Abstract
Background
Paeonia lactiflora is the main active ingredient of peony decoction, which is used to treat ulcerative colitis (UC) in traditional Chinese medicine (TCM). Network pharmacology indicates the multiple interactions among genes, proteins, and metabolites associated with diseases and drugs from the network perspective, which shows the multi-component and multi-target attributes of TCM. This study predicted the pharmacological mechanism of Paeonia lactiflora in the treatment of UC by network pharmacological method.
Material/Methods
Chemical constituents of Paeonia lactiflora were searched from TCMSP data, gene names of target sites were extracted from UniProt database, and disease targets of ulcerative colitis were obtained from the CTD disease database. Use Venny online tools to obtain common targets for drugs and diseases. The DAVID database was used to enrich GO and KEGG for the common target, and the related functions and pathways were obtained. Cytoscape 3.7.1 was used to construct the ‘drug-compound-target-disease’ network.
Results
There are 70 common target genes between Paeonia lactiflora and UC. GO analysis showed that the biological functions of the common target genes of Paeonia lactiflora and UC include response to lipopolysaccharide, response to estradiol, response to drug, positive regulation of nitric oxide biosynthetic process, and steroid hormone-mediated signaling pathway. Enrichment of the KEGG signaling pathway mainly involves signaling pathways, including Pathways in cancer, TNF signaling pathway, Tuberculosis, Hepatitis B, and Toxoplasmosis.
Conclusions
The network pharmacology intuitively shows the multi-component, multi-target, and multi-channel pharmacological effects of Paeonia lactiflora on UC, and provides a scientific basis for studying the mechanism of the effect of Paeonia lactiflora on UC.
MeSH Keywords: Colitis, Ulcerative; Medicine, Chinese Traditional; Pharmacologic Actions
Background
Peony decoction (Baishao) is composed of Paeonia lactiflora, Betelang, Rhubarb, Scutellaria baicalensis, Coptis chinensis, Angelica sinensis, first-class cinnamon, licorice, and banksia rose. It can effectively relieve abdominal pain, fecal pus and blood, and tenesmus [1]. Experimental studies and clinical application show that peony decoction has obvious therapeutic effects on ulcerative colitis [2,3]. Paeonia lactiflora is one of the main active ingredients of peony decoction. The pathogenesis of ulcerative colitis is complex and Paeonia lactiflora contains many ingredients; therefore, its mechanism in treatment of ulcerative colitis needs to be further clarified. It is important to explore the mechanism of Paeonia lactiflora in the treatment of ulcerative colitis from the perspective of systems biology by using the method of network pharmacology.
Network pharmacology is a research method for designing multi-target drug molecules based on the theory of systems biology and network analysis of biological systems by selecting specific signal nodes (Nodes). Network pharmacology emphasizes multi-channel regulation of signaling pathways in order to improve the therapeutic effect of drugs, reduce toxic and adverse effects, enhance the success rate of clinical trials of new drugs, and reduce the cost of drug research and development [4,5]. After thousands of years of clinical practice, traditional Chinese medicine has accumulated rich experience. Traditional Chinese medicine and its prescriptions are multi-component, multi-channel, and multi-target, and show advantages in the treatment and prevention of diseases [6]. However, because the mechanism of traditional Chinese medicine is difficult to quantify, its scientific nature has been questioned. At present, network pharmacology has been applied in the field of traditional Chinese medicine, which provides a basis for revealing the mechanism of action of traditional Chinese medicine [7]. For example, Yu et al. found that in the progression of asthma, 8 major putative targets of Yin-Huang-Qing-Fei capsule were associated with the inflammatory process [8]. Chen et al. reported that 34 proteins and 28 related pathways of YXST may explain the mechanism by which Yangxinshi tablet acts on heart failure and myocardial infarction [9]. Zhang et al. found 66 QiXueHe Capsule candidate targets that may be used to treat menstrual disorders [10]. The purpose of the present study was to use network pharmacology to analyze the mechanism by which Paeonia lactiflora is effective in treating ulcerative colitis, and to provide a reference for further experimental verification.
