Abstract
Objective: There are no safety or absorption studies to guide topical timolol therapy for treatment of chronic wounds. This study was undertaken to address this gap.
Approach: A prospective, observational, cross-sectional comparative study of timolol plasma levels in patients after topical administration to a chronic wound, compared with levels in patients after timolol ocular administration for the indication of glaucoma.
Results: There was no statistically significant difference in the average plasma level of timolol in wound as compared with glaucoma patients. No bradycardia or wheezing was observed after administration.
Innovation: We determined the single time point concentration of timolol in plasma 1 h after application of timolol 0.5% gel-forming solution to debrided chronic wounds, providing insight as to the safety of this emerging off-label treatment.
Conclusion: The topical application of timolol for chronic wounds shares the same safety profile as the widely used application of ocular administration for glaucoma.
Keywords: timolol, adrenergic receptor, catecholamine, chronic wounds, glaucoma, absorption

Roslyn Rivkah Isseroff, MD

Sara Evona Dahle, DPM, MPH
Introduction
Timolol is a nonselective, beta1/beta2 adrenergic receptor antagonist that has been widely used for many years as an ocular topical formulation for the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. Recent clinical experience has broadened its off-label use for a number of dermatologic indications, including infantile hemangiomas, and most recently, chronic wounds. While extensive systemic absorption studies have been performed for the ocular administration of the drug to assure safety, to date no studies have documented absorption of timolol postapplication to chronic wounds, where the absence of an intact epidermis would facilitate dermal or subcutaneous penetration and the potential for systemic cardiac effects. To address this information gap, a single-center, open-label, prospective, observational, cross-sectional comparative study was undertaken to determine the plasma levels of timolol in patients after topical administration to a chronic wound, and compare these levels with those of patients after ocular administration of the same drug formulation for the indication of glaucoma.
Clinical Problem Addressed
Chronic wounds have been estimated to affect 8.2 million patients in the United States,1 and in many cases these wounds do not heal when treated with the current standard of care alone. Application of timolol 0.5% gel-forming solution to the wound bed is being increasingly done as an off-label treatment to improve the healing of chronic wounds2–4; however, no studies have been performed to examine the safety of this route of administration of timolol in adults to date. The aim of this study is to address this current lack of safety information.
Materials and Methods
Clinical study
This study was approved by the Institutional Review Board of the VA Northern California Health Care System. The primary purpose of this study was the determination and comparison of plasma concentrations of timolol in two patient groups: those given topical timolol for the treatment of recalcitrant wounds (the wound group) and those taking timolol by ocular instillation for the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma (the glaucoma group). For the wound group, patients who were documented to have any type of chronic wound (persistence >30 days with minimal improvement), and were identified from clinic appointment lists using a Health Insurance Portability and Accountability Act (HIPAA) authorization waiver, were invited to participate in this study during their normally scheduled return visit to the wound clinic. Patients who met inclusion and exclusion criteria and gave informed consent were enrolled.
For the glaucoma group, an electronic medical records pharmacy data pull identified patients with a diagnosis of glaucoma with active prescriptions for timolol maleate 0.5% gel-forming solution. Patients who met the inclusion and exclusion criteria were contacted by telephone to confirm their interest and were enrolled after providing informed consent at their next appointment in the eye clinic. For both groups, inclusion criteria included the following: age >18 years; using physician-prescribed timolol as directed; and the ability to read, understand, and sign informed consent for blood draw and release of medical information forms. Exclusion criteria included the following: patient not currently prescribed timolol or currently taking oral metoprolol (excluded as this is used as an internal standard for chromatographic analysis); history of any type of heart block; history of bradycardia (heart rate <60 beats per minute [bpm]); history of documented hypotension; and history of asthma or chronic obstructive pulmonary disease.
In patients with wounds, baseline vital signs were measured, and lungs auscultated before application of topical timolol to the wound bed. If a heart rate <60 bpm or wheezing on auscultation was noted before application, subjects were excluded. If no bradycardia or wheezing was observed, clinicians then debrided and measured each wound, and applied the number of drops of timolol as calculated based on the wound area (cm2). Once the gel spread over the wound bed, the wound was covered with a nonadherent dressing. The patient's vital signs were monitored and lungs auscultated again 20 min after application of timolol, a time point chosen to coincide with the onset of action.5 This was to ensure no drop in pulse >3 bpm and to monitor for development of wheezing after application of timolol. A single vial blood draw was performed 1 h after application of the prescribed dose of timolol, a single, time point chosen to coincide with the peak concentration in plasma after ocular application of the solution.6
In glaucoma patients routinely using timolol, vital signs were not measured before administration of the drug. Timolol application was performed per existing physician prescription (one or two drops) under direct observation of study personnel. For both groups, one 6 mL dipotassium edetate (K2·EDTA) vacutainer of blood was drawn 1 h after drug administration. After centrifugation, the plasma from both groups was frozen at −80°C until assay.
