Abstract
Brain stimulation offers an alternative to focal resection for the treatment of focal drug-resistant epilepsy. Chronic subthreshold cortical stimulation is an individualized biomarker-informed open-loop continuous electrical stimulation approach targeting the seizure onset zone and surrounding areas. Before permanent implantation, trial stimulation is performed during invasive monitoring to assess stimulation efficacy as well as to optimize stimulation location and parameters by modifying interictal EEG biomarkers. We present clinical and neurophysiological results from a retrospective analysis of 21 patients, showing a median percent reduction in seizure frequency of 100% and responder rate of 89% with a median follow-up of 27 months. About 40% of patients were free of disabling seizures for a 12-month period or longer. We find that stimulation-induced decreases in delta (1–4 Hz) power and increases in alpha and beta (8–20 Hz) power during trial stimulation correlate with improved long-term clinical outcomes. These results suggest chronic subthreshold cortical stimulation may be an effective alternative approach to treating focal drug-resistant epilepsy and that short-term stimulation-related changes in spectral power may be a useful interictal biomarker and relate to long-term clinical outcome.
Keywords: focal epilepsy, chronic subthreshold cortical stimulation, EEG biomarkers, brain stimulation, trial stimulation
Focal drug-resistant epilepsy remains challenging to treat. Chronic subthreshold cortical stimulation (CSCS) offers a novel individualized treatment approach that targets the seizure onset zone with continuous electrical stimulation. Stimulation-related changes in EEG biomarkers during trial stimulation may help guide implantation of permanent electrodes.
Graphical Abstract
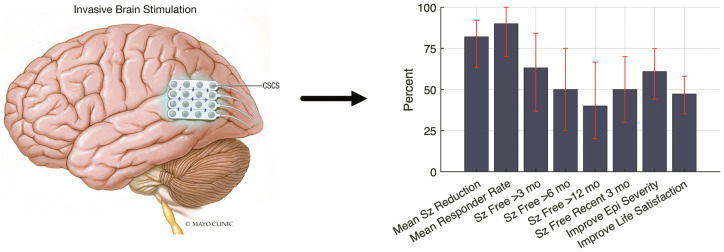
Graphical Abstract.
Introduction
Epilepsy affects young patients disproportionately and is the neurological disorder with the fifth highest morbidity burden following stroke, migraine, meningitis and dementia (Feigin et al., 2017). Focal drug-resistant epilepsy remains common with a prevalence of approximately 1.5 per 1000 (Hirtz et al., 2007; Wyllie, 2015). Although surgical resection is generally the most effective treatment, it is not an option if the seizure focus involves cortex that is eloquent. Chronic subthreshold cortical stimulation (CSCS) is an open-loop brain stimulation technique that lowers seizures probability (Lundstrom et al., 2016; Kerezoudis et al., 2018) and offers an alternative therapy to resection (Téllez-Zenteno et al., 2010), deep brain stimulation (Salanova et al., 2015) or responsive stimulation (Bergey et al., 2015). Although brain stimulation is effective, determining precisely where and how to stimulate remains a fundamental challenge. A key component of CSCS is trial stimulation, which entails continuous electrical stimulation performed for an extended time period during pre-surgical invasive EEG monitoring. Trial stimulation is performed to assess stimulation efficacy and to help determine optimal stimulation location and parameters (Lundstrom et al., 2016, 2017). Prior work has correlated epileptiform spike rate reduction with continuous stimulation (Velasco et al., 2000; Lundstrom et al., 2018), and recent evidence suggests that short-term stimulation generally decreases spectral power for 4–40 Hz (Westin et al., 2019). Despite this, there remains a paucity of evidence that short-term stimulation-related EEG changes, i.e. EEG biomarkers, can predict the long-term efficacy of stimulation to reduce seizure burden at the individual level. In this study, we present results from 21 consecutive patients treated with CSCS. We find stimulation-related relative decreases in delta (1–4 Hz) power and relative increases in alpha and beta (8–20 Hz) power correlate with long-term clinical improvement.