Material and Methods
Major compounds and related targets of Paeonia lactiflora were obtained from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database. The screening criteria were: oral bioavailability (OB) >30% and drug-like (DL) properties >0.15. The genes corresponding to the target were queried through the UniProt database. With ‘Ulcerative colitis’ as the key word, the CTD disease database was used to search for disease target genes of ulcerative colitis. The Venny online tool was used to obtain common target genes for drugs and diseases. GO and KEGG enrichment analysis of common target genes were performed using the DAVID database to obtain the related functions and pathways. The screening criterion was FDR <0.05. Cytoscape 3.7.1 was used to construct the ‘drug-compound-target gene-disease’ network.
Results
Drug target prediction
The chemical constituents and targets of Paeonia lactiflora were predicted in the TCMSP database. A total of 13 compounds of Paeonia lactiflora were obtained, as shown in Table 1. We could not obtain related targets from 5 of them (11alpha, 12alpha-epoxy-3beta-23-dihydroxy-30-norolean-20-en-28, 12beta-olide, albiflorin_qt, benzoyl paeoniflorin, Lactiflorin, and paeoniflorin_qt). We entered the target name and selected “person” as the species in the UniProt database. As a result, 71 genes corresponding to the target were identified.
Table 1.
Compounds in Paeonia lactiflora.
| Mol ID | Molecule name | OB (%) | DL |
|---|---|---|---|
| MOL000492 | (+)-catechin | 54.83 | 0.24 |
| MOL001919 | (3S,5R,8R,9R,10S,14S)-3,17-dihydroxy-4,4,8,10,14-pentamethyl-2,3,5,6,7,9-hexahydro-1H-cyclopenta[a]phenanthrene-15,16-dione | 43.56 | 0.53 |
| MOL001910 | 11alpha,12alpha-epoxy-3beta-23-dihydroxy-30-norolean-20-en-28,12beta-olide | 64.77 | 0.38 |
| MOL001928 | Albiflorin_qt | 66.64 | 0.33 |
| MOL001930 | Benzoyl paeoniflorin | 31.27 | 0.75 |
| MOL000358 | beta-sitosterol | 36.91 | 0.75 |
| MOL000422 | Kaempferol | 41.88 | 0.24 |
| MOL001921 | Lactiflorin | 49.12 | 0.8 |
| MOL000211 | Mairin | 55.38 | 0.78 |
| MOL001918 | Paeoniflorgenone | 87.59 | 0.37 |
| MOL001924 | Paeoniflorin | 53.87 | 0.79 |
| MOL001925 | Paeoniflorin_qt | 68.18 | 0.4 |
| MOL000359 | Sitosterol | 36.91 | 0.75 |
Disease target prediction
By searching the keyword ‘ulcerative colitis’ in the CTD disease database, 14 775 disease targets were obtained.
Intersection target
A total of 70 common targets for drugs and diseases are obtained using the Venny online tools, as shown in Figure 1.
Figure 1.
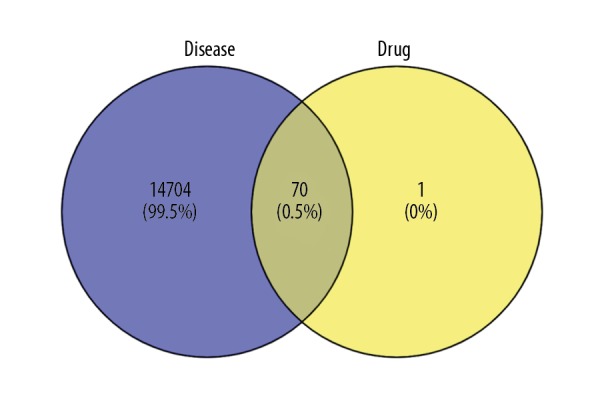
Venn diagram of disease and drug targets.