Chromatographic analysis of timolol in plasma
Timolol in patient plasma was measured with reverse-phase high-performance liquid chromatography with ultraviolet absorbance detection using a modification of our published procedure.7 Samples were spiked with metoprolol (used as an internal standard) and then purified using cation-exchange cartridges as previously described, modified with use of a larger cartridge (Supelclean LC-WCX cartridges, 3 mL tube volume, 500 mg sorbent), and setting the mean value of three drug-free plasma samples as the analytical baseline. The limit of detection was calculated as three standard deviations of the assay results from the drug-free plasma and was, on average, 50 pg/mL timolol in plasma. The limit of quantification was 165 pg/mL timolol in plasma. More details regarding instrumentation and sample preparation are provided in the Supplementary Data.
Results
A total of 40 patients who had been prescribed topical timolol were recruited for the study: 20 in each group, diagnosed with either a chronic wound or glaucoma. Of the 20 wound patients, 6 patients had an additional wound that was also treated with topically applied timolol. Any wound type was included; however, most wounds were venous ulcers (46.2%). Wounds were located in the following primary locations: foot, ankle, leg, and scalp. All patients were male, with an average age of 70 years in the wound group, and 76 years of age in the glaucoma group. The ages for both groups ranged between 55 and 85 years. Other patient demographics and baseline wound characteristics are described in Table 1.
Table 1.
Patient demographics and baseline wound characteristics
| Patient Demographics | Chronic Wound | Glaucoma | p-Value |
|---|---|---|---|
| [n = 20]a | [n = 20] | ||
| Mean (SD) | Mean (SD) | ||
| Age in years | 69.6 (11.27) | 76.1 (12.98) | 0.65 |
| Body mass index | 26.9 (5.98) | 25.57 (5.11) | 0.37 |
| HgbA1cb | 6.9 (1.37) | 6.1 (0.96) | 0.47 |
| Percentage of smokers | 6% | 2% | 0.69 |
| Gender (male/female ratio) | 20/0 | 20/0 | — |
| Body surface area (BSA) 1.80 1.76 | |||
| Wound Characteristics | n | % | |
|---|---|---|---|
| Wound location | |||
| Foot | 5 | 19.2 | |
| Ankle | 2 | 7.7 | |
| Leg | 17 | 65.4 | |
| Scalp | 2 | 7.7 | |
| Wound etiology | |||
| Venous | 12 | 46.2 | |
| Diabetic | 5 | 19.2 | |
| Pressure | 3 | 11.5 | |
| Trauma | 4 | 15.3 | |
| Arterial | 1 | 3.9 | |
| Mixed (venous/arterial) | 1 | 3.9 | |
| Wound size at baseline | Mean, cm2 | SD | |
| Foot | 1.53 | 1.60 | |
| Ankle | 1.35 | 0.21 | |
| Leg | 6.50 | 10.03 | |
| Scalp | 13.5 | 14.93 | |
Demographics of patients enrolled in the study.
Within the chronic wound group, there were 20 patients with a total of 26 wounds, while there were 20 patients in the glaucoma group.
Ten of the patients in the chronic wound group had diabetes, and six in the glaucoma group had diabetes.
HgbA1c, hemoglobin A1c; SD, standard deviation.
Vital signs were monitored immediately before and 20 min after administration of timolol in all the patients in the wound group, and were found to be within normal limits. There was no drop of heart rate >3 bpm noted after administration of timolol. No patient developed wheezing after administration of the drug. As in clinical practice, vital signs were not measured before treatment for those receiving timolol for glaucoma.
Timolol dosage for the wound group was determined based on the wound size after debridement as described in a published protocol and summarized in Table 2.8 Each milliliter of 0.5% solution contains an amount of timolol maleate equivalent to 5 mg timolol. The volume of a drop from the timolol dispenser varies from 30 to 50 μL.9 We used the average value of 40 μL per drop; thus one drop of a 0.5% timolol solution contains 200 μg of timolol. For any wounds >3 cm2, the maximum dose applied was three drops. In the cases where patients had multiple wounds, the wound areas were combined to determine the total timolol dose. For the glaucoma group, one drop was applied to either one, or both eyes, with an average dose of 1.42 drops/patient. The average number of drops used for the wound and glaucoma groups is outlined in Table 2.
Table 2.
