoian et al., 2017). Indeed, activation of 5-HT2BR can induce a mitogenic effect on valvular endothelial cells and has resulted in the discontinued development of some compounds (Roth, 2007). Perhaps one of the most recognizable compounds to be withdrawn under Food and Drug Administration recommendation is the 5-HT reuptake blocker fenfluramine (Rothman et al., 2000), a compound currently in clinical trial as an add-on therapy for Dravet syndrome patients with, as yet, no reported negative cardiovascular events (Ceulemans et al., 2012; Ceulemans et al., 2016). Once approved as an anorectic, it was later removed from the market after heart valve defects were noted in ∼25% of patients (Abenhaim et al., 1996; Khan et al., 1998). Norfenfluramine, the active metabolite of fenfluramine is a potent activator of 5-HT2BR and may contribute to these observed heart valve defects (Fitzgerald et al., 2000). In the scn1lab zebrafish Dravet syndrome model, fenfluramine is effective in suppressing spontaneous seizures (Dinday and Baraban, 2015; Sourbron et al., 2016), additionally, norfenfluramine was able to restore the swimming behaviour or scn1lab mutant larvae to wild-type levels (Stage I) and reduce spontaneous seizure events in some, but not all larvae (5 out of 8 larvae; Fig. 6) suggesting that this active metabolite may contribute to the anti-seizure activity of fenfluramine.
Our previous working hypothesis proposed that clemizole, lorcaserin, trazodone and fenfluramine could increase gamma-aminobutyric acid (GABA)-mediated synaptic inhibition through direct activation of (i) 5-HT2ARs, which are expressed throughout the central nervous system; or (ii) 5-HT2CRs which are expressed on a subpopulation of inhibitory interneurons (Liu et al., 2007). However, newly synthesized clemalogues with selective affinity for 5-HT2A or 5-HT2C receptor subtypes were not consistently observed to inhibit spontaneous seizures in our zebrafish model whereas those with 5-HT2BR binding properties were effective. The histamine receptor (H1) is also a common site of action for clemizole (a first-generation antihistamine) and many of the compounds described in this manuscript. This receptor was excluded as the mechanism of action site because antihistamines are often contraindicated as anti-seizure medications in paediatric epilepsies (Miyata et al., 2011). Additionally, prior screening of more than 40 different H1-receptor binding compounds failed to identify any other antihistamines with anti-seizure activity (Griffin et al., 2017).
Zebrafish have a single 5-HT2BR orthologue, which has 62.0% protein identity compared to the human protein (Griffin et al., 2017). While htr2b is expressed in the zebrafish brain (Theodoridi et al., 2017), the precise cell type in the larval central nervous system where this receptor is expressed has not been established. In the mammalian brain, prominent central nervous system expression of 5-HT2BR has been reported on astrocytes (Sanden et al., 2000; Zhang et al., 2010), and receptor activation can result in elevated calcium signalling. 5-HT2R mediated enhancement of astrocyte signalling could (i) release glutamate onto GABAergic interneurons causing a potentiation of inhibitory interneuron signalling (Kang et al., 1998), or (ii) provide positive feedback autoregulation to somatostatin-expressing GABAergic interneurons innervating the dendrites of excitatory pyramidal neurons (Matos et al., 2018). Additionally, neuronal 5HT2BR expression has been reported in Purkinje cells (Choi and Maroteaux, 1996) and neurons in the dorsal raphe nuclei (Diaz et al., 2012). Optogenetic stimulation of these raphe neurons has been shown to suppress both hippocampal and cortical neuronal activity, as well as Sudden Unexplained Death in Epilepsy in mice (Wang et al., 2015; Lottem et al., 2016; Zhang et al., 2018). There is also evidence to suggest that 5-HT2B receptors directly modulate serotonin levels independently of the serotonin transporter (Hertz et al., 2015). Finally, published evidence for 5-HT2BR expression on microglia (Krabbe et al., 2012; Kolodziejczak et al., 2015) could suggest a modulatory role as part of the tripartite synapse (Ji et al., 2013). Each of these possibilities are presented as potential mechanisms of action for discussion purposes only and merit further investigation using the pharmacological tools developed here.
