Abstract
Significance: Spinal cord injury (SCI) is a neurological disorder that resulted from destroyed long axis of spinal cord, affecting thousands of people every year. With the occurrence of SCI, the lesions can form cystic cavities and produce glial scar, myelin inhibitor, and inflammation that negatively impact repair of spinal cord. Therefore, SCI remains a difficult problem to overcome with present therapeutics. This review of cell therapeutics in SCI provides a systematic review of combinatory therapeutics of SCI and helps the realization of regeneration of spinal cord in the future.
Recent Advances: With major breakthroughs in neurobiology in recent years, present therapeutic strategies for SCI mainly aim at nerve regeneration or neuroprotection. For nerve regeneration, the application approaches are tissue engineering and cell transplantation, while drug therapeutics is applied for neuroprotection. Cell therapeutics is a new approach that treats SCI by cell transplantation. Cell therapeutics possesses advantages of neuroprotection, immune regulation, axonal regeneration, neuron relay formation, and remyelination.
Critical Issues: Neurons cannot regenerate at the site of injury. Therefore, it is essential to find a repair strategy for remyelination, axon regeneration, and functional recovery. Cell therapeutics is emerging as the most promising approach for treating SCI.
Future Directions: The future application of SCI therapy in clinical practice may require a combination of multiple strategies. A comprehensive treatment of injury of spinal cord is the focus of the present research. With the combination of different cell therapy strategies, future experiments will achieve more dramatic success in spinal cord repair.
Keywords: spinal cord injury, regeneration, anatomical structure, cell therapeutics, neurotrophic factors

Yingji Mao, PhD

Wenguo Cui, PhD
Scope and Significance
Cell therapeutics is emerging as the most promising approach for treating spinal cord injury (SCI). This review summarizes cell therapeutics with respect to challenges in regeneration, cell types, and related neurotrophic factors. The work described here will contribute to improving multifaceted combination of strategies for SCI regeneration in clinical application.
Translational Relevance
The mechanism of cell therapeutics is widely defined as the direct or indirect interaction between transplanted cells and host cells, which changes the local microenvironment and thereby affects the histological or functional results after SCI. By now, many experimental repair strategies developed by different research groups have focused on promoting axonal growth for various cell transplants by improving the inhospitable central nervous system (CNS) environment.
Clininal Relevance
Traumatic SCIs are generally caused by external trauma such as car accidents, falls, and violent acts, which can cause disability or even mortality, as well as loss of sensory and motor function. In the past, treatment of SCI was generally palliative such as preventing damage from worsening, treating complications, and guiding patients to deal with their disabilities. Fortunately, with major breakthroughs in neurobiology in recent years, more effective interventions were invented such as cell therapeutics, which will provide new approaches to accelerate functional recovery after SCI.
Discussion of Findings and Relevant Literature
Anatomical structure of spinal cords
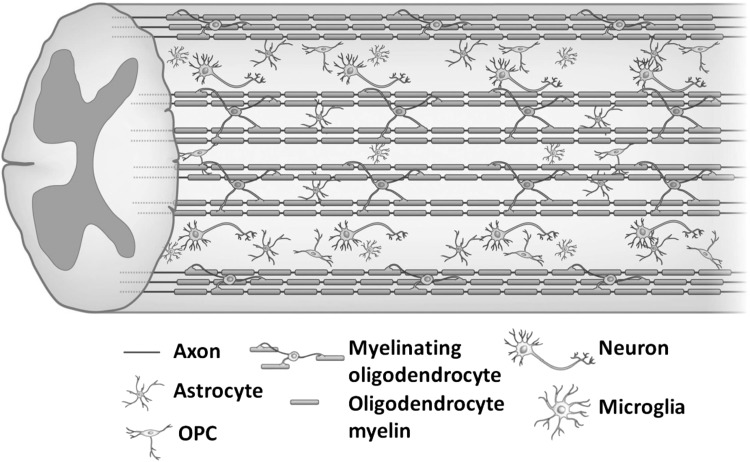
Spinal cord originates at the medulla of the bottom of brain and reaches the first lumbar vertebra through occipital foramen. In the center of spinal cord, there is a gray area with a butterfly shape, which is called gray matter.1 The gray matter is composed of numerous neuronal cell bodies, dendrites, a few myelinated and unmyelinated axons, glial cells, and capillaries.2 White matter surrounds the central gray matter, consisting of oligodendrocytes, astrocytes, and microglia (Fig. 1). Oligodendrocyte precursor cells (OPCs) are distributed throughout the white and gray matter. Oligodendrocytes are distributed in the CNS. They are located near the cell body of neurons and around nerve fibers, and their protruding ends expand into a flat membrane and wrap the axons of neurons to form an insulating myelin sheath structure. At the same time, it can assist the efficient transmission of bioelectrical signals and maintain and protect the normal functions of neurons.1 While astrocytes are related to the blood/brain barrier that separates the CNS from proteins and cells in the blood.1 Microglia play vital roles in synaptic pruning and remodeling, removing debris from both developmental and damaged cells.3,4 The axons are surrounded by a myelin sheath formed by Schwann cells (SCs), and the outer layer is surrounded by the endoneurium. Next, the individual axons converge to form fascicles. Finally, the individual nerve fascicles are surrounded by the epineurium of a loose fibrocollagenous tissue component, which combines to form the nerve trunk.5
Figure 1.
Schematic representation of the spinal cord. Reproduced with permission from Assinck et al.125
SCI animal models and application limitations
Choosing an animal SCI model is a very important step for spinal cord repair experiment. Rats and mice are the first choice of SCI animal models because they are inexpensive and easy to preserve.6,7 Although a primate SCI model is closer to humans, its use is limited due to ethical issues. At present, the most commonly used SCI models are contusion, compression, hemisection, and transection models.