Timolol dosing
| Wound Size (cm2) | Number of Drops | Timolol Dosage (μg/day) | Timolol Dosage (μL/day) |
|---|---|---|---|
| One Drop ∼40 μL | |||
| <0.5 | 1 | 200 | 40 |
| >0.5–0.9 | 1 | 200 | 40 |
| >1.0–1.9 | 1 | 200 | 40 |
| >2.0–2.9 | 2 | 400 | 80 |
| >3.0 | 3 | 600 | 120 |
| Groups | Number | Average Number of Drops (SD) | Average Dose Delivered (μg) |
|---|---|---|---|
| Chronic Wound | 26 | 1.95 (0.999) | 390 |
| Glaucoma | 20 | 1.42 (0.643) | 284 |
Protocol for determination of topical timolol dosing per unit area of wound. The average dose of timolol applied to each wound or to each eye is expressed as a drop, or as μg drug. Based on the calculation of an average of 40 μL per drop of solution, with each milliliter of a 0.5% solution containing 5 mg of timolol, one drop of the 0.5% timolol solution contains an average of 200 μg of timolol.
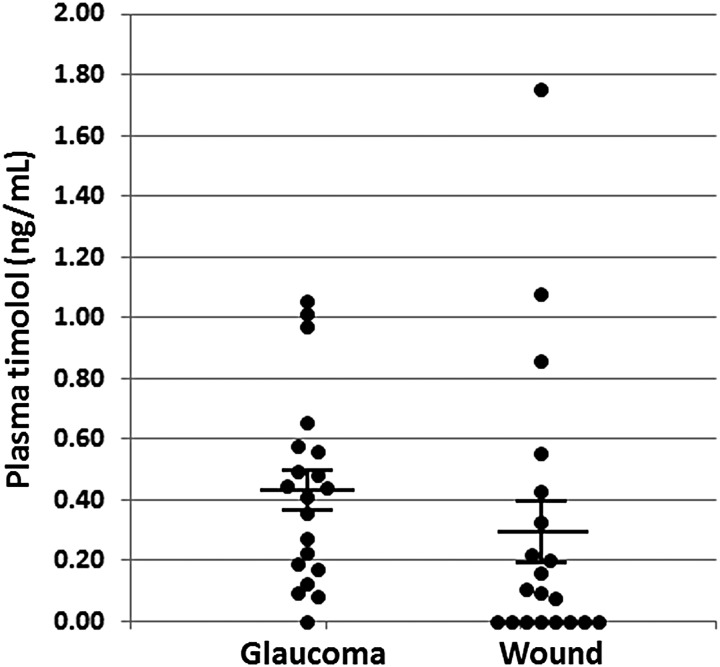
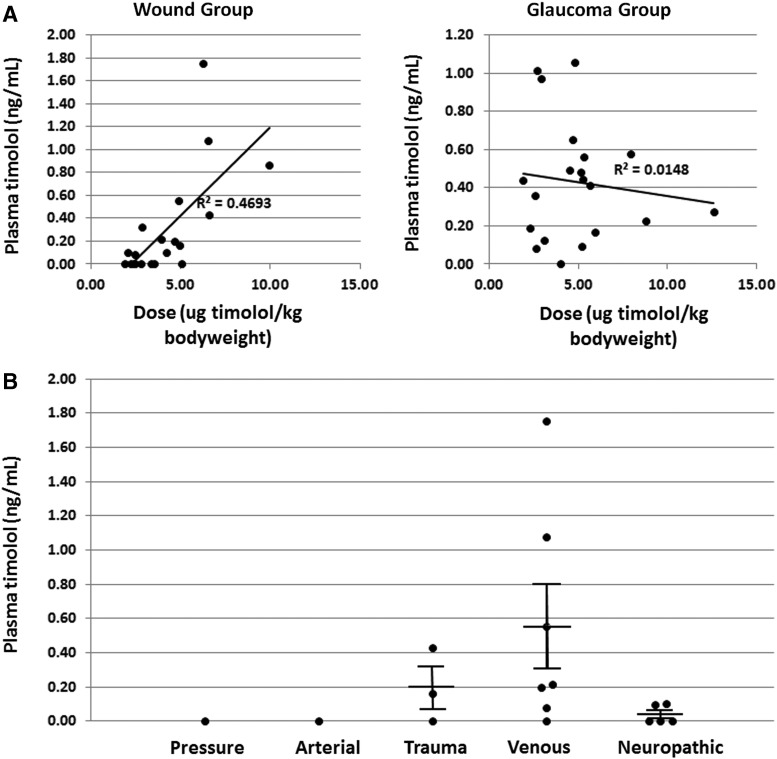
Plasma concentrations of timolol did not differ significantly between the glaucoma (mean = 0.43 ± 0.07 ng/mL; median = 0.43 ng/mL, IQR = 0.38 ng/mL) and wound (mean = 0.29 ± 0.10 ng/mL; median = 0.10 ng/mL, IQR = 0.36 ng/mL) patients receiving the drug topically (Fig. 1). Pearson's correlation coefficient (PCC) was calculated from a linear regression of dose versus plasma timolol concentration for both the wound and glaucoma groups (Fig. 2A). In the wound group, systemic levels were found to be correlated with timolol dose/kg body weight (PCC = 0.69). Interestingly, no such correlation was observed for the glaucoma group (PCC = 0.12), likely because the average dose was lower, and doses did not vary as widely as in the wound group. There was no statistically significant difference in the mean plasma levels of the drug in the different wound categories (e.g., venous, diabetic, pressure ulcer) (Fig. 2B). In both groups, there was no correlation between drug plasma level and body mass index (PCC = 0.24, data not shown).