In conclusion, our results imply a 5-HT2BR mechanism of action for several serotonergic compounds currently in clinical development for Dravet syndrome and suggest that a zebrafish-based strategy may represent a promising avenue to the discovery and development of drugs for treating catastrophic epilepsies of childhood.
Supplementary Material
Acknowledgements
We would like to thank members of the Baraban laboratory for their useful discussions during the course of these studies; Matthew Dinday and Kyla Hamling in particular for their support in zebrafish facility maintenance.
Glossary
Abbreviations
- AEDs
antiepileptic drugs
- dpf
days post-fertilization
- FDA
Food and Drug Administration
- SAR
structure–activity relationship
- SUDEP
Sudden Unexplained Death in Epilepsy
- 5-HT
serotonin
- 5-HTR
serotonin receptor
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) R01 (NS079214 to S.C.B.); University of California, San Francisco UCSF Catalyst Award to S.C.B.; and the Dravet syndrome Foundation Fellowship to A.G.
Competing interests
S.C.B. is a co-Founder and Scientific Advisor for EpyGenix Therapeutics.
References
- Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med 1996; 335: 609–16. [DOI] [PubMed] [Google Scholar]
- Applegate CD, Tecott LH. Global increases in seizure susceptibility in mice lacking 5-HT2C receptors: a behavioral analysis. Exp Neurol 1998; 154: 522–30. [DOI] [PubMed] [Google Scholar]
- Baraban SC. Forebrain electrophysiological recording in larval zebrafish. J Vis Exp 2013; 50104.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun 2013; 4: 2410.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 2005; 131: 759–68. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 1999; 38: 1083–152. [DOI] [PubMed] [Google Scholar]
- Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, et al. Automated design of ligands to polypharmacological profiles. Nature 2012; 492: 215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Thomas EA, Danielson PE, Sutcliffe JG. The 5HT5A serotonin receptor is expressed predominantly by astrocytes in which it inhibits cAMP accumulation: a mechanism for neuronal suppression of reactive astrocytes. Glia 1996; 17: 317–26. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol (Lond) 2010; 588: 1849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans B, Boel M, Leyssens K, Van Rossem C, Neels P, Jorens PG, et al. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia 2012; 53: 1131–9. [DOI] [PubMed] [Google Scholar]
- Ceulemans B, Schoonjans AS, Marchau F, Paelinck BP, Lagae L. Five-year extended follow-up status of 10 patients with Dravet syndrome treated with fenfluramine. Epilepsia 2016; 57: e129–134. [DOI] [PubMed] [Google Scholar]
- Choi DS, Maroteaux L. Immunohistochemical localisation of the serotonin 5-HT2B receptor in mouse gut, cardiovascular system, and brain. FEBS Lett 1996; 391: 45–51. [DOI] [PubMed] [Google Scholar]
- Cooper MS, McIntosh A, Crompton DE, McMahon JM, Schneider A, Farrell K, et al. Mortality in Dravet syndrome. Epilepsy Res 2016; 128: 43–7. [DOI] [PubMed] [Google Scholar]
- De la Torre JC, Kawanaga HM, Mullan S. Seizure susceptibility after manipulation of brain serotonin. Arch Int Pharmacodyn Ther 1970; 188: 298–304. [PubMed] [Google Scholar]
- De la Torre JC, Mullan S. A possible role for 5-hydroxytryptamine in drug-induced seizures. J Pharm Pharmacol 1970; 22: 858–9. [DOI] [PubMed] [Google Scholar]
- Diaz SL, Doly S, Narboux-Neme N, Fernandez S, Mazot P, Banas SM, et al. 5-HT(2B) receptors are required for serotonin-selective antidepressant actions. Mol Psychiatry 2012; 17: 154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinday MT, Baraban SC. Large-scale phenotype-based antiepileptic drug screening in a zebrafish model of Dravet syndrome. eNeuro 2015; 2. doi: 10.1523/ENEURO.0068-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravet C. The core Dravet syndrome phenotype. Epilepsia 2011; 52 Suppl 2: 3–9. [DOI] [PubMed] [Google Scholar]
- Dravet C, Oguni H. Dravet syndrome (severe myoclonic epilepsy in infancy). Handb Clin Neurol 2013; 111: 627–33. [DOI] [PubMed] [Google Scholar]