A contusion model is generated by striking an exposed spinal cord with a heavy fall, which is normally achieved by an impactor consisting of an animal restraint device and a computer. By adjusting the height, impulse, velocity, power, and weight, the degree of damage can be easily controlled.8 Specific segments of spinal cord can be selected to replicate the contusion model in different degrees and different parts of spinal cord. The model retains the integrity of the dura, and it is close to the pathophysiological characteristics and changing rules of human SCI. Infinite Horizon impactor is the most commonly used instrument for creating spinal cord contusion at present. It can quickly strike the exposed spinal cord with a stainless steel impactor at the tip and then immediately retract.9 It does not cause crush injury due to the short stay of the impactor in the spinal cord. However, compared with the crosscut model, it is difficult to distinguish the tissue at the original site from the regenerated tissue in the contusion model after repair. In addition, due to the influence of many factors (such as animal size, fixed body position, and spinal cord exposure), the injury degree of experimental animals is not consistent, leading to differences among individuals.
The compression model simulates SCI caused by space-occupying lesions in the spinal canal, which can be created by using balloons, tweezers, arterial clamps, heavy weights, or other materials.10,11 Similar to the injury model caused by impactor, the compression model can be produced in the spinal cord in different parts and degrees by adjusting compression position, compression time, and intensity. The pathophysiological process of acute compression injury model is similar to that of the spinal cord impingement model, with diffuse hemorrhagic necrosis and edema in the early stage and cyst and glial scar in the later stage. Similar to the contusion model, it is difficult to distinguish the regenerated tissue from the original tissue.
The hemisection model is formed by cutting the left or right part of spinal cord. Since one side of the spinal cord is undamaged, the bladder and bowel functions are preserved. Moreover, the postoperative care of the animals is easy and the survival rate is high, which are suitable for studying the axon sprouting at the junction between the injured side and the uninjured side of the spinal cord.8 The back hemisection seriously affects the stability, speed, and accuracy of the crawling process of animals.8 Due to the nonuniformity of the hemisection, the injury models of animals are different. In addition, it is difficult to determine whether axons at the injured site regenerate from the hemisection or sprouting at the unresected site.
The method of cutting the entire spinal cord with a sharp instrument with the dura cut completely is called the transection model.12 The transection model reflects the regeneration of axons in SCI, because the axons are completely separated at the injury site and there is no remaining axon. Therefore, the regeneration of axons can be evaluated at the injury site. The transection model completely destroys the axon fibers and the connected neurons in the spinal cord, leading to paralysis and serious complications, and postoperative animal mortality is higher. Compared with other models, postoperative nursing is more challenging. For example, complications in the canine model of complete spinal cord transection include deep vein thrombosis, pressure ulcers, muscle spasms, osteoporosis, urinary tract infections, and respiratory complications.13
In summary, different SCI models have different advantages and disadvantages regarding different experimental purposes. The contusion and compression models are closely related to the pathophysiology of patients with SCI in clinical practice, while the hemisection and transection are more valuable for the study of spinal axonal regeneration. Therefore, to make the animal model more valuable, the primate model of spinal cord transection is the best research program.
Challenges in SCI regeneration
The occurrence of SCI will lead to cystic cavity and glial scar.14 Cells at the initial stage of injury swell and the damaged cells secrete toxins,15 promoting necrosis of the damaged cells and resulting in formation of cystic cavity surrounded by glial scar.16 Moreover, the inhibitory molecules and inflammation generated after injury also inhibit regeneration of spinal cords.17
Cystic cavity formation
After initial injury of spinal cord and the followed necrosis, a fluid-filled cavity develops, which can limit axon regeneration and cell migration.18 Several studies demonstrated that cystic cavity could extend to other undamaged spinal segments around the injury, resulting in cell death and loss of function of undamaged spinal segments. Moreover, formation of cystic cavity generates a physical barrier to restrict neurotrophic factor infiltration and inhibit signal transduction. Cell transplantation was expected to reduce cystic cavity formation, restore the signal in the spinal cord, and facilitate axon regeneration.19,20
Glial scars and chondroitin sulfate proteoglycan
Many of the limiting factors associated with glial scar are closely related to the extracellular matrix (ECM) of chondroitin sulfate proteoglycan (CSPG) produced by mature reactive astrocytes.21 At the early stage of injury, the glial scar is necessary to prevent spreading of damage to surrounding tissue and spare the delicate surrounding tissue.22 Meanwhile, the glial scar constitutes a physical and molecular barrier at the lesion site, which forms a major impediment to axonal regeneration.23 At present, the most common strategy for laboratory degradation of CSPG is using an enzyme called chondroitinase ABC (ChABC). This enzyme attenuates CSPG inhibitory activity by cleaving CSPG glycosaminoglycan chains, and thereby benefits regeneration of axon and recovery of locomotor and proprioceptive behaviors.24,25
Myelin inhibitors
Three major myelin-derived inhibitors have been identified in reports, neurite outgrowth inhibitor (Nogo), myelin-associated glycoprotein (MAG), and oligodendrocyte myelin glycoprotein (OMgp). All the inhibitors possess potential inhibitory activity on neurite outgrowth in vitro.26–28 Nogo, a membrane protein mainly expressed by oligodendrocytes, has negative effects with growth inhibition and growth cone collapse by binding to receptors on the membrane of neurons.29 MAG, a myelin-related protein expressed by oligodendrocytes, is the first protein found to inhibit the outgrowth of neurites in vitro.28 OMgp is expressed around the axons of oligodendrocyte-like glia in the CNS, which can inhibit neurite outgrowth.26 Although they do not share the same sequence homology, the three classic myelin-associated inhibitors transmit signals via the common receptor, the Nogo receptor (NgR), meaning that these inhibitors play important roles in retracing growth cones and restricting axon regeneration.26,30
Inflammation
An obvious inflammatory response following SCI was confirmed, and microglia and macrophages were found to play important roles in inflammation.31 In fact, microglial cells are unique immune cells in the CNS and act as sensors for disruption of homeostasis in CNS. Their acute activation has a protective role focusing on elimination of damaging factors. However, chronic and uncontrolled activation leads to sustained release of proinflammatory cytokines and neurotoxic molecules in the surrounding environment, which can contribute to neurotoxic consequences.31 Furthermore, as different phenotype macrophages impact differently, macrophages can be divided into two types according to the phenotype, with proinflammatory effect of M1 and anti-inflammatory effect of M2.32,33 Unfortunately, M1 macrophage-mediated proinflammatory effect was reported to be predominant in SCI rat and mouse models.33 This suggests that the timing of inflammatory changes and the subsequent related reactions play decisive roles in the recovery or deterioration of the condition.