Figure 1.
Systemic levels of timolol in the glaucoma and chronic wound groups. Plasma was collected from the patients in the glaucoma group and the chronic wound group 1 h after administration of topical timolol 0.5% gel-forming solution to the eye or wound surface. Plasma levels were determined as outlined in the Materials and Methods section. Nonparametric analysis was used to report findings. Kolmogorov–Smirnov normal test showed that eye group data were normally distributed (0.200); however, the wound group data were not normally distributed (0.001). The means plasma levels of both groups were not significantly different (p = 0.26).
Figure 2.
Systemic levels of timolol by dose/body weight and by wound type. (A) The administered timolol dose per kg body weight plotted against the systemic plasma timolol level. Linear regression analysis of the data: R-squared, fitness of the data to the regression line is 0.47 in the chronic wound group and 0.01 in the glaucoma group. (B) The mean plasma level of timolol in patients in each category of wound was tallied. The difference in group means is not statistically significant.
Discussion
The topical application of timolol for dermatologic therapeutic use began in 2010 when first reported for the treatment of infantile hemangiomas.10 More recently, the off-label use of topically applied timolol has been extended to the treatment of chronic cutaneous ulcers. The rationale underpinning this approach derives from preclinical studies that have documented multiple proreparative functional outcomes of blockade of the beta adrenergic receptor in wound cells.11 Beta adrenergic receptor antagonists prevent receptor activation by the endogenous catecholamine agonists, epinephrine and norepinephrine, that are present in the wound and generated by keratinocytes upon injury.12,13 Binding of the agonists to the keratinocyte receptor results in impaired epithelial migration, which translates to delayed re-epithelialization in both in vitro and in vivo wound models.14–17 Receptor activation in dermal fibroblasts delays wound contraction.18 In a murine wound model, elevation of systemic epinephrine levels results in increased neutrophil trafficking to the wound, increased neutrophil dwell time, and maintenance of a proinflammatory cytokine wound milieu.19 All of these outcomes are reversed by beta adrenergic receptor blockade.12,17,19,20 Since chronic wounds are characterized by persistent inflammation and impairment of keratinocyte migration at the wound edge,21 the logical next step was the translation of the preclinical findings to a clinical therapeutic to improve healing.
Timolol, a nonspecific beta1/beta2 adrenergic receptor antagonist, is readily available as an FDA-approved drug for topical administration for the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma, and has been increasingly used as an off-label therapy for nonhealing wounds. A number of cases of chronic ulcers of diverse etiology, including venous ulcers, trauma, surgery, and inflammatory conditions, that have responded to this topical treatment have been reported.2,3,22–25 A prospective observational study of 60 patients with venous leg ulcers, 30 treated with topical timolol and 30 with standard of care, demonstrated efficacy of topical timolol for improving healing.4 A randomized clinical trial to determine efficacy in diabetic foot ulcers is currently underway.26 The increasing use of topical timolol for the indication of chronic ulcers and the lack of safety studies make a formal study of the safety of this treatment all the more valuable.
There are an increasing number of case reports documenting the use of topically applied timolol as a treatment for various other dermatologic diseases or dermatoses. Most reports are limited to single or few cases, but point to the potential of this therapy across multiple etiologies. The early reports of use to improve hemangiomas have propelled application to other entities with a vascular proliferative component, such as angiofibromas, angiosarcoma, lymphatic malformations, Kaposi's sarcoma, and pyogenic granuloma.27–29 The tissue reparative functions noted in the preclinical studies have prompted use in chronic wounds of multiple etiologies, including venous and diabetic ulcers and pyoderma gangrenosum (reviewed in Chen and Tsai27). Indeed, currently there are five clinical trials examining the efficacy of topical timolol in nonglaucoma, nonhemangioma, dermatologic conditions.30 The increasing use of timolol for multiple dermatologic conditions propels the need for cutaneous absorption and safety studies.