GO analysis of drug-disease intersection target gene
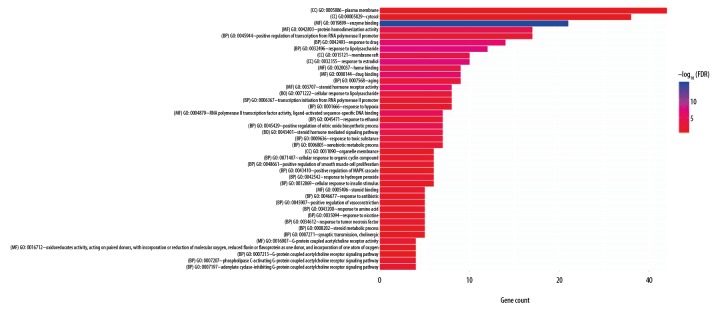
We imported 70 common target genes into the DAVID database and selected “person” as the species and set FDR at <0.05, yielding 41 enrichment results. The enrichment of biological processes is shown in Table 2. The ggplot2.R package was used for visualization, and the results are shown in Figure 2. The biological processes include response to lipopolysaccharide, response to estradiol, response to drug, positive regulation of nitric oxide biosynthetic process, and steroid hormone mediated signaling pathway.
Table 2.
GO functional enrichment analysis of common targets (top 10).
| Biological processes | GO ID | Related targets | FDR |
|---|---|---|---|
| Response to lipopolysaccharide | 0032496 | OPRM1, VCAM1, CASP3, CYP1A1, CASP9, PTGS2, JUN, CASP8, SLPI, CYP1A2, LBP, SELE | 9.99E-08 |
| Response to estradiol | 0032355 | CASP3, CASP9, PTGS2, SLC6A4, CASP8, ESR1, F7, CAT, CYP1A2, GSTP1 | 1.90E-07 |
| Response to drug | 0042493 | ICAM1, CASP3, IL6, CYP1A1, PTGS2, SLC6A2, JUN, BCL2, RELA, SLC6A4, PPARG, ADRA1A, CAT, STAT1 | 4.40E-07 |
| Positive regulation of nitric oxide biosynthetic process | 0045429 | OPRM1, AKT1, ICAM1, IL6, PTGS2, ESR1, INSR | 3.38E-05 |
| Steroid hormone mediated signaling pathway | 0043401 | PGR, NR1I3, NR1I2, RXRA, PPARG, NR3C2, ESR1 | 1.93E-04 |
| Aging | 0007568 | VCAM1, AKT1, IL6, CYP1A1, CASP9, RELA, JUN, ADRA1A, CAT | 5.98E-04 |
| Cellular response to lipopolysaccharide | 0071222 | ICAM1, IL6, RELA, MAPK8, LBP, NOS2, CD14, GSTP1 | 6.43E-04 |
| Xenobiotic metabolic process | 0006805 | CYP3A4, NR1I2, CYP1B1, PTGS1, CYP1A2, GSTP1, AHR | 4.00E+00 |
| Response to toxic substance | 0009636 | CYP1B1, BAX, BCL2, SLC6A4, PON1, GSTP1, AHR | 1.00E+00 |
| Positive regulation of transcription from RNA polymerase II promoter | 0045944 | AR, IL6, RXRA, RELA, PPARG, ESR1, STAT1, AHR, PGR, AKT1, ADRB2, NR1I3, NR1I2, NCOA2, JUN, PPP3CA, IKBKB | 3.00E+00 |
Figure 2.
GO functional enrichment. The X-axis represents the number of genes enriched in function, the y-axis GO function annotation, and the color represents the significance of enrichment. The bluer the color, the higher the significance.
Analysis of KEGG pathway enrichment
We imported 70 common target genes into the DAVID database, selected “person” as the species, and set FDR as <0.05, producing 41 enrichment results. The enrichment of biological processes is shown in Table 3. The ggplot2.R (3.2.0 Version) package was used for visualization and the results are shown in Figure 3. The target gene network of Paeonia lactiflora-ulcerative colitis is mainly related to Pathways in cancer, TNF signaling pathway, Tuberculosis, Hepatitis B, and Toxoplasmosis.
Table 3.