Materials and methods
The Mayo Clinic Institutional Review Board approved this retrospective analysis of 21 consecutive patients with drug-resistant focal epilepsy who were implanted with a permanent stimulation pulse generator for CSCS between 2011 and 2018 (Table 1). A prior study (Lundstrom et al., 2016) describes the initial 13 patients of this cohort. Patients underwent an evaluation with intracranial electroencephalography (iEEG) monitoring for potential surgical resection. The seizure onset zone (SOZ) was found to include eloquent cortex and surgical resection was not performed. Patients were offered a 1- to 4-day therapeutic trial of continuous electrical stimulation (biphasic; frequency, 2–100 Hz; pulse width, 90–450 µ;s; voltage amplitude, 1–6 V in voltage mode) targeting the SOZ and surrounding tissue using the already-implanted temporary electrodes use for invasive monitoring. The primary purpose of trial stimulation is to optimize stimulation location and stimulation parameters, which is accomplished via a subjective assessment of EEG epileptiform activity in response to stimulation (Lundstrom et al., 2017, 2016). This approach uses the spatial electrode coverage provided during epilepsy surgery evaluations and allows stimulation to be applied to both the SOZ and surrounding electrodes. Trial stimulation is limited to a maximum of 16 electrode contacts given available pulse generators. Permanent stimulation hardware (16-contact Medtronic PrimeAdvanced Neurostimulator with Medtronic platinum-iridium surgical leads or Medtronic 3387, 3389 or 3391 deep brain stimulation electrodes) was implanted at the time of temporary subdural electrode explanation (n = 17) or several months following stereoelectroencephalography explanation (n = 3; Kerezoudis et al., 2018). One patient underwent intraoperative iEEG with permanent electrode placement immediately following intraoperative monitoring. After the permanent implant, stimulation parameters were altered according to clinical judgement at follow-up appointments. With clinical guidance, patients may alter one stimulation parameter, typically voltage amplitude, at home via the Medtronic patient programmer.
Table 1.
Patient summary data
| Pt # | Age/handed NESS/gender | Seizure type | MRI lesion | Location | Comment |
|---|---|---|---|---|---|
| 1 | 56/R/M | Reflex | Bilateral temporal and frontal encephalomalacia | Left peri-rolandic region | |
| 2 | 9/R/M | FIAS | Left parietal FCD, L MTS | Left parietal lobe | Second implant 6 months later due to electrode movement |
| 3 | 14/R/F | FMS | Right parietal FCD, polymicrogyria | Right parietal lobe | Stimulator later removed due to pain |
| 4 | 15/R/F | FIAS | Left MCA infarct | Left peri-rolandic region | |
| 5 | 26/R/M | FIAS | Left frontal FCD | Left frontal lobe | La Crosse encephalitis age 7 |
| 6 | 27/L/F | FIAS | Right temporal FCD | Right temporal lobe | |
| 7 | 39/R/F | EPC motor | Left parietal atrophy | Left peri-rolandic region | Possible Rasmussen’s encephalitis |
| 8 | 6/R/F | FIAS | Right frontal FCD | Right peri-rolandic region | |
| 9 | 16/L/M | FIAS | Left central FCD | Left peri-rolandic region | |
| 10 | 27/R/M | Reflex | Right mesial parietal FCD | Right peri-rolandic region | |
| 11 | 22/R/M | FAS (Sensory Motor) | Non-lesional | Right frontal lobe | |
| 12 | 7/R/M | FIAS | Left parietal FCD | Left peri-rolandic region | |
| 13 | 17/R/F | FMS | Right hemispheric infarct | Right peri-rolandic region | |
| 14 | 19/R/M | FIAS | Left parietal occipital encephalomalacia | Left parieto-occipital lobe | |
| 15 | 20/R/F | FMS | Right central FCD | Right peri-rolandic region | |
| 16 | 26/R/M | FMS | Right peri-insula encephalomalacia, | Right insula | Perinatal right MCA stroke |
| Right hemisphere atrophy | |||||
| 17 | 22/R/F | FIAS & FMS | Enhancing posterior left temporal tuber | Left temporal tuber | Genetically confirmed tuberous sclerosis |
| 18 | 19/R/F | FBTCS | Left caudate head atrophy | Left frontal lobe | |
| 19 | 36/L/F | FAS (Sensory Motor) | Non-lesional | Right peri-rolandic region | |
| 20 | 31/R/M | FIAS | Left posterior temporal FCD | Left temporo-parietal lobe | |
| 21 | 41/R/M | FIAMS | Non-lesional | Left insula |
EPC = epilepsia partialis continua; F = female; FAS = focal onset aware seizures; FBTCS = focal to bilateral tonic-clonic seizures; FCD = focal cortical dysplasia; FIAMS = focal onset impaired awareness motor onset seizures; FIAS = focal onset impaired awareness seizures; FMS = focal motor seizures; L = left; M = male; MCA = middle cerebral artery; MTS = mesial temporal sclerosis; Pt = patient; R = right.