Timolol has been used for topical glaucoma therapy for decades. Absorption and safety studies have been performed for this indication for use of timolol.31 It is extremely well tolerated in the eye, but may have systemic side effects despite ocular administration.32 Orally administered timolol must first be detoxified in the liver, resulting in <50% of the drug available for absorption into the systemic circulation.33 However, when applied to the eye, 80% of the drop enters the nasolacrimal passages and can be systemically absorbed. Plasma timolol concentrations after ophthalmic timolol 0.5% solution application are reported to range from 0.87 to 2.45 ng/mL, with a mean value of 1.39 ng/mL at 1 h and of 1.03 ng/mL at 3 h after administration.34 Interestingly, ophthalmic timolol gel has been shown to have lower systemic bioavailability than timolol ophthalmic solution. In adults, peak plasma concentrations after timolol gel instillation averaged <0.3 ng/mL,6 far below the systemic therapeutic range for cardiac effect (10–40 ng/mL).35
Safety studies and contraindications are well documented for topical use of timolol for glaucoma. Contraindications include overt heart failure, second- or third-degree atrioventricular block, and sinus bradycardia. Bronchial asthma is a contraindication as well. Ocular beta blockers must be used with care in poorly controlled diabetics, patients taking beta agonists or xanthines for bronchodilation, or in patients taking beta agonists for inotropic support in early heart failure. Many of these interactions occur infrequently but are important to consider.36
Safety guidelines and contraindications are being developed for the cutaneous indication of topical timolol for pediatric hemangiomas. Two clinical trials in children with infantile hemangiomas concluded that topical timolol was a safe and effective therapeutic option for small superficial hemangiomas, although no systemic drug levels were measured.37 There is, however, one study that reported mild adverse events (AEs) for bradycardia and hypotension, detected in 6% of a total of 36 pediatric patients who received ocularly applied timolol 0.5% solution.38 A more recent prospective trial measured plasma timolol levels in infants receiving topical timolol for hemangiomas, finding levels ranging from 0.3 to 1.6 ng/mL and no AEs.39 However, plasma samples in this study were taken at 3–4.5 h post-timolol administration, when values are decreased relative to peak absorption time of 1–2 h.6 A current clinical trial is underway to evaluate the efficacy, safety, and pharmacokinetics of topical timolol treatment of hemangiomas in infants.40
While topical usage has been evaluated for safety in the infant population, pharmacokinetics and drug responses differ considerably between infants and adults.41 In adults, pharmacokinetics for ocularly administered timolol have demonstrated that timolol is subject to a moderate first pass effect.42 Eighty percent of the oral dose is metabolized in the liver by hydrolytic cleavage of the morpholine ring with subsequent oxidation, whereas 20% is eliminated unchanged in urine.6 Cytochrome P450 enzyme CYP2D6 is extensively involved in its hepatic metabolism. Topical timolol for ocular use half-life varies from 2.0 to 5 h according to the polymorphism exhibited by CYP2D6.6,42 Half-lives of 3.7 and 7.5 h were reported in extensive and poor metabolizers, respectively.43 Timolol bioavailability is ∼60%.43 Its apparent volume of distribution is 1.3–1.7 L/kg,43 and the total body clearance is 463 mL/kg/h6. Plasma protein binding is ∼10%. Timolol crosses the placenta.
This study addresses the gap in knowledge as to the safety of topically applied timolol in the adult population for the indication of chronic wounds, where the absence of an intact epidermis would be expected to facilitate systemic absorption. For comparison, we considered ocular instillation of timolol for the indication of glaucoma due to its long history of safe usage and minimal risk of systemic effects. We found that plasma levels were equivalent in the wound and glaucoma treatment groups; the mean concentrations were 0.29 ± 0.10 ng/mL and 0.43 ± 0.07 ng/mL, respectively (Fig. 1). Although the likelihood of cardiac adverse effects increases with plasma levels >0.7 ng/mL, such events are rare in the absence of predisposing factors.9 Only three patients (15% of the wound cohort) had plasma timolol levels >0.7 ng/mL after receiving either three or four drops (where one patient received four drops) of timolol gel-forming solution on their wounds, as did three patients (15%) in the eye cohort after ocular instillation of timolol. In addition, all values were well below the therapeutic range for systemic beta blockade to achieve cardiac effects (10–40 ng/mL), for orally administered timolol in normal adults.35 This implies that the safety profile for topical application of timolol on chronic wounds is similar to that of ocular instillation of timolol for glaucoma, which is established as a safe treatment.