Cell therapeutics
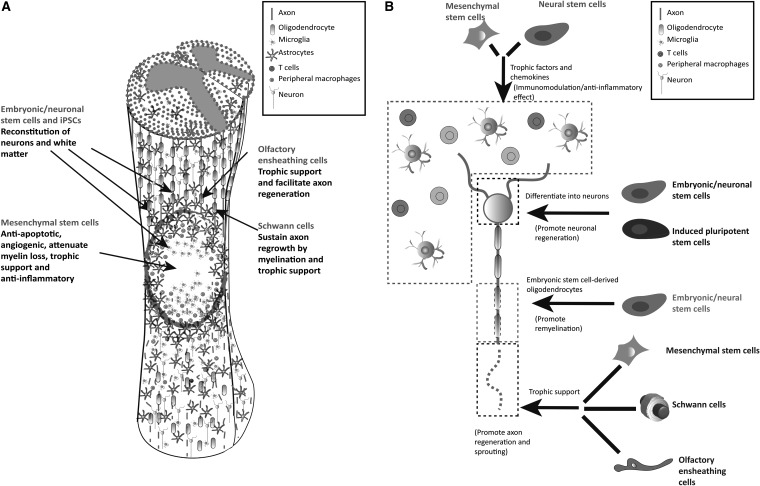
Cell therapies hold the potential for neuroprotection as well as neuroregeneration in the context of SCI. Importantly, however, with multiple targets and stimuli-responsive functions, cells have been used to regulate inflammatory responses, provide nutritional support, form scaffolding, axon remyelin, replace cells, and enhance plasticity (Fig. 2).34 Exploiting this potential mechanism, a variety of cells from several different tissue sources have been investigated for treating SCI (Table 1).
Figure 2.
(A) Targets and related mechanisms of different cells in SCI. (B) Repair mechanism of cells in SCI. Reproduced with permission from Vismara et al.34 SCI, spinal cord injury.
Table 1.
Examples of existing cell tissue engineering studies
| Cells | Source | Species | SCI model | Cotransplant material | Relevant mechanism |
|---|---|---|---|---|---|
| SCs | Sciatic nerve | Rat | CS is a lateral hemisection of the spinal cord | Alginate hydrogel scaffold and BDNF | Neuroprotection axon growth remyelin38 |
| OECs | Olfactory bulb | Rat | T9 spinal cord is completely transected | Olfactory sheath cytokines | Neuroprotection axon growth, functional connectivity48,49 |
| M2 macrophages | Mice | Mice | T12 spinal cord impingement injury | NSC | Decreased myelin-related glycoprotein increased angiogenesis and promoted axon regeneration6,55 |
| Fibroblasts | Catkin | Cat | T11/T12 spinal cord is completely transected | BDNF and NT-3 | Neuroprotection secretes extracellular matrix, promotes axon growth, and resheathing60 |
| NSCs | Rat brain | Rat | T8/T9 complete transection of spinal cord | Bifunctional scaffold combing collagen and EGFR antibody | Promotes neuronal differentiation and remyelin7 |
| ESCs | Embryo | Rat | C4/C5 spinal cord radiation injury | The oligodendrocytes are differentiated | Axons regenerate and myelin76 |
| BMSCs | Bone marrow of femur and tibia in rats | Rat | T9–T10 spinal cord hemisection | Crosslinked hydrogel scaffold with hyaluronic acid and adipate dihydrazide | Neuroprotection, immune regulation, axon regeneration79 |
| iPSCs | Genetic modification of human fetal lung fibroblasts | Rat | Spinal cord T8–T9 compression injury | — | Promote axon regeneration, angiogenesis, and motor function recovery11 |
BDNF, brain-derived neurotrophic factor; BMSC, bone marrow mesenchymal stem cell; ESCs, embryonic stem cells; iPSC, induced pluripotent stem cell; NSC, neural stem cell; NT-3, neurotrophin-3; OECs, olfactory ensheathing cells; SCs, Schwann cells; SCI, spinal cord injury.