KEGG functional enrichment analysis of common targets (top 10).
| Term | Fold enrichment | Related targets | FDR |
|---|---|---|---|
| hsa05200: Pathways in cancer | 5.739 | PRKCA, AR, IL6, PTGS2, RXRA, RELA, PPARG, STAT1, MMP1, AKT1, CASP3, CASP9, JUN, BAX, BCL2, CASP8, MAPK8, NOS2, IKBKB, GSTP1 | 4.45E-07 |
| hsa04668: TNF signaling pathway | 12.647 | VCAM1, AKT1, ICAM1, CASP3, IL6, PTGS2, RELA, JUN, CASP8, MAPK8, IKBKB, SELE | 1.69E-06 |
| hsa05152: Tuberculosis | 8.9197 | AKT1, CASP3, IL6, CASP9, BCL2, RELA, BAX, CASP8, MAPK8, LBP, PPP3CA, NOS2, STAT1, CD14 | 3.06E-06 |
| hsa05161: Hepatitis B | 10.11 | PRKCA, IL6, RELA, STAT1, AKT1, CASP3, CASP9, BCL2, BAX, JUN, CASP8, MAPK8, IKBKB | 3.49E-06 |
| hsa05145: Toxoplasmosis | 11.277 | AKT1, CASP3, CASP9, RELA, BCL2, CASP8, MAPK8, ALOX5, NOS2, IKBKB, STAT1 | 3.40E-05 |
| hsa04620: Toll-like receptor signaling pathway | 10.639 | AKT1, IL6, RELA, JUN, CASP8, MAPK8, LBP, IKBKB, STAT1, CD14 | 3.26E-04 |
| hsa04932: Non-alcoholic fatty liver disease (NAFLD) | 8.2151 | AKT1, CASP3, IL6, RELA, JUN, RXRA, BAX, CASP8, MAPK8, IKBKB, INSR | 6.91E-04 |
| hsa04210: Apoptosis | 14.551 | AKT1, CASP3, CASP9, RELA, BAX, BCL2, CASP8, IKBKB | 0.001105 |
| hsa05164: Influenza A | 7.1292 | PRKCA, AKT1, ICAM1, IL6, CASP9, RELA, JUN, PRSS1, MAPK8, IKBKB, STAT1 | 0.002545 |
| hsa05204: Chemical carcinogenesis | 11.277 | GSTM1, CYP3A4, GSTM2, CYP1B1, CYP1A1, PTGS2, CYP1A2, GSTP1 | 0.006317 |
Figure 3.
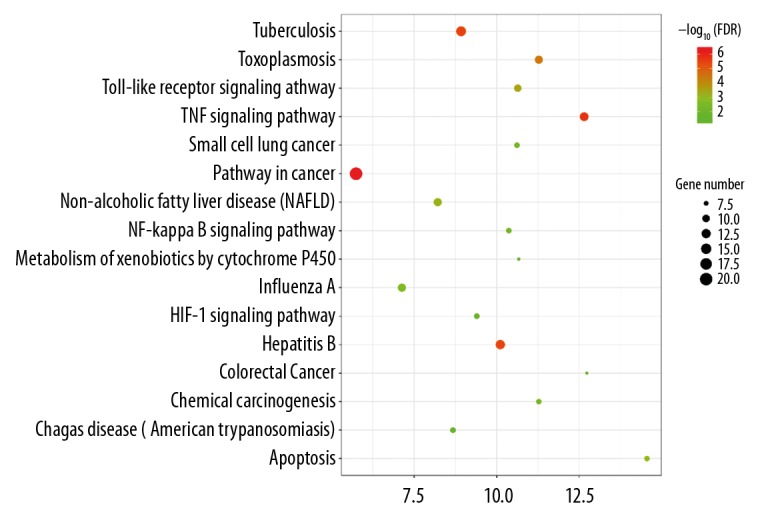
KEGG pathway enrichment.
Drug-compound-target-disease network
Cytoscape 3.7.1 software was used to construct the ‘drug-compound-target-disease’ network of Paeonia lactiflora, and the interaction among drugs, compounds, diseases, and targets was obtained. The results are shown in Figure 4.