There are, however, some limitations to this study. Potential differences in drop size of the applied drug were not taken into consideration. A recent study has shown that drop size, and thus dose of drug delivered, can vary significantly with formulation, brand, and the individual user.44 However, all of the patients in this study received the identical formulation (0.5% timolol gel-forming solution) from the same supplier, as sourced by the Veterans Administration pharmacy, thus limiting variability based on those factors. Other factors may influence absorption rate, including location of the lesion and the vascularity of the underlying tissue, as has been demonstrated for timolol absorption from scalp and facial hemangiomas.39 Ophthalmologic literature reports that the total tear volume is ∼30 μL, thus, increasing drop size does not increase dose delivered to the cornea.45
There are a number of limitations to this study. It was not powered for stratification by wound location or type, and larger series will be needed to determine if these factors impact absorption and systemic levels of the drug. In addition, due to the patient population at clinical site (VA), no female patients were examined. Another limitation of this study was the selection of a single time point for determination of the peak plasma timolol concentration after application to chronic wounds. While the peak plasma concentration of timolol after ocular instillation is 1 h post-treatment,6 and thus was chosen for this study, selection of multiple time points may be ideal for determination of peak plasma concentrations after application on wounds. Another limitation of our study is potential patient selection bias during screening phase. It is possible that some patients who were “less tolerant” to timolol may have been screened out since only patients in whom timolol was already prescribed were enrolled in this study. Those with any prior AE in response to a beta blocker were excluded as all patients who were enrolled were receiving ongoing treatment with timolol. Another safety measure limitation was that baseline and postapplication vital signs were not obtained for the glaucoma group, per their ongoing clinical practice. Additional studies will be needed to address these questions.
Conclusion
The study findings support the conclusion that topical application of timolol to chronic wounds has the same safety profile as its use in glaucoma, and may be used in patients following the same precautions as noted for ocular use. In addition, no patient experienced bradycardia or hypotension postadministration of the drug. Together, these findings suggest that topical timolol, in the doses used in this study (one to two drops/day), may be safely applied in patients who have no contraindications, as an adjunctive therapy for chronic wounds with little potential for cardiac effects of systemic beta adrenergic receptor blockade.
Innovation
Chronic wounds are difficult to treat and pose a significant economic burden for patients. Application of timolol 0.5% gel-forming solution to the wound bed is increasingly being done to improve healing of chronic wounds, but safety information for this application is currently limited. Our study provides evidence that the application of timolol 0.5% gel-forming solution to chronic wounds is safe for most patients in the absence of predisposing factors, and that the safety profile for this application is similar to that of the widely used ocular instillation of timolol for treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
Key Findings
The mean single point plasma concentration of timolol 1 h after topical application to chronic wounds was 0.29 ± 0.10 ng/mL, well below the margin of systemic therapeutic action.
No patients developed wheezing or bradycardia after receiving 0.5% timolol solution on their chronic wounds.
The measured plasma concentrations of timolol were not different after topical application to chronic wounds and ocular instillation for treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma (p = 0.27), used here as a benchmark for its long history of safe usage.
Supplementary Material
Acknowledgments and Funding Sources
None to declare.
Abbreviations and Acronyms
- AE
adverse event
- bpm
beats per minute
- HgbA1c
hemoglobin A1c
- HPLC
high-performance liquid chromatography
- PCC
Pearson's correlation coefficient
- QC
quality control
- SD
standard deviation
- SPE
solid phase extraction
- TEA
triethylamine
- VA
Veteran's Affairs
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author(s) listed. No ghostwriters were used to write this article.
About the Authors
Anthony Cole Gallegos, BS, is an analytical chemist who devised the methodology for detection of timolol in plasma. Michael James Davis, MD, was a research fellow with UC Davis Dermatology, is currently an intern, and will be a dermatology resident at Dartmouth in July 2018. Catherine N. Tchanque-Fossuo, MD, MS, was a research fellow with UC Davis Dermatology and is currently a dermatology resident at the University of New Mexico. Kaitlyn West, BS, is a clinical research coordinator at the VA NCHCH. Angela Eisentrout-Melton, MNS, FNP-C, is the wound care and ostomy nurse-practitioner supervisor at VA NCHCS. Thomas R. Peavy, PhD, is a professor in the Department of Biological Sciences at CSUS. Roy W. Dixon, PhD, is professor and chairman of the Department of Chemistry at CSUS. Roma P. Patel, MD, MBA, is Chief of Ophthalmology, VA NCHCS and assistant professor of clinical ophthalmology at UC Davis. Sara Evona Dahle, DPM, MPH, is Chief of Podiatry, VA NCHCS and assistant professor of clinical dermatology at UC Davis. Roslyn Rivkah Isseroff, MD, is Chief of Dermatology, VA NCHCS and professor of dermatology at UC Davis.