Therapy using SCs
SCs are located in the peripheral nervous system (PNS). They are arranged in strings and wrap the axons of the peripheral nerve fibers one by one. In the medullated nerve fibers, the SCs form the myelin sheath and the myelin sheath forming cells of the PNS.35 SCs have the longest transplant history of any type of cell used in SCI therapy and are widely recognized in the field of SCI therapy as the most promising transplant donor for the regeneration of spinal cord axons.36 In part, it is because SCs can produce several beneficial factors, such as increased trophic factors, ECM, and cell adhesion molecules.37
Transplantation of SCs provides neuroprotection and can reduce cyst and glial scar formation, promote axonal regeneration and myelinization, and effectively improve functional outcome (Fig. 3).38,39 However, no significant change in functional recovery was found in transplantation with SCs alone. The SCs were transplanted to the SCI site alone and the therapeutic effect was not satisfactory due to their low survival rate.40 Necrosis and apoptosis of transplanted SCs occurred mainly in early stage, which can be attributed to harmful local microenvironment, hypoxic levels, M1-mediated inflammatory effects, and cell-mediated immune responses.41 Some combinatorial strategies were invented to overcome these deficiencies. For example, Moradi et al. demonstrated that the use of BD PuraMatrix peptide hydrogel combined with SCs can be used as a scaffold to promote the proliferation of SCs and reduce the number of astrocytes in the T10 segment of rat spinal cord after moderate compression injury, thereby limiting the formation of glial scar and promoting the recovery of motor function.42 Combined with neurotrophic factors and ChABC, transplantation of SCs showed more significant repair effects on SCI.43–45 Studies have also tested the use of genetically modified SCs to secrete bifunctional neurotrophin and ChABC and were transplanted into a moderate thoracic spinal cord contusion injured rats. As shown in the Glial Scars and Chondroitin Sulfate Proteoglycan section, ChABC-modified SCs inhibit the activity of CSPG, which facilitates myelination of axons and increases the number of spinal intrinsic axons in the graft and surrounding host tissue.19
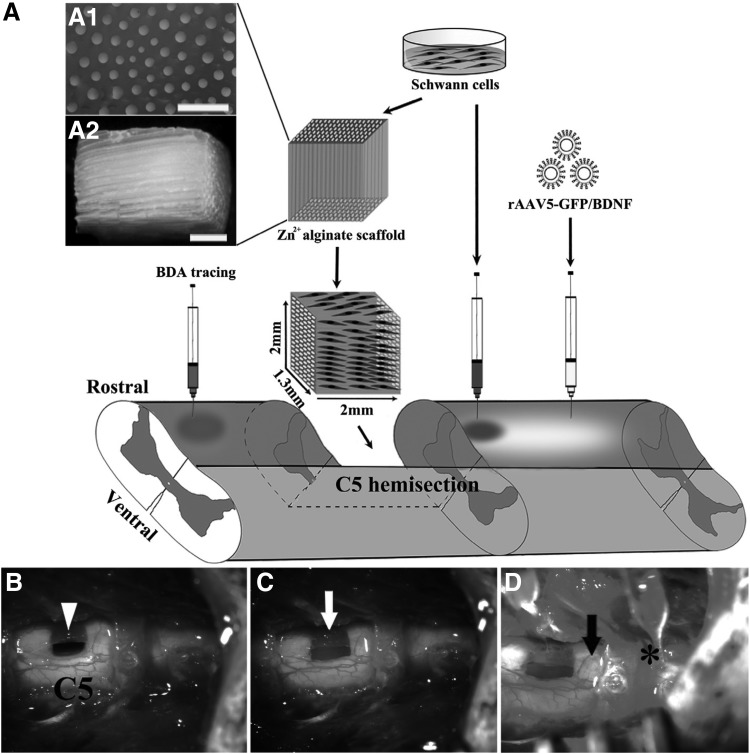
Figure 3.
Lesion paradigm and experimental procedures. (A) Unilateral spinal cord resection of 1.5–2 mm was performed at the level of C5 in rats. Schwann cell-seeded alginate hydrogel was transplanted to the lesion site. Under the control of tetracycline regulatory promoter, caudally injected AAV5 expressing BDNF. Studies have demonstrated a significant increase in the number of regenerating axons and myelination. (A1) Cross-sectional and (A2) longitudinal view of the capillary lumen. (B) Spinal cord hemisection lesion (arrowhead); (C) alginate scaffold for SCs (white arrow); (D) AAV5 (*) or SCs were injected into the caudal spinal cord (black arrow). Reproduced with permission from Liu et al.38 BDNF, brain-derived neurotrophic factor; SCs, Schwann cells.
Therapy using olfactory ensheathing cells
The transplantation of olfactory ensheathing cells (OECs) is considered to be one of the most promising approaches for enhancing axon regeneration and functional recovery after SCI. OECs can be obtained by nasal biopsies from the olfactory mucosa and olfactory bulb.46,47 Furthermore, OECs hold great potential to create a positive microenvironment for axon regeneration, regulating glial scar formation and axon remyelination, reconstructing neural tissue, and counteracting diffusion of inhibitory factors released by axons of dead neurons in vitro.48
Many experimental studies have revealed that after implantation of OECs in the injury model, the postoperative motor function and respiratory function of the rats receiving OECs recover more significantly compared with the control group (Fig. 4).49,50 After implantation of OECs in a completely transverse rat model with sciatic nerve injury, Radtke et al. were able to demonstrate that OEC grafts provided nutritional support and bridged lesion sites, allowing axon regeneration and myelin to improve functional prognosis.47 In addition, after SCI, fibroblasts and CSPG invaded the site of injury and form glial scar, which had the side effects of obstructing axon regeneration and cell infiltration. In contrast to SCs, OECs can penetrate this barrier and promote spinal cord regeneration and functional recovery.51 Although numerous studies have reported that OECs help improve neurological function, treatment methods remain inconsistent, and this variability may stem from different olfactory cell populations before transplantation to the damaged site. Therefore, a method of identifying and purifying OECs is needed first in clinic, and then transplanted therapy can be carried out.52 These studies will help prepare for the clinical use of OEC transplantation and make it reliable in the treatment of SCI.