- Ek F, Malo M, Åberg Andersson M, Wedding C, Kronborg J, Svensson P, et al. Behavioral analysis of dopaminergic activation in zebrafish and rats reveals similar phenotypes. ACS Chem Neurosci 2016; 7: 633–46. [DOI] [PubMed] [Google Scholar]
- Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 2010; 51: 1650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, et al. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol 2000; 57: 75–81. [PubMed] [Google Scholar]
- Griffin A, Hamling KR, Hong S, Anvar M, Lee LP, Baraban SC. Preclinical animal models for Dravet syndrome: seizure phenotypes, comorbidities and drug screening. Front Pharmacol 2018; 9: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A, Hamling KR, Knupp K, Hong S, Lee LP, Baraban SC. Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain 2017; 140: 669–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A, Krasniak C, Baraban SC. Advancing epilepsy treatment through personalized genetic zebrafish models. Prog Brain Res 2016; 226: 195–207. [DOI] [PubMed] [Google Scholar]
- Grone BP, Qu T, Baraban SC. Behavioral comorbidities and drug treatments in a zebrafish scn1lab model of Dravet syndrome. eNeuro 2017; 4. doi: 10.1523/ENEURO.0066-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Rothman DL, Li B, Peng L. Chronic SSRI stimulation of astrocytic 5-HT2B receptors change multiple gene expressions/editings and metabolism of glutamate, glucose and glycogen: a potential paradigm shift. Front Behav Neurosci 2015; 9: 25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Miyauchi J, Tsirka SE. Microglia: an active player in the regulation of synaptic activity. Neural Plast 2013; 2013: 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci 1998; 1: 683.. [DOI] [PubMed] [Google Scholar]
- Kauppila LA, Amorim, C. Bentes I, Peralta AR. Trazodone: a new antiepileptic drug for Dravet syndrome? Int J Epilepsy 2018; 05: 99–103. [Google Scholar]
- Khan MA, Herzog CA, St Peter JV, Hartley GG, Madlon-Kay R, Dick CD, et al. The prevalence of cardiac valvular insufficiency assessed by transthoracic echocardiography in obese patients treated with appetite-suppressant drugs. N Engl J Med 1998; 339: 713–8. [DOI] [PubMed] [Google Scholar]
- Kilian M, Frey HH. Central monoamines and convulsive thresholds in mice and rats. Neuropharmacology 1973; 12: 681–92. [DOI] [PubMed] [Google Scholar]
- Knupp KG, Wirrell EC. Treatment strategies for Dravet syndrome. CNS Drugs 2018; 32: 335–50. [DOI] [PubMed] [Google Scholar]
- Kolodziejczak M, Bechade C, Gervasi N, Irinopoulou T, Banas SM, Cordier C, et al. Serotonin modulates developmental microglia via 5-HT2B receptors: potential implication during synaptic refinement of retinogeniculate projections. ACS Chem Neurosci 2015; 6: 1219–30. [DOI] [PubMed] [Google Scholar]
- Krabbe G, Matyash V, Pannasch U, Mamer L, Boddeke HW, Kettenmann H. Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav Immun 2012; 26: 419–28. [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience 2007; 146: 1677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W. Animal models of seizures and epilepsy: past, present, and future role for the discovery of antiseizure drugs. Neurochem Res 2017a; 42: 1873–88. [DOI] [PubMed] [Google Scholar]
- Löscher W. The search for new screening models of pharmacoresistant epilepsy: is induction of acute seizures in epileptic rodents a suitable approach? Neurochem Res 2017b; 42: 1926–38. [DOI] [PubMed] [Google Scholar]
- Lottem E, Lorincz ML, Mainen ZF. Optogenetic activation of dorsal raphe serotonin neurons rapidly inhibits spontaneous but not odor-evoked activity in olfactory cortex. J Neurosci 2016; 36: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos M, Bosson A, Riebe I, Reynell C, Vallée J, Laplante I, et al. Astrocytes detect and upregulate transmission at inhibitory synapses of somatostatin interneurons onto pyramidal cells. Nat Commun 2018; 9: 4254.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata I, Saegusa H, Sakurai M. Seizure-modifying potential of histamine H1 antagonists: a clinical observation. Pediatr Int 2011; 53: 706–8. [DOI] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M. Serotonin 1A receptors on astrocytes as a potential target for the treatment of Parkinson's disease. Curr Med Chem 2016; 23: 686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoian T, Jagadeesh G, Saulnier M, Simpson N, Ravindran A, Yang B, et al. Regulatory forum review*: utility of in vitro secondary pharmacology data to assess risk of drug-induced valvular heart disease in humans: regulatory considerations. Toxicol Pathol 2017; 45: 381–8. [DOI] [PubMed] [Google Scholar]