Clinical assessments
Patients were contacted via telephone by one of us (F.K.) not involved in patient clinical care. Patients were queried regarding their seizure frequency and longest periods of seizure freedom as well as their subjective rating (scale of 1–10) of epilepsy severity and overall life satisfaction. For seizure frequency, patients were asked what their average seizure frequency was for the 3-month period just prior to implant and for the most recent 3-month period. Answers were compared with prior records and any discrepancies resolved with the patients. Three of 21 patients were unable to be reached, and only the available data from the medical record was used.
Neurophysiological assessments
Data were acquired with Natus EMU 128Fs (bandwidth 0.1–940 Hz), Natus Quantum (bandwidth 0.01–4 kHz) or Neuralynx ATLAS (bandwidth 0.16–5000 Hz) electrophysiology systems. Data were filtered and down-sampled to 500 or 512 Hz. For 13 of the 21 patients, data from the trial stimulation were able to be analysed. Four 15-min epochs of iEEG data were examined for each patient. Two epochs separated by approximately 2 h were taken from either the first or second morning of admission. Two epochs were also taken from one of the last two mornings of the admission when continuous stimulation was ongoing, similar to the analysis described previously (Lundstrom et al., 2016, 2018). For each patient, electrode contacts were grouped according to their distance from the clinically determined SOZ electrode contacts: SOZ (0–1 cm), Bridge (1–2 cm) and non-SOZ (2–4 cm). Contacts >4 cm away from the SOZ were not considered. The total number of contacts examined per patient was: 47, 35, 29, 36, 62, 43, 55, 46, 57, 41, 50, 62 and 39. Suitable stimulation frequencies during invasive monitoring were determined to be: 2, 2, 2, 2, 2, 2, 2, 40, 2, 7, 2, 4 and 2 Hz. To determine these frequencies, stimulation frequency was typically initiated at 2 Hz and increased as needed to minimize interictal epileptiform discharges and seizure activity.
Potential interictal epileptiform discharges were quantified using the suggested published parameters of a previously validated iEEG spike detector (Barkmeier et al., 2012). For all epochs, within a 4 ms window, epileptiform spikes that occurred at the stimulation frequency, half the frequency, or one-quarter the stimulation frequency for all epochs were excluded to account for stimulation artefact, as described previously (Lundstrom et al., 2016). Power spectra and power in band were calculated via Welch’s method using forward and reverse fourth-order Butterworth filters for zero-phase filtering. To minimize stimulus artefact, power spectra were median filtered with a sliding window size of 2 Hz (truncated as needed) for frequencies >0.5 Hz. Delta (1–4 Hz) and alpha and beta (8–20 Hz) power bands were normalized by power from 1 to 20 Hz. The analysis focused on power in the <20 Hz band, as power in the SOZ above 20 Hz showed a flat frequency spectrum suggestive of artefact. For clinical outcomes, the fractional improvement was used for epilepsy severity (1-S/B), and the logarithm of the ratio of seizure frequencies (B/S) or seizure freedom periods (S/B), where B and S represent the baseline and stimulation periods, respectively. To avoid dividing by zero, 1/12 was added to zero values of seizure frequency (per month). Logarithmic ratios were used given the exponential distributions observed in Fig. 1b and c.
Figure 1.