Figure 4.
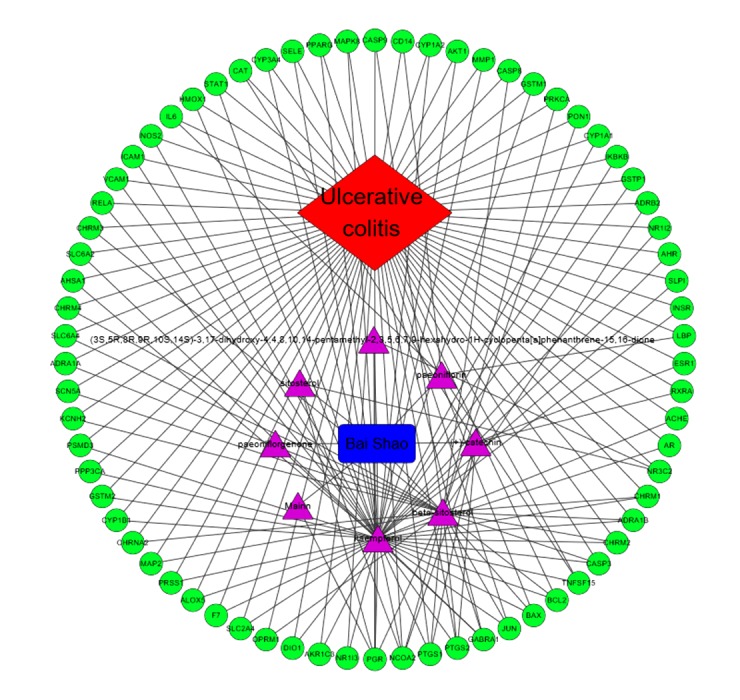
Drug-compound-target-disease network map.
Discussion
The occurrence of ulcerative colitis (UC) involves genetic, biochemical, psychological, social, and environmental factors [11]. Many scholars have studied the pathogenesis of UC in terms of infection factors, immune factors, and genetic factors [12,13]. In view of the complex pathogenesis of ulcerative colitis, it is difficult for a single target drug to have a better therapeutic effect, and multi-target combination drug therapy has become the trend of UC treatment in the future [14]. Scholars regard traditional Chinese medicine as a library of combinatorial compounds [15,16]. Because of the multi-target effect of TCM, it has become an important source of UC therapeutic drugs [17,18]. By exploring the relationship between drugs and diseases from a holistic perspective, network pharmacology is holistic and systematic, which provides a new strategy for the study of traditional Chinese medicine.
Paeonia lactiflora is an important traditional Chinese medicine, which has been widely used in anti-inflammatory, analgesic, and immune regulation for hundreds of years [19]. In this study, the TCMSP database was used to predict the chemical composition and targets of Paeonia lactiflora. A total of 13 compounds of Paeonia lactiflora were found, of which 5 compounds had no corresponding target. Further target prediction indicated that 71 targets were related to 8 active ingredients. UC disease targets were analyzed using the CTD network online analysis platform, and 70 common target genes between drug and disease were obtained using the Venny online tool. Our exploration of the biological function of target genes of Paeonia lactiflora in the treatment of UC showed that the common target genes between Paeonia lactiflora and UC included OPRM1, VCAM1, CASP3, CYP1A1, CASP9, IL6, BCL2, AKT1, PTGS2, JUN, CASP8, SLPI, and CYP1A2. These target genes are involved in biological processes, including response to lipopolysaccharide, response to estradiol, response to drug, positive regulation of nitric oxide biosynthetic process, steroid hormone-mediated signaling pathway, aging, cellular response to lipopolysaccharide, xenobiotic metabolic process, response to toxic substance, and positive regulation of transcription from RNA polymerase II promoter. This also indicates that UC involves abnormalities in many biological processes in vivo, similar to the results of previous pathological studies of UC. For example, Obana et al. reported ulcerative colitis was related to a promoter polymorphism of the lipopolysaccharide receptor gene, CD14 [20]. Crotty reported ulcerative colitis resulted from a reactive xenobiotic metabolite that is conjugated before excretion into the bile [21]. Principi et al. reviewed the estrogen receptors had been reported to have anti-inflammatory and anti-tumor roles in the colon, suggesting a translational potential that could prevent and/or treat UC [22]. These results suggest that Paeonia lactiflora could be used in the treatment of UC by improving these biological processes.