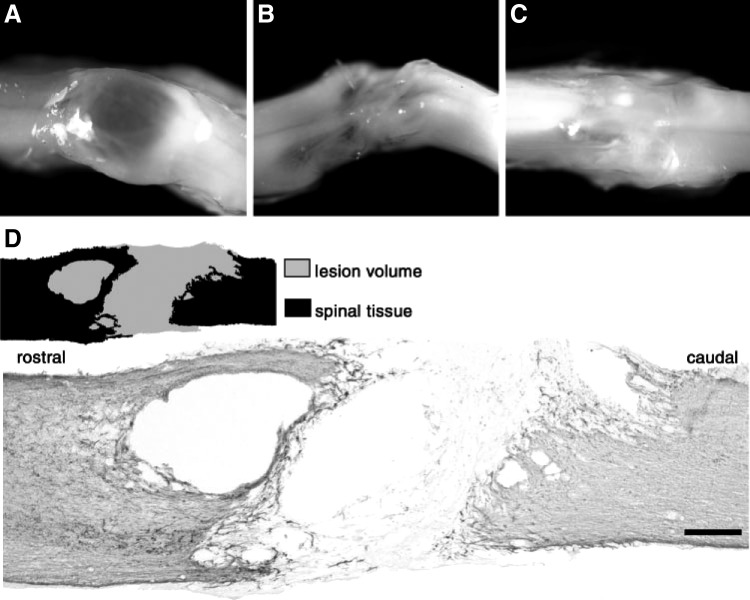
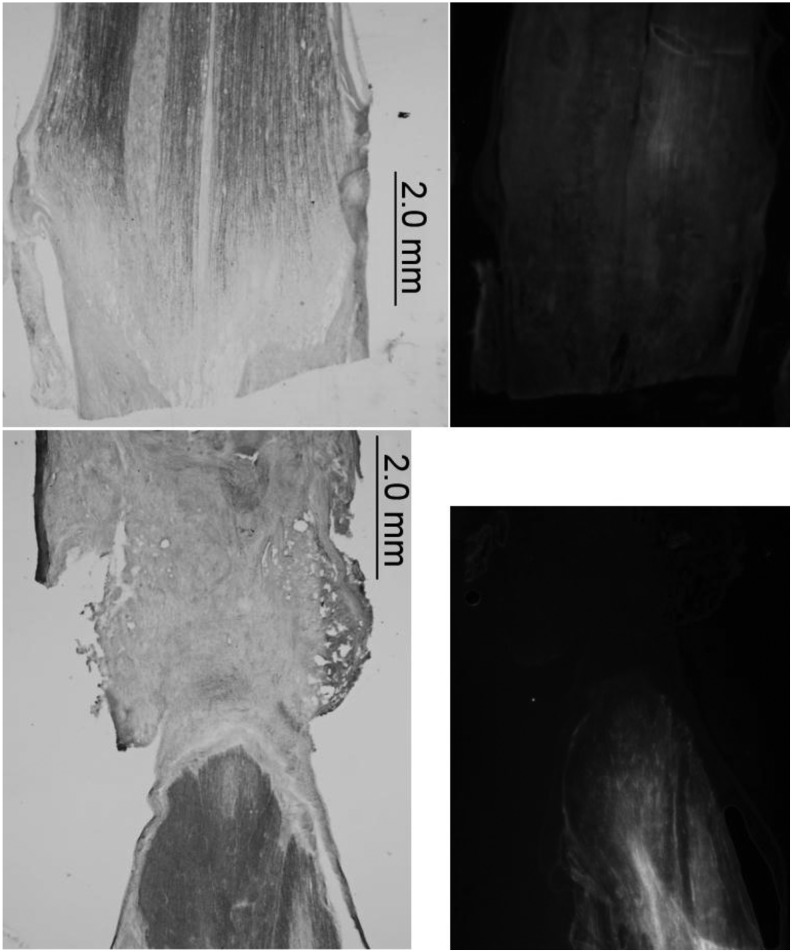
Figure 4.
OEG transplantation at he transection site. (A) A spinal cord form a media-untrained rat: large transparent cavitation appears in the injury site. (B) A second media-untrained rat: much less cavitation is apparent in the lesion site. (C) An OEG-trained rat: pronounced cavitation disappears in the injury site. (D) Immunohistochemical staining of GFAP: the black area and the gray in drawing represent the GFAP-positive tissue and the GFAP-negtive transection site, respectively. Reproduced with permission from Kubasak et al.49
Therapy using activated macrophage
The immune system can not only protect body's tissue from damage but also promote the rehabilitation of already damaged tissues. Furthermore, several studies have demonstrated that inflammatory responses can have both proinflammatory and anti-inflammatory components.53 The proinflammatory phenotype is involved in fighting with infection, removing dead and dying cells, and repairing wounds. The anti-inflammatory phenotype is associated with the natural breakdown of the early inflammatory response and recovering the tissue to a normal state.54
Research carried out by Kristina A. Kigerl has shown that adequately activated macrophages can have a positive effect on SCI.33 For example, transplantation of M2 macrophages could support neuroprotection and regeneration in different animal models.32,55 It has been shown that transplantation of macrophages can decrease myelin-related glycoprotein, and promote axon regeneration and myelination.6,56,57 Another study has demonstrated that neural stem/precursor cell transplantation led to a decrease in the proportion of classical M1 macrophages and promoted the rehabilitation of the injured cord (Fig. 5).55 To study the effect of macrophages on globoid cell leukodystrophy (GLD), the Yoichi Kondo laboratory obtained the macrophage-deficient twitcher mouse model through crossbred, and the experimental results showed that the overall effect of macrophages in GLD was to prolong the life span of mice and alleviate neurological symptoms by promoting myelination.57
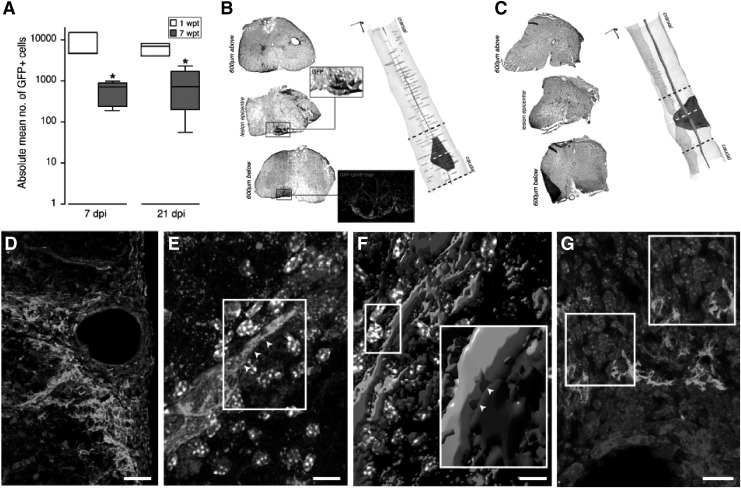
Figure 5.
(A) Quantification of transplanted fGFP+ NPCs in vivo at either 1 or 7 weeks post transplantation (wpt). *P ≤ 0.05. Representative axial images of the GFP staining for stereological quantifications at 1 (B) or 7 (C) weeks after NPC transplantation. (D) Image of an ‘atypical perivascular niche’. (E) Image of GFP (green) NPCs contacting F4/80+ macrophages via connexin43+ cellular junctions (red; arrowheads). (F) 3D reconstruction of the confocal Z-stack in (E). The magnified inset shows structural junctional connexin43 pattern (red; arrowheads). (G) In a perivascular spinal cord area GFP (green) NPCs and B220+ putative B lymphocytes (blue) not establishing connexin43+ (red) mediated junctional coupling. Reproduced with permission from Cusimano et al.55
Therapy using fibroblast
Fibroblasts are the cells that make up connective tissue and secrete ECM molecules. These cells are not difficult to obtain and can easily expand in culturing, making them attractive for use in cell therapy.58 However, besides glial scar, the scar after SCI can also be a fibrotic scar. Fibrotic scar is mainly caused by excessive deposition of ECM molecules secreted by fibroblasts, which plays the same role in inhibiting axon growth. However, compared with glial scar, fibrotic scar has no strong inhibition on axons.59 Fibroblasts presently used in the laboratory are modified or combined with other therapeutic strategies.