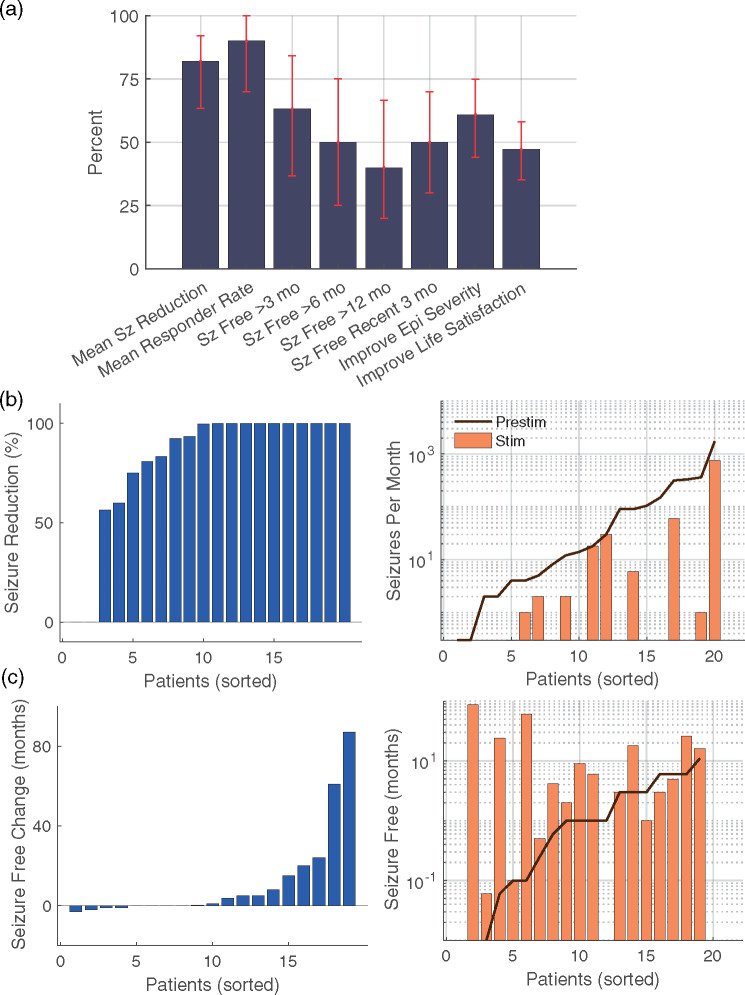
Clinical results from CSCS comparing the 3-month period prior to permanent implantation with the most recent 3-month period. (a) Summary of clinical results, where Sz = seizure and Epi = epilepsy (n = 20). Error bars reflect bootstrapped 95% confidence intervals. (b) For seizure frequency, reported change with stimulation (left panel) and reported frequency before and during stimulation assessed over 3-month periods (right panel). (c) For the longest period of seizure freedom, reported change with stimulation (left panel) and longest reported seizure-free period before and during stimulation (right panel). X-axes reflect data for each patient, sorted in order from least to greatest
Statistical analysis
Pearson correlation was used to assess the linear correlations between the clinical outcomes of epilepsy severity, seizure frequency and longest seizure freedom and changes in interictal epileptiform discharge rate and spectral power. Data were consistent with those from a normal distribution via the Anderson–Darling test (5% significance level). Error bars in figures reflect bootstrapped 95% confidence intervals (bias corrected and accelerated, 10 000 iterations), which is a statistical resampling technique that relies on random sampling with replacement of the distribution to provide a non-parametric and robust confidence interval estimate (Press, 1992).
Data availability
The data that support the findings of this study are available upon request.
Results
Twenty-one patients (10 female, three left-handed) received a permanent implant for CSCS (Table 1) with mean age of 23.6 years (range 6–56) and a median follow-up time of 27 months (range 3–101). One-third of patients were <19 years of age at the time of implant. Twelve patients received permanent stimulation with implanted Medtronic deep brain stimulation electrodes, whereas the remaining patients were implanted with subdural electrodes, as previously described (Kerezoudis et al., 2018). Ten of 21 patients had electrodes implanted in the peri-Rolandic region. In the 3-month period immediately following stimulation initiation, the responder rate (i.e. at least 50% seizure reduction) was 79%; median reduction in seizure frequency was 93% (57–99%); mean improvement in epilepsy severity was 4.6 (3.4–5.9); and mean improvement in life satisfaction was 2.9 (1.8–4.0), with bootstrapped 95% confidence intervals in parentheses. In the most recent 3-month period (Table 2 and Fig. 1), the responder rate was 89%; the median reduction in seizure frequency was 100% (82–100%); mean improvement in seizure frequency was 5.0 (3.5–6.2); and mean improvement in life satisfaction was 3.4 (2.5–4.5). Ten of 20 were free of disabling seizures for the past 3 months at most recent follow-up. In total, 63% experienced seizure-free periods of at least 3 months, 50% with periods of at least 6 months and 40% with periods of at least 12 months. Across patients, the distribution of seizure frequency (Fig. 1b) and seizure-free periods (Fig. 1c) was logarithmically distributed.
Table 2.