KEGG pathway enrichment analysis results were mainly related to Pathways in cancer, TNF signaling pathway, Tuberculosis, Hepatitis B, Toxoplasmosis, Toll-like receptor signaling pathway, Non-alcoholic fatty liver disease (NAFLD), Apoptosis, Influenza A, and Chemical carcinogenesis. This is consistent with the previous research results of UC molecular biology. For example, TNF-α, as a pro-inflammatory and regulatory factor, plays an important role in the development of UC and disease progression because TNF promotes the release of pro-inflammatory factors [23]. In addition, it can work with interferon to change the barrier function of intestinal epithelial cells, enhance the permeability of intestinal mucosa and vascular wall, destroy the integrity of intestinal mucosa, and form ulcers [24]. Toll-like receptors are important mediators of innate defense of intestinal mucosa and play an important role in maintaining intestinal mucosa and intestinal microecology. Published research confirmed there is a relationship between nonsynonymous variants in the TLR1, TLR2, and TLR6 genes and extensive colonic disease in UC [25]. Therefore, it is speculated that Paeonia lactiflora exerts its pharmacodynamic effects by affecting the related signaling pathways by acting on the related targets. Previous studies have confirmed the role of Paeonia lactiflora in ulcerative colitis, as well as our results. For example, Liu et al. has confirmed that Paeonia lactiflora can treat UC by reducing the expression of pro-inflammatory factors, increasing the body’s antioxidant effect, and reducing intestinal mucosal permeability in mice [26]. Fang et al. found that paeoniflorin significantly reduced the levels of TNF-alpha, IL-1, IL-10, 5-HT, TLR4, MyD 88, and NF-kappa B p65, and upregulated the expression of Tollip in ulcerative colon by significantly reducing the levels of TNF-alpha, IL-1, IL-10, and 5-HT [27].
The present study focused on inflammation-related signaling pathways, including the TNF signaling pathway and Toll-like receptor signaling pathway. These pathways mainly involve the genes VCAM1, AKT1, ICAM1, CASP3, IL6, PTGS2, RELA, JUN, CASP8, MAPK8, IKBKB, SELE, LBP, STAT1, and CD14. These genes have also been reported to be involved in the mechanism of UC. For example, PTGS2 participates in inflammatory regulation and antioxidant response [28]. IL6 gene expression is closely related to the progression of IBD [29]. JUN is a proto-oncogene and plays a key role in inflammation. Many inflammatory factors activate JUN directly or indirectly. Activated JUN further regulates the expression and regulation of related inflammatory factors, and then participates in the inflammatory response [30]. Therefore, Paeonia lactiflora has anti-inflammatory and immune effects by acting on these genes.
Although the present research revealed the mechanism of Paeonia lactiflora in the treatment of ulcerative colitis from the perspective of network pharmacology, it still has some limitations. The main limitation of this study is the lack of experimental verification, which will be further expanded in future research.
Conclusions
The results of this analysis show that Paeonia lactiflora acts on multiple targets and plays a therapeutic role on UC through multiple pathways. These predicted targets and pathways are consistent with the pharmacological effects reported in the literature. In addition, there are few reports about some targets in the results, which could provide clues for further study of the molecular mechanism of potential targets of Paeonia lactiflora in the treatment of UC. This study confirmed the therapeutic effect of the traditional Chinese medicine Paeonia lactiflora on UC by use of network pharmacological method, which embodies the multi-component, multi-target and integrated regulation of traditional Chinese medicine prescriptions, and provides a basis for further study of the pharmacological mechanism of Paeonia lactiflora in treatment of UC.
Appendix.