Studies found that the neurotrophin brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) secreted by modified autologous fibroblasts could promote recovery of stepping, oligodendrocyte proliferation, and axon myelination in the SCI cat (Fig. 6).60 Furthermore, transplantation of Wnt-containing alginate scaffolds secreting fibroblasts was considered to promote axonal regeneration and functional recovery after SCI.61 On the contrary, through in vitro gene therapy, BDNF, nerve growth factor (NGF), and NT-3 were delivered to the early injured spinal cord by modified fibroblasts, which proved to be effective in inducing axon regeneration, filling the diseased cavity, and restoring spinal cord function in adult rats.62,63 Transplanted fibroblasts secrete cytokines that alter neurite recognition of NG2 glycoprotein inhibitor components following SCI, suggesting that they can also facilitate axon regeneration even in glial scar areas that are widely expressed in CSPG.62
Figure 6.
The spinal cord was completely severed creating a 3–5-mm-long pocket formed by the dura mater and bordered at the rostral and caudal edges of the cut spinal cord. The rostral end of the lesion site, about 1 mm from the edge of the lesions tissue, was injected with a micro-ruby tracer and the caudal end with micro-emerald. Reproduced with permission from Krupka et al.60
Therapeutics using stem cells
Therapy using neural stem cells
Neural stem cells (NSCs) are pluripotent progenitors or stem cells that have the ability to self-renew and can be isolated from the subventricular zone of hippocampus of brain or the central canal of the spinal cord.64 Most importantly, NSCs facilitated recovery of spinal cords with the ability to differentiate into neurons and oligodendrocytes and to replace the lost cells within the lesion site.65 NSC was able to secrete a variety of neurotrophic molecules that inhibit cell death, as well as promote axon regeneration and remyelination. In addition, NSCs can also reduce lesion volume, inhibit scar tissue formation, elicit anti-inflammatory effects, and improve electrophysiological and motor functional recovery.64–66
Due to the microenvironment after SCI, NSCs at the injury site mainly tend to differentiate into glial cells, suggesting that NSCs may need to be predifferentiated before implantation (Fig. 7).7 Neuronal restricted precursors (NRP) and glial restricted precursors (GRP) differentiated from spinal cord or NSCs were transplanted into rats with C4 spinal cord lateral funiculus injuries. Unlike NSCs, NRP and GRP could be differentiated into desired lineage, such as neurons and oligodendrocytes. Experiment results showed that the mixed lineage-restricted precursor cells filled the cavity, differentiated into mature CNS cells, and repaired the damaged sites.67 The NSCs expressing green fluorescent protein were combined with fibrin matrix containing growth factor mixture and transplanted to the injury site of complete spinal cord transection in rats, and the results showed that NSCs differentiated into many kinds of cells, including neurons, and the axons formed rich synapses with host cells after large growth, which promoted the formation of electrophysiological relays and led to the recovery of motor function in rats.68 Alternatively, the rationale behind the use of NSC-conditioned medium in SCI treatments focuses on reducing the expression of inflammatory cytokines in M1 macrophages and damaged spinal cord tissues, as well as reducing systematic inflammation.65
Figure 7.
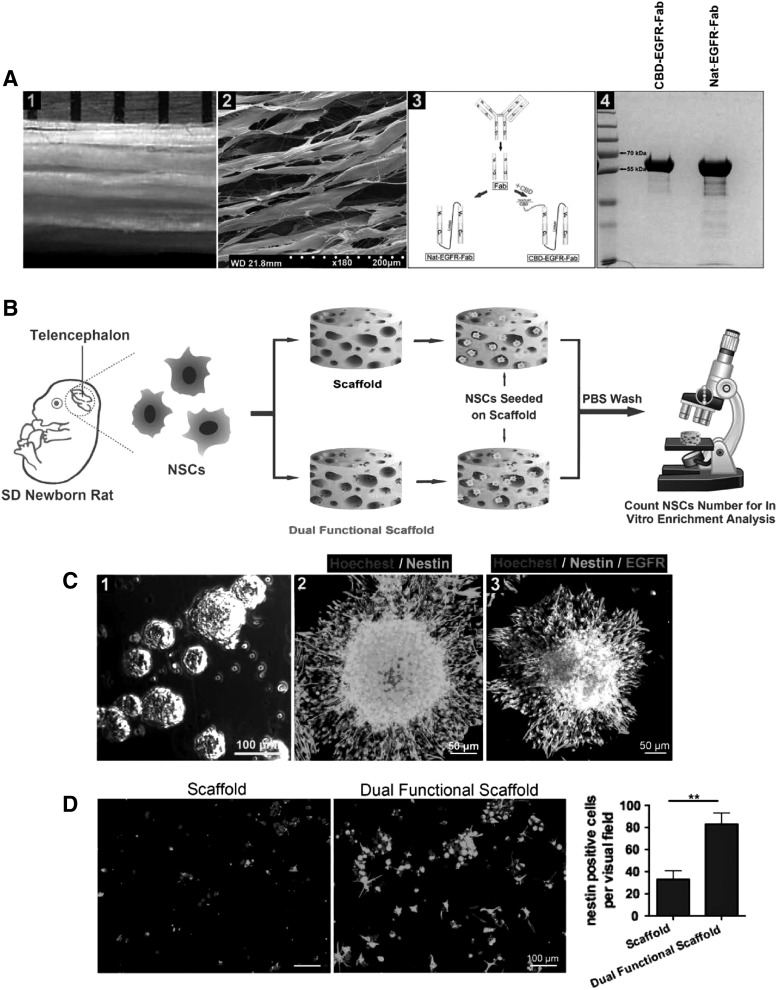
(A) (1) The macroscopic image of collagen scaffold. (2) The SEM image of scaffold. (3) The schematic diagram of the construction of recombinant protein. (4) SDS-PAGE analysis of Nat-EGFR-Fab and CBD-EGFR-Fab. (B) The experiment process of capturing and retaining NSCs by the dual functional scaffold in vitro. (C) (1) Image of neurospheres. (2) Image of the neurosphere immunostained with nestin. (3) Nestin and EGFR double staining showing the neurosphere. (D) Analysis of the number of nestin positive cells at the two scaffolds. **p < 0.01. Reproduced with permission from Xu et al.7 NSC, neural stem cell.