Results summary
| Disabling seizure/mo |
Longest seizure-free period (mo) |
Epilepsy severity (worst, 10) |
Life satisfaction (best, 10) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt # | Follow-up (mo) | Before | After | Improve (%) | Before | After | Improve | Before | After | Improve | Before | After | Improve |
| 1 | 27 | 2 | 0 | 100 | 6 | 3 | −3 | 10 | 5 | 5 | 1 | 6 | 5 |
| 2 | 22 | 18 | 18 | 0 | 0.1 | 0.1 | 0 | 6 | 6 | 0 | 4 | 4 | 0 |
| 3 | 31 | 4 | 0.3 | 93 | 1 | 2 | 1 | 8 | 3 | 5 | 6 | 8 | 2 |
| 4 | 33 | 0.3 | 0 | 100 | 1 | 9 | 8 | 9 | 7 | 2 | 5 | 5 | 0 |
| 5 | 32 | 0.3 | 0 | 100 | 11 | 16 | 5 | 7 | 2 | 5 | 6 | 9 | 3 |
| 6 | 35 | 12 | 2 | 83 | 1 | 6 | 5 | 5 | 2 | 3 | 5 | 8 | 3 |
| 7 | 34 | 30 | 30 | 0 | 0 | 0 | 0 | 10 | 7 | 3 | 4 | 4 | 0 |
| 8 | 35 | 14 | 0 | 100 | 6 | 5 | −1 | 8 | 2 | 6 | 2 | 8 | 6 |
| 9 | 30 | 360 | 1 | 100 | 3 | 3 | 0 | 8 | 9 | −1 | 6 | 8 | 2 |
| 10 | 16 | N/A | N/A | N/A | N/A | N/A | N/A | 8 | 0 | 8 | 2 | 4 | 2 |
| 11 | 37 | 315 | 60 | 81 | 0.01 | 0.06 | 0.05 | 7 | 5 | 2 | 8 | 9 | 1 |
| 12 | 61 | 150 | 0 | 100 | 0.1 | 61 | 61 | 10 | 0 | 10 | 2 | 8 | 6 |
| 13 | 101 | 105 | 0 | 100 | 0 | 87 | 87 | 9 | 0 | 9 | 2 | 10 | 8 |
| 14 | 26 | 2 | 0 | 100 | 6 | 26 | 20 | 8 | 0 | 8 | 2 | 8 | 6 |
| 15 | 25 | 330 | 0 | 100 | 0.06 | 24 | 23.94 | 8 | 0 | 8 | 4 | 10 | 6 |
| 16 | 23 | 90 | 0 | 100 | 3 | 18 | 15 | 8 | 0 | 8 | 5 | 10 | 5 |
| 17 | 12 | 5 | 2 | 60 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 18 | 10 | 4 | 1 | 75 | 3 | 1 | −2 | 6 | 5 | 1 | 6 | 8 | 2 |
| 19 | 4 | 1725 | 750 | 57 | 1 | 0 | −1 | 8 | 3 | 5 | 3 | 6 | 3 |
| 20 | 3 | 90 | 6 | 93 | 0.25 | 0.5 | 0.25 | 9 | 3 | 6 | 3 | 7 | 4 |
| 21 | 4 | 8 | 0 | 100 | 0.6 | 4.2 | 3.6 | 7 | 1 | 6 | 4 | 8.5 | 4.5 |
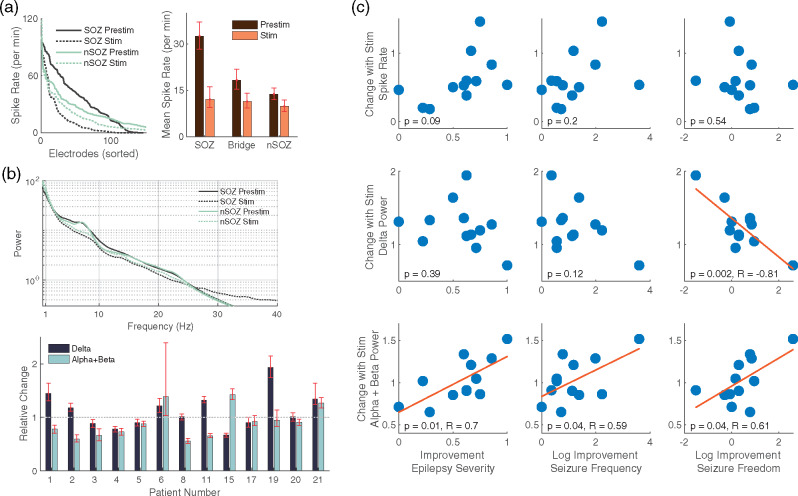
Interictal epileptiform discharge rates were significantly higher in the SOZ compared with the non-SOZ (Fig. 2a). Electrical stimulation decreased overall interictal discharge rates (Fig. 2a). Stimulation decreased the mean spectral power most notably in the 4–10 Hz frequency band (Fig. 2b, upper panel) but had variable effects on an individual patient basis (Fig. 2b, lower panel). Given work associating epileptiform spike rates (Lundstrom et al., 2018) and delta power activity (Boly et al., 2017) with the SOZ as well as activity in the alpha and beta power bands with stimulation (Westin et al., 2019), we wanted to see whether these short-term stimulation-related EEG changes would predict long-term clinical outcomes. The mean epileptiform spike rate decreased with stimulation for 11 of 13 patients; however, the magnitude of decrease did not correlate with clinical outcome (Fig. 2c, top row). Changes in delta (1–4 Hz) power were correlated with improvement in seizure freedom rates, whereas changes in alpha and beta (8–20 Hz) power were correlated with improvement in epilepsy severity, seizure frequency and seizure freedom (Fig. 2c, middle and bottom rows). These correlations do not reach levels of significance when the most negative and positive outlying data points are removed, which correspond to patients 15 and 19. When corrected for multiple comparisons, the delta power change with seizure freedom rates and the alpha and beta power change with epilepsy severity remained significant. Long-term clinical outcomes were improved when delta power was relatively less with short-term stimulation or alpha and beta power was relatively more. Clinical outcome was not correlated with pre-stimulation delta or alpha and beta power alone.