Supplementary Material
References
- 1. Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care (New Rochelle). 2019;8:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lev-Tov H, Dahle S, Moss J, Isseroff RR. Successful treatment of a chronic venous leg ulcer using a topical beta-blocker. J Am Acad Dermatol 2013;69:e204–e205 [DOI] [PubMed] [Google Scholar]
- 3. Tang JC, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg 2012;38:135–138 [DOI] [PubMed] [Google Scholar]
- 4. Thomas B, Kurien JS, Jose T, Ulahannan SE, Varghese SA. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg Venous Lymphat Disord 2017;5:844–850 [DOI] [PubMed] [Google Scholar]
- 5. Zimmerman TJ, Kaufman HE. Timolol, a beta-adrenergic blocking agent for the treatment of glaucoma. Arch Ophthalmol 1977;95:601–604 [DOI] [PubMed] [Google Scholar]
- 6. Shedden AH, Laurence J, Barrish A, Olah TV. Plasma timolol concentrations of timolol maleate: timolol gel-forming solution (TIMOPTIC-XE) once daily versus timolol maleate ophthalmic solution twice daily. Doc Ophthalmol 2001;103:73–79 [DOI] [PubMed] [Google Scholar]
- 7. Gallegos A, Peavy T, Dixon R, Isseroff RR. Development of a novel ion-pairing UPLC method with cation-exchange solid-phase extraction for determination of free timolol in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2018;1096:228–235 [DOI] [PubMed] [Google Scholar]
- 8. U.S. National Library of Medicine. Beta adrenergic antagonist for the healing of chronic DFU (BAART-DFU). ClinicalTrials.gov 2018; https://clinicaltrials.gov/ct2/show/NCT03282981?term=NCT03282981&rank=1 (last accessed December30, 2018)
- 9. Maenpaa J, Pelkonen O. Cardiac safety of ophthalmic timolol. Expert Opin Drug Saf 2016;15:1549–1561 [DOI] [PubMed] [Google Scholar]
- 10. Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol 2010;128:255–256 [DOI] [PubMed] [Google Scholar]
- 11. Pullar CE, Manabat-Hidalgo CG, Bolaji RS, Isseroff RR. beta-Adrenergic receptor modulation of wound repair. Pharmacol Res 2008;58:158–164 [DOI] [PubMed] [Google Scholar]
- 12. Sivamani RK, Pullar CE, Manabat-Hidalgo CG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med 2009;6:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sivamani RK, Shi B, Griffiths E, et al. Acute wounding alters the beta2-adrenergic signaling and catecholamine synthetic pathways in keratinocytes. J Invest Dermatol 2014;134:2258–2266 [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Hoffman BB, Isseroff RR. Beta-adrenergic receptor activation inhibits keratinocyte migration via a cyclic adenosine monophosphate-independent mechanism. J Invest Dermatol 2002;119:1261–1268 [DOI] [PubMed] [Google Scholar]
- 15. Pullar CE, Chen J, Isseroff RR. PP2A activation by beta2-adrenergic receptor agonists: novel regulatory mechanism of keratinocyte migration. J Biol Chem 2003;278:22555–22562 [DOI] [PubMed] [Google Scholar]
- 16. Pullar CE, Grahn JC, Liu W, Isseroff RR. Beta2-adrenergic receptor activation delays wound healing. FASEB J 2006;20:76–86 [DOI] [PubMed] [Google Scholar]
- 17. Pullar CE, Zhao M, Song B, et al. Beta-adrenergic receptor agonists delay while antagonists accelerate epithelial wound healing: evidence of an endogenous adrenergic network within the corneal epithelium. J Cell Physiol 2007;211:261–272 [DOI] [PubMed] [Google Scholar]
- 18. Pullar CE, Isseroff RR. Beta 2-adrenergic receptor activation delays dermal fibroblast-mediated contraction of collagen gels via a cAMP-dependent mechanism. Wound Repair Regen 2005;13:405–411 [DOI] [PubMed] [Google Scholar]
- 19. Kim MH, Gorouhi F, Ramirez S, et al. Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta2-adrenergic receptor-mediated upregulation of IL-6. J Invest Dermatol 2014;134:809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pullar CE, Le Provost GS, O'Leary AP, Evans SE, Baier BS, Isseroff RR. beta2AR antagonists and beta2AR gene deletion both promote skin wound repair processes. J Invest Dermatol 2012;132:2076–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle) 2014;3:445–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braun LR, Lamel SA, Richmond NA, Kirsner RS. Topical timolol for recalcitrant wounds. JAMA Dermatol 2013;149:1400–1402 [DOI] [PubMed] [Google Scholar]
- 23. Millsop JW, Trinh N, Winterfield L, Berrios R, Hutchens KA, Tung R. Resolution of recalcitrant pyogenic granuloma with laser, corticosteroid, and timolol therapy. Dermatol Online J 2014;20:pii: [PubMed] [Google Scholar]
- 24. Beroukhim K, Rotunda AM. Topical 0.5% timolol heals a recalcitrant irradiated surgical scalp wound. Dermatol Surg 2014;40:924–926 [DOI] [PubMed] [Google Scholar]
- 25. Liu DY, Fischer R, Fraga G, Aires DJ. Collagenase ointment and topical timolol gel for treating idiopathic pyoderma gangrenosum. J Am Acad Dermatol 2014;71:e225–e226 [DOI] [PubMed] [Google Scholar]
- 26. U.S. National Library of Medicine. Beta Adrenergic antagonist for the healing of chronic DFU. ClinicalTrials.gov 2018; https://clinicaltrials.gov/ct2/results?cond=&term=NCT03282981&cntry=&state=&city=&dist=(last accessed December13, 2018).