Access to resources:
TCMSP: http://lsp.nwu.edu.cn/tcmsp.php
CTD: http://ctdbase.org/
DAVID: https://david.ncifcrf.gov/
UniProt: https://www.uniprot.org/
Venny: http://bioinfogp.cnb.csic.es/tools/venny/
ggplot2.R(3.2.0 Version): https://cran.r-project.org/web/packages/ggplot2/
Cytoscape 3.7.1: https://cytoscape.org/
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.He DY, Dai SM. Anti-Inflammatory and immunomodulatory effects of Paeonia Lactiflora Pall., a traditional Chinese herbal medicine. Front Pharmacol. 2011;2:10. doi: 10.3389/fphar.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li HB, Cheng LL. [Shaoyao decoction combined with acupuncture in the treatment of active ulcerative colitis]. Jilin Journal Traditional Chinese Medicine. 2018;38:331–34. [in Chinese] [Google Scholar]
- 3.Yang L, Tang YP, Gong YX, et al. [Meta-analysis of shaoyao decoction combined with aminosalicylic acid in the treatment of ulcerative colitis]. Chinese Journal of Integrated Traditional and Western Medicine on Digestion. 2017;25:168–73. [in Chinese] [Google Scholar]
- 4.Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25:1110–11. doi: 10.1038/nbt1007-1110. [DOI] [PubMed] [Google Scholar]
- 5.Zhang A, Sun H, Yang B, Wang X. Predicting new molecular targets for rhein using network pharmacology. BMC Syst Biol. 2012;6:20. doi: 10.1186/1752-0509-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Wang Y, Fan X, Qu H, et al. Strategies and techniques for multi-component drug design from medicinal herbs and traditional Chinese medicine. Curr Top Med Chem. 2012;12:1356–62. doi: 10.2174/156802612801319034. [DOI] [PubMed] [Google Scholar]
- 7.Hao DC, Xiao PG. Network pharmacology: A Rosetta Stone for traditional Chinese medicine. Drug Dev Res. 2014;75(5):299–312. doi: 10.1002/ddr.21214. [DOI] [PubMed] [Google Scholar]
- 8.Yu GH, Zhang YQ, Ren WQ, et al. Network pharmacology-based identification of key pharmacological pathways of Yin-Huang-Qing-Fei capsule acting on chronic bronchitis. Int J Chron Obstruct Pulmon Dis. 2017;12:85–94. doi: 10.2147/COPD.S121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Cao Y, Zhang H, et al. Network pharmacology-based strategy for predicting active ingredients and potential targets of Yangxinshi tablet for treating heart failure. J Ethnopharmacology. 2018;219:359–68. doi: 10.1016/j.jep.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Xia M, Su J, et al. A network pharmacology-based strategy deciphers the underlying molecular mechanisms of Qixuehe Capsule in the treatment of menstrual disorders. Chin Med. 2017;12:23. doi: 10.1186/s13020-017-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinozaki M, Sawada T, Watanabe T, et al. [Influencing factors on occurrence and relapse in ulcerative colitis]. Nihon Rinsho. 1999;57:2420–25. [in Japanese] [PubMed] [Google Scholar]
- 12.Sarlos P, Kovesdi E, Magyari L, et al. Genetic update on inflammatory factors in ulcerative colitis: Review of the current literature. World J Gastrointest Pathophysiol. 2014;5:304–21. doi: 10.4291/wjgp.v5.i3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ananthakrishnan AN, Oxford EC, Nguyen DD, et al. Genetic risk factors for Clostridium difficile infection in ulcerative colitis. Aliment Pharmacol Ther. 2013;38:522–30. doi: 10.1111/apt.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao B, Zhang Z, Viennois E, et al. Combination therapy for ulcerative colitis: Orally targeted nanoparticles prevent mucosal damage and relieve inflammation. Theranostics. 2016;6:2250–66. doi: 10.7150/thno.15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu F, Kong L, Zou H, Lei X. Progress on the screening and analysis of bioactive compounds in traditional chinese medicines by biological fingerprinting analysis. Comb Chem High Throughput Screen. 2010;13:855–68. doi: 10.2174/138620710793360356. [DOI] [PubMed] [Google Scholar]