Figure 2.
Electrophysiological results with correlation to clinical results. (a) Spike rate for each electrode in the SOZ and in the non-SOZ for the pre-stimulation and stimulation conditions (left panel). Mean spike rate was decreased during stimulation (right panel) for SOZ (n = 146), Bridge (n = 160) and non-SOZ (n = 286) electrodes. (b) Mean spectral power for all electrodes in the SOZ and non-SOZ regions before and during stimulation (upper panel). At the individual patient level, changes in delta as well as alpha and beta activities were variable (lower panel, n = 97, 70, 58, 72, 104, 86, 110, 92, 114, 82, 100, 124, 78). Error bars reflect bootstrapped 95% confidence intervals. (c) Pearson’s correlation between changes in spike rate, delta power (1–4 Hz), alpha and beta power (8–20 Hz) and three clinical outcome measures: Epilepsy Severity, Seizure Frequency and Longest Period of Seizure Freedom. Displayed data are consistent with those from a normal distribution via the Anderson–Darling test (5% significance level). The 95% confidence intervals for the correlation coefficients were −0.81 [−0.43 to −0.94], 0.70 [0.22 to 0.91], 0.59 [0.03 to 0.87] and 0.61 [0.05 to 0.88]. Using the Bonferroni correction for multiple tests within each a priori outcome measure (i.e. P < 0.0167), the correlation for delta band power change with seizure freedom and alpha and beta band power change with epilepsy severity remains significant. These correlations do not reach levels of significance when the most negative and positive outlying data points are removed
Discussion
These findings suggest that open-loop continuous electrical stimulation such as CSCS is a promising alternative therapy for neocortical epilepsy and that trial stimulation is useful for optimizing electrical stimulation location and estimating prognosis. Given challenges in patient selection and determining stimulation location, EEG biomarkers that predict long-term prognosis would be enormously helpful in improving stimulation-related clinical outcomes and could be beneficial for cortical resection as well. Short-term stimulation could provide a means of better determining the geometry of the SOZ, thus assisting in determining resection margins or permanent electrode placement. The location for chronic stimulation in these patients was often in the fronto-parietal region near the eloquent motor cortex and insula as well as neocortical frontal, temporal, parietal and occipital locations. Mesial temporal structures were not targeted. Typically, there were no MRI lesions concordant with the SOZ. Evidence suggests CSCS is a safe and feasible therapy (Kerezoudis et al., 2018) with minimal complications and is reversible. One concern with continuous stimulation in eloquent cortex is the possibility of inducing unwanted side effects (van Blooijs et al., 2017). Long-term negative side effects were not reported, and stimulation amplitude was lowered for any transient negative side effects. Reported beneficial side effects were reported in two patients and included decreased irritability with improved motivation and improved motor function (Starnes et al., 2019).