- Roth BL. Drugs and valvular heart disease. N Engl J Med 2007; 356: 6–9. [DOI] [PubMed] [Google Scholar]
- Roth BL, Lopez E, Patel S, Kroeze WK. The multiplicity of serotonin receptors: uselessly diverse molecules or an embarrassment of riches? Neuroscientist 2000; 6: 252–62. [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 2000; 102: 2836–41. [DOI] [PubMed] [Google Scholar]
- Sanden N, Thorlin T, Blomstrand F, Persson PA, Hansson E. 5-Hydroxytryptamine2B receptors stimulate Ca2+ increases in cultured astrocytes from three different brain regions. Neurochem Int 2000; 36: 427–34. [DOI] [PubMed] [Google Scholar]
- Schoonjans A, Paelinck BP, Marchau F, Gunning B, Gammaitoni A, Galer BS, et al. Low-dose fenfluramine significantly reduces seizure frequency in Dravet syndrome: a prospective study of a new cohort of patients. Eur J Neurol 2017; 24: 309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourbron J, Schneider H, Kecskes A, Liu Y, Buening EM, Lagae L, et al. Serotonergic modulation as effective treatment for Dravet syndrome in a zebrafish mutant model. ACS Chem Neurosci 2016; 7: 588–98. [DOI] [PubMed] [Google Scholar]
- Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov 2011; 10: 507–19. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, et al. Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature 1995; 374: 542.. [DOI] [PubMed] [Google Scholar]
- Theodoridi A, Tsalafouta A, Pavlidis M. Acute exposure to fluoxetine alters aggressive behavior of zebrafish and expression of genes involved in serotonergic system regulation. Front Neurosci 2017; 11: 223.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolete P, Knupp K, Karlovich M, DeCarlo E, Bluvstein J, Conway E, et al. Lorcaserin therapy for severe epilepsy of childhood onset: A case series. Neurology 2018; 91: 837–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia 2006; 47: 21–6. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Tupal S, Mhaskar Y, Faingold CL. Abnormal serotonin receptor expression in DBA/2 mice associated with susceptibility to sudden death due to respiratory arrest. Epilepsy Research 2010; 88: 183–8. [DOI] [PubMed] [Google Scholar]
- Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, et al. Structural features for functional selectivity at serotonin receptors. Science 2013; 340: 615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Romani S, Lustig B, Leonardo A, Pastalkova E. Theta sequences are essential for internally generated hippocampal firing fields. Nat Neurosci 2015; 18: 282–8. [DOI] [PubMed] [Google Scholar]
- Yonezawa A, Yoshizumi M, Ebiko M, Ise S-N, Watanabe C, Mizoguchi H, et al. Ejaculatory response induced by a 5-HT2 receptor agonist m-CPP in rats: differential roles of 5-HT2 receptor subtypes. Pharmacol Biochem Behav 2008; 88: 367–73. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhao H, Zeng C, Van Dort C, Faingold CL, Taylor NE, et al. Optogenetic activation of 5-HT neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 mouse SUDEP model. Neurobiol Dis 2018; 110: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li B, Lovatt D, Xu J, Song D, Goldman SA, et al. 5-HT2B receptors are expressed on astrocytes from brain and in culture and are a chronic target for all five conventional ‘serotonin-specific reuptake inhibitors’. Neuron Glia Biol 2010; 6: 113–25. [DOI] [PubMed] [Google Scholar]
- Ziobro J, Eschbach K, Sullivan JE, Knupp KG. Current treatment strategies and future treatment options for Dravet syndrome. Curr Treat Options Neurol 2018; 20: 52.. [DOI] [PubMed] [Google Scholar]
- Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov 2005; 4: 35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and Supplementary material.