- 16.Wang CY, Bai XY, Wang CH. Traditional Chinese medicine: A treasured natural resource of anticancer drug research and development. Am J Chin Med. 2014;42:543–59. doi: 10.1142/S0192415X14500359. [DOI] [PubMed] [Google Scholar]
- 17.Chen XL, Wen Y, Wu ZC, et al. Development of a traditional Chinese medicine syndrome-specific scale for ulcerative colitis: The large intestine dampness-heat syndrome questionnaire. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/4039019. 4039019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye B, Shen H, Lu Y, Wang YQ. Clinical observations on 100 cases of ulcerative colitis treated with the method of clearing away heat, expelling dampness, promoting blood circulation and healing ulcer. J Tradit Chin Med. 2010;30:98–102. doi: 10.1016/s0254-6272(10)60022-2. [DOI] [PubMed] [Google Scholar]
- 19.Parker S, May B, Zhang C, et al. Pharmacological review of bioactive constituents of paeonia lactiflora pallas and paeonia veitchii lynch. Phytother Res. 2016;30:1445–73. doi: 10.1002/ptr.5653. [DOI] [PubMed] [Google Scholar]
- 20.Obana N, Takahashi S, Kinouchi Y, et al. Ulcerative colitis is associated with a promoter polymorphism of lipopolysaccharide receptor gene, CD14. Scand J Gastroenterol. 2002;37:699–704. doi: 10.1080/00365520212504. [DOI] [PubMed] [Google Scholar]
- 21.Crotty B. Ulcerative colitis and xenobiotic metabolism. Lancet. 1994;343:35–38. doi: 10.1016/s0140-6736(94)90882-6. [DOI] [PubMed] [Google Scholar]
- 22.Principi M, Barone M, Pricci M, et al. Ulcerative colitis: From inflammation to cancer. Do estrogen receptors have a role? World J Gastroenterol. 2014;20:11496–504. doi: 10.3748/wjg.v20.i33.11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh K, Xiao SD. Inflammatory bowel disease: A survey of the epidemiology in Asia. J Dig Dis. 2009;10:1–6. doi: 10.1111/j.1751-2980.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 24.Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999;34:66–74. doi: 10.1007/s005350050218. [DOI] [PubMed] [Google Scholar]
- 25.Pierik M, Joossens S, Van Steen K, et al. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm Bowel Dis. 2006;12:1–8. doi: 10.1097/01.mib.0000195389.11645.ab. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Li LT, Ji XQ, et al. [Effects and mechanism of couplet medicines of Scutellaria baicalensis-Paeonia lactiflora on improving ulcerative colitis in mice]. China Pharmacy. 2018;29:356–60. [in Chinese] [Google Scholar]
- 27.Fang XH, Wu X, Zhu XM, et al. Albiflorin attenuates inflammatory injury by regulating the TLR4 signaling pathway and its negative regulating factor Tollip in experimental models of ulcerative colitis. J Chinese Pharmaceutical Sci. 2016;5:366–72. [Google Scholar]
- 28.Mansour DF, Saleh DO, Mostafa RE. Genistein ameliorates cyclophosphamide – induced hepatotoxicity by modulation of oxidative stress and inflammatory mediators. Open Access Maced J Med Sci. 2017;5:836–43. doi: 10.3889/oamjms.2017.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pizarro TT, De La Rue SA, Cominelli F. Role of interleukin 6 in a murine model of Crohn’s ileitis: Are cytokine/anticytokine strategies the future for IBD therapies? Gut. 2006;55:1226–27. doi: 10.1136/gut.2005.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YS, Ahn Y, Hong MH, et al. Rosuvastatin suppresses the inflammatory responses through inhibition of c-Jun N-terminal kinase and Nuclear Factor-kappaB in endothelial cells. J Cardiovasc Pharmacol. 2007;49:376–83. doi: 10.1097/FJC.0b013e31804a5e34. [DOI] [PubMed] [Google Scholar]