The FDA-approved brain stimulation technique that targets neocortical foci directly is Neuropace Responsive NeuroStimulation (RNS; Bergey et al., 2015). Recent work has evaluated the efficacy of RNS for neocortical epilepsy for 126 patients with mean follow-up of 6.1 years (Jobst et al., 2017), reporting a 58% median reduction in seizure frequency and 55% responder rate. CSCS results were 100% and 89%, respectively. Similarly, seizure-free periods of at least 3, 6 and 12 months for RNS were reported as 37%, 26% and 14%, respectively (Jobst et al., 2017). In this study, we report 3, 6 and 12-month minimum periods free of disabling seizures as 63%, 50% and 40%, respectively. Five-year follow-up data from deep brain stimulation of the anterior nucleus thalamus show 69% median seizure reduction, 68% responder rate and 16% with seizure-free periods of at least 6 months (Salanova et al., 2015). The encouraging results reported here for CSCS are from a small group of highly selected patients and thus must be regarded with some caution.
A key challenge in brain stimulation is determining the proper stimulation location and most efficacious parameters from a nearly infinite set of possibilities without any clear way to quickly determine efficacy. During invasive EEG monitoring once surgical resection has been deemed undesirable, we have typically stimulated for a 2- to 3-day period to help guide stimulation location and parameter choice prior to permanent implantation for CSCS (Lundstrom et al., 2016; Kerezoudis et al., 2018). A primary component has involved the subjective assessment of interictal epileptiform activity in response to continuous stimulation, where a decrement of interictal epileptiform activity has been viewed favourably (Velasco et al., 2000; Lundstrom et al., 2016). In this study, we find that although interictal epileptiform activity decreased in response to stimulation in the majority of patients, the degree of suppression did not correlate with improved outcomes at the individual level (Fig. 2c). Perhaps suppression of interictal epileptiform activity better correlates with short-term clinical outcomes (Velasco et al., 2000) rather than long-term outcomes. Our clinical protocol has been to initiate stimulation at low frequencies (e.g. 2 Hz) and increase as needed. Empirically, we found that low frequencies could be efficacious and during Trial Stimulation lead to reduced artefact contamination. Long-term low-frequency stimulation leads to improved battery life.
When comparing EEG spectral power in the pre-stimulation epochs, we found that trial stimulation could either increase or decrease spectral power in delta, alpha and beta frequency bands. However, when considering the clinical outcome, relatively less delta and more alpha and beta power correlated with a favourable long-term outcome. Prior work has linked increases in delta activity (Tononi and Cirelli, 2014) and decreases in alpha and beta activities (Crone et al., 1998) to activated cortex. During sleep, delta activity is increased near the SOZ (Boly et al., 2017) and beta activity is decreased (Lundstrom et al., 2019). For delta activity and activity less than ∼2 Hz, distinct inhibitory and excitatory mechanisms may be contributing (Lundstrom et al., 2019), and a straightforward explanation for these results may be lacking. Nonetheless, in patients with the most favourable outcomes chronic stimulation may decrease cortical excitability.
The interpretation of these results is limited by the size of the patient cohort, the retrospective nature of the analysis, and a heterogeneous patient population. Results must be interpreted with caution due to the lack of control group or blinding. Further, the presented electrophysiologic data come from 13 patients, and thus results must be interpreted with caution. For patients with short follow-up times, results could also be influenced by an implantation effect, the tendency of seizure frequency to decline following implant surgery (Fisher et al., 2010; Morrell and RNS System in Epilepsy Study Group, 2011). Despite these limitations, the clinical results suggest that targeting the seizure focus and surrounding regions using short-term trial stimulation before permanent device implantation could improve long-term chronic stimulation efficacy.
Acknowledgements
We thank Michal Kucewicz and Doug Sheffield for helpful comments. Graphical abstract image used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
Glossary
Abbreviations
- CSCS =
chronic subthreshold cortical stimulation
- EEG =
electroencephalography
- iEEG =
intracranial electroencephalography
- SOZ =
seizure onset zone
Funding
Data collection was supported by grants R01 NS092882 (GW) and R01 NS078136 (MS) from the National Institutes of Health National Institute of Neurological Disease and Stroke (NINDS). B.N.L. was supported by the Mayo Clinic Foundation and the NIH NINDS (K23NS112339).