- 27. Chen L, Tsai TF. The role of beta-blockers in dermatological treatment: a review. J Eur Acad Dermatol Venereol 2018;32:363–371 [DOI] [PubMed] [Google Scholar]
- 28. Oberlin KE. Expanding uses of propranolol in dermatology. Cutis 2017;99:E17–E19 [PubMed] [Google Scholar]
- 29. Prabha N, Chhabra N, Arora R. Beta-blockers in dermatology. Indian J Dermatol Venereol Leprol 2017;83:399–407 [DOI] [PubMed] [Google Scholar]
- 30. U.S. National Library of Medicine. Clinical trials, search term timolol and skin. ClinicalTrials.gov 2018; https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT02774590+OR+NCT02422017+OR+NCT03452072+OR+NCT03579160+OR+NCT03282981 (last accessed December12, 2018).
- 31. McMahon P, Oza V, Frieden IJ. Topical timolol for infantile hemangiomas: putting a note of caution in “cautiously optimistic”. Pediatr Dermatol 2012;29:127–130 [DOI] [PubMed] [Google Scholar]
- 32. Leier CV, Baker ND, Weber PA. Cardiovascular effects of ophthalmic timolol. Ann Intern Med 1986;104:197–199 [DOI] [PubMed] [Google Scholar]
- 33. Farkouh A, Frigo P, Czejka M. Systemic side effects of eye drops: a pharmacokinetic perspective. Clin Ophthalmol 2016;10:2433–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Passo MS, Palmer EA, Van Buskirk EM. Plasma timolol in glaucoma patients. Ophthalmology 1984;91:1361–1363 [DOI] [PubMed] [Google Scholar]
- 35. Bobik A, Jennings G, Ashley P, Korner PI. Timolol pharmacokinetics and effects on heart rate and blood pressure after acute and chronic administration. Eur J Clin Pharmacol 1979;16:243–249 [Google Scholar]
- 36. Zimmerman TJ. Topical ophthalmic beta blockers: a comparative review. J Ocul Pharmacol 1993;9:373–384 [DOI] [PubMed] [Google Scholar]
- 37. Chan H, McKay C, Adams S, Wargon O. RCT of timolol maleate gel for superficial infantile hemangiomas in 5- to 24-week-olds. Pediatrics 2013;131:e1739–e1747 [DOI] [PubMed] [Google Scholar]
- 38. Plager DA, Whitson JT, Netland PA, et al. Betaxolol hydrochloride ophthalmic suspension 0.25% and timolol gel-forming solution 0.25% and 0.5% in pediatric glaucoma: a randomized clinical trial. J AAPOS 2009;13:384–390 [DOI] [PubMed] [Google Scholar]
- 39. Borok J, Gangar P, Admani S, Proudfoot J, Friedlander SF. Safety and efficacy of topical timolol treatment of infantile haemangioma: a prospective trial. Br J Dermatol 2018;178:e51–e52 [DOI] [PubMed] [Google Scholar]
- 40. U.S. National Library of Medicine. Efficacy, safety and pharmacokinetics of topical timolol in infants with infantile hemangioma. ClinicalTrials.gov 2018; https://clinicaltrials.gov/ct2/show/NCT02913612 (last accessed December13, 2019).
- 41. Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol 2015;79:395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shedden A, Laurence J, Tipping R, Timoptic XESG. Efficacy and tolerability of timolol maleate ophthalmic gel-forming solution versus timolol ophthalmic solution in adults with open-angle glaucoma or ocular hypertension: a six-month, double-masked, multicenter study. Clin Ther 2001;23:440–450 [DOI] [PubMed] [Google Scholar]
- 43. Vlasses PH, Ribeiro LG, Rotmensch HH, et al. Initial evaluation of transdermal timolol: serum concentrations and beta-blockade. J Cardiovasc Pharmacol 1985;7:245–250 [DOI] [PubMed] [Google Scholar]
- 44. Yu J, Keuter T, Schilter K, et al. Variability of delivery of timolol for the treatment of infantile hemangiomas. Pediatr Dermatol 2017;34:458–460 [DOI] [PubMed] [Google Scholar]
- 45. Bartlett J, Jaanas S. Clinical Ocular Pharmacology, 5th ed., Oxford, United Kingdom: Butterworth-Heinemann, 2007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.