Therapy using embryonic stem cells
Embryonic stem cells (ESCs) are pluripotent cells that can differentiate into many cell types and have the capacity of continuous self-renewing.69 Indeed, it was demonstrated that ESCs are capable of differentiating into specific neural lineage, including neurons, oligodendrocytes, and astrocytes, both in vitro and in vivo.70,71 After transplantation, these stem cell-derived populations can replenish lost cell types, provide trophic support for axon regeneration, remyelinate surviving axons, and deliver immunomodulatory, anti-inhibitory factors to form relay circuits that contribute to functional recovery.72–74
Xie et al. predifferentiated mouse ESCs (mESCs) in neural progenitors by adding retinoic acid to embryoid body cultured for 4 days. Their results demonstrated that the combination of electrospun fiber scaffolds and mESCs of predifferentiated neural progenitor cells not only promoted neuronal differentiation but also limited the glial scar formation and guided the neurite outgrowth.69,70 Iwai et al. transplanted ESC-derived neural stem/progenitor cells (ESC-NS/PCs) into the marmoset SCI C5 Contusive model, and implanted 14 days after the injury. Implantation of ESC-NS/PCs led to tissue retention at the site of injury, regeneration of corticospinal tract (CST) fibers, axonal regeneration, and angiogenesis compared with the control group. The combination of cells resulted in functional recovery without tumorigenicity.75 Furthermore, others have demonstrated that myelinating OPCs derived from mESCs and transplanted into a mouse SCI model gave significantly enhanced remyelination and functional recovery (Fig. 8).76 Interestingly, in the model of cervical SCI in nude mice, after treatment with human ESC-derived OPCs, the cystic cavity at the injury site was significantly reduced and the retention of myelinated axons was increased.77
Figure 8.
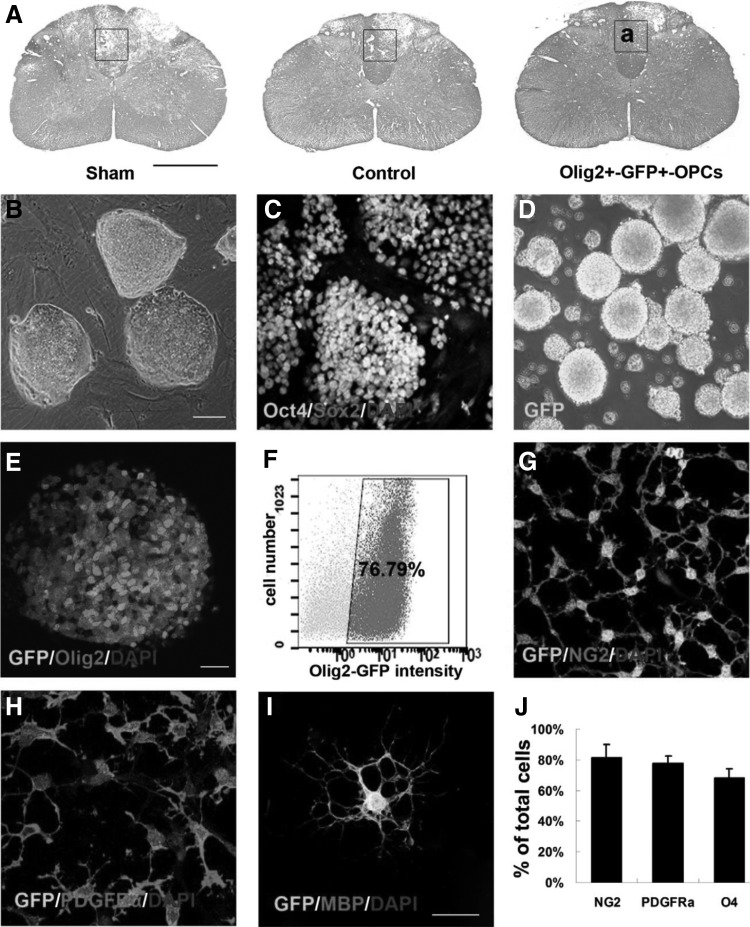
(A) LFB/H&E staining images of normal spinal cord. Enlargement of framed area in (a) for observation of immunostaining. (B) Image of mESCs colonies on mouse embryonic fibroblast. (C) Image of Oct4/Sox2 immunostaining of mESCs. (D) Image of Olig2+-GFP+ spheres at day 12. (E) Image of Olig2 immunostaining in GFP+ spheres. (F) The percentage of GFP+ cells determined by FACS at day 12. Images of NG2 (G) and PDGFRα (H) immunostaining in GFP+-OPCs at day 14. (I) Image of the GFP+-oligodendrocyte immunostained with MBP. (J) Quantification and comparison of the number of cells expressed NG2, PDGFRα and O4. Reproduced with permission from Sun et al.76
Therapy using bone marrow mesenchymal stem cells
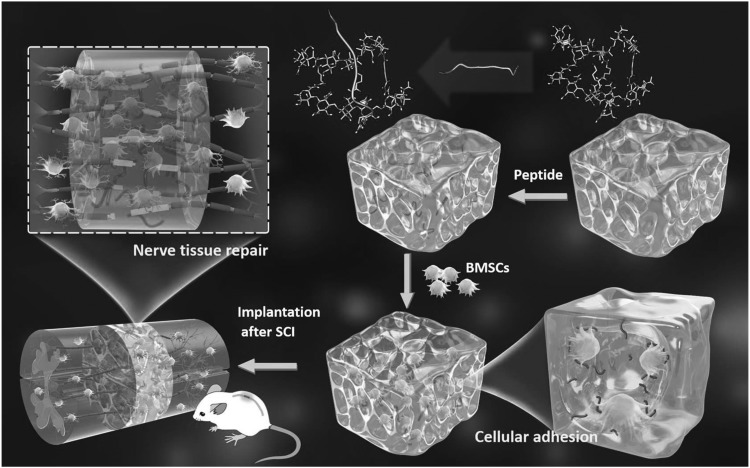
Most of bone marrow mesenchymal stem cells (BMSCs) used in preclinical experiments are obtained from humans or rodents. They are widely used because they are easy to extract, culture, and can be used for autologous transplantation.78 Most importantly, BMSCs have been shown to reduce inhibitory scar tissue/cavity formation, preserve axons, increase myelin sparing, and ultimately lead to anatomical and functional recovery of SCI animal models (Fig. 9).78–80 In addition, BMSCs can also promote transformation of macrophages from the M1 proinflammatory phenotype to M2 anti-inflammatory phenotype, and reduce acute inflammatory response in SCI, and thereby promote functional recovery.78
Figure 9.