Competing interests
Drs. M.S., G.W. and J.V.G. have the rights to receive future royalties from the licensing of technology related in this research. Mayo Clinic has a financial interest related to this research. Mayo Clinic is co-owner of Cadence Neuroscience Inc., the development of which has been assisted by Drs. B.N.L., M.S., G.W. and J.V.G.
References
- Barkmeier DT, Shah AK, Flanagan D, Atkinson MD, Agarwal R, Fuerst DR, et al. High inter-reviewer variability of spike detection on intracranial EEG addressed by an automated multi-channel algorithm. Clin Neurophysiol 2012; 123: 1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 2015; 84: 810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blooijs DV, Huiskamp GJM, Leijten FSS.. Is brain-responsive neurostimulation in eloquent cortex without symptoms? Epilepsia 2017; 58: 1487.. [DOI] [PubMed] [Google Scholar]
- Boly M, Jones B, Findlay G, Plumley E, Mensen A, Hermann B. et al. Altered sleep homeostasis correlates with cognitive impairment in patients with focal epilepsy. Brain 2017; 140: 1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP.. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain 1998; 121 (Pt 12): 2301–15. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Abajobir AA, Hassen Abate K, Abd-Allah F, Abdulle AM, Abera SF, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol 2017; 16: 877–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010; 51: 899–908. [DOI] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R.. How common are the ‘common; neurologic disorders? Neurology 2007; 68: 326–37. [DOI] [PubMed] [Google Scholar]
- Jobst BC, Kapur R, Barkley GL, Bazil CW, Berg MJ, Bergey GK, et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia 2017; 58: 1005–14. [DOI] [PubMed] [Google Scholar]
- Kerezoudis P, Grewal SS, Stead M, Lundstrom BN, Britton JW, Shin C, et al. Chronic subthreshold cortical stimulation for adult drug-resistant focal epilepsy: safety, feasibility, and technique. J Neurosurg 2018; 129: 533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom BN, Boly M, Duckrow R, Zaveri HP, Blumenfeld H.. Slowing less than 1 Hz is decreased near the seizure onset zone. Sci Rep 2019; 9: 6218.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom BN, Meisel C, Van Gompel J, Stead M, Worrell G.. Comparing spiking and slow wave activity from invasive electroencephalography in patients with and without seizures. Clin Neurophysiol 2018; 129: 909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom BN, Van Gompel J, Britton J, Nickels K, Wetjen N, Worrell G, et al. Chronic subthreshold cortical stimulation to treat focal epilepsy. JAMA Neurol 2016; 73: 1370.. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Worrell GA, Stead M, Van Gompel JJ.. Chronic subthreshold cortical stimulation: a therapeutic and potentially restorative therapy for focal epilepsy. Expert Rev Neurother 2017; 17: 661–6. [DOI] [PubMed] [Google Scholar]
- Morrell MJ; RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011; 77: 1295–1304. [DOI] [PubMed] [Google Scholar]
- Press WH. Numerical recipes in C: the art of scientific computing. 2nd edn Cambridge; New York: Cambridge University Press; 1992. [Google Scholar]
- Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015; 84: 1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes K, Brinkmann BH, Burkholder D, Van Gompel J, Stead M, Lundstrom BN.. Two cases of beneficial side effects from chronic electrical stimulation for treatment of focal epilepsy. Brain Stimul 2019; 12: 1077. [DOI] [PubMed] [Google Scholar]
- Téllez-Zenteno JF, Ronquillo LH, Moien-Afshari F, Wiebe S.. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 2010; 89: 310–18. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C.. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014; 81: 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco M, Velasco F, Velasco AL, Boleaga B, Jimenez F, Brito F, et al. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia 2000; 41: 158–69. [DOI] [PubMed] [Google Scholar]
- Westin K, Lundstrom BN, Van Gompel J, Cooray G.. Neurophysiological effects of continuous cortical stimulation in epilepsy—spike and spontaneous ECoG activity. Clin Neurophysiol 2019; 130: 38–45. [DOI] [PubMed] [Google Scholar]
- Wyllie E, editor. Wyllie’s treatment of epilepsy. 6th edn Philadelphia, PA: Wolters Kluwer; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request.