The spinal cord was transected to make 1.5 mm gaps in rat T9–10 segment. Peptide-modified scaffold is developed based on hyaluronic acid and an adhesive peptide PPFLMLLKGSTR to promote the adhesive growth of BMSCs. The implantation of BMSC-embedded scaffold can not only significantly promote the integration of nerve tissue and the regeneration of neurons but also inhibit the formation of glial scar and the spread of inflammatory cells. Reproduced with permission from Li et al.79 BMSC, bone marrow mesenchymal stem cell.
Scaffolds with BMSCs secreting neurotrophic factors are used to address the problems of acute and secondary injury, including neuronal deficiency, axonal breakage, glial scar barrier, and inflammatory responses.80 NT-3 gene-modified BMSCs were reported to inhibit glial scar formation, improve the microenvironment in injured spinal cord, promote nerve regeneration, and increase locomotor function recovery.81 A pair of studies from Zhao et al.'s laboratory reported the use of genetically modified BMSCs expressing cerebral dopamine neurotrophic factor (CDNF) to treat SCI at T10 in rats. The CDNF-expressing cells have a strong anti-inflammatory effect at the lesion site compared with normal BMSCs. By inhibiting the neuroinflammatory response after SCI, the production of proinflammatory cytokines PGE2 and IL-1β can be reduced, thereby promote motor function and nerve recovery of the injured spinal cord.82 Moreover, the ability of exosomes derived from bone MSCs (BMSCs-Exos) possesses robust proangiogenic properties, which makes it an attractive agent to study improved functional behavioral recovery effects after traumatic SCI. BMSC-Exo treatment inhibits inflammation, reduces neuronal cell apoptosis, suppresses glial scar formation, attenuates lesion size, and promotes axonal regeneration.83
Therapy using induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are reprogrammed somatic cells in mouse or human fibroblasts by transplanting SOX2, OCT3/4, tumor suppressor Krüppel-like factor 4 (KLF4), proto-oncogene c-MYC, and other genes, which may become a preferred cell source for SCI treatment in human patients because there are no ethical issues involved.84–86 Moreover, iPSCs possess the ability to differentiate into neural precursor cells, oligodendrocytes, astrocytes, neural crest cells, neurons, and mesenchymal stem cells.87 These cells can promote axon regeneration, bridge disease cavities, and generate functional recovery by replacing missing cells or regulating the microenvironment at the site of injury (Fig. 10).11,88,89
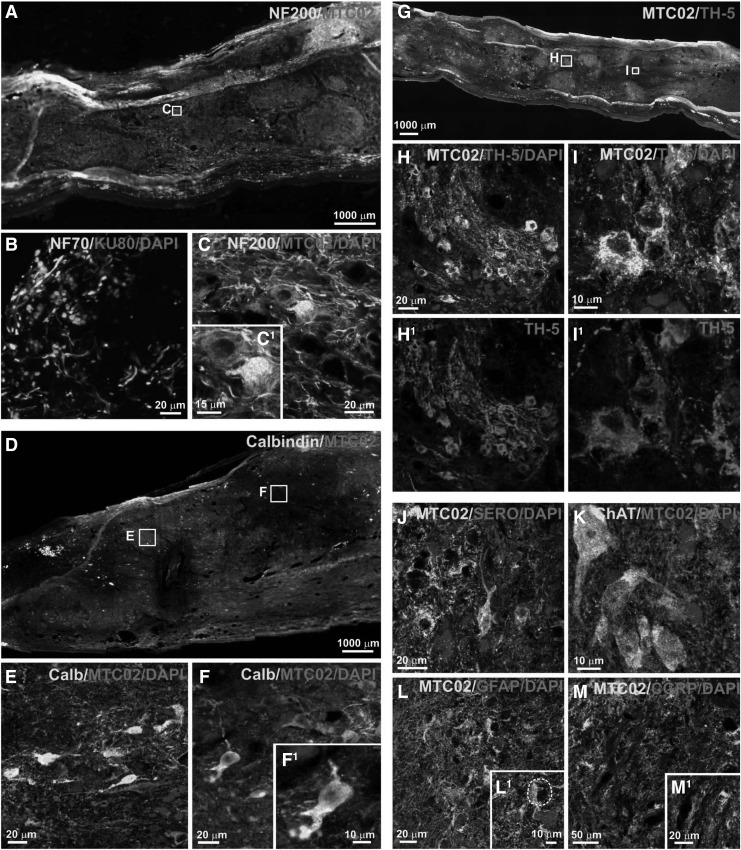
Figure 10.
iPS-NP survival and migration after transplantation at 17 weeks. Image of infiltrated by endogenous cells and cell elements positive for NF200 (A, C), GFAP (L and L1–higher magnification of L) and CGRP (M and M1–higher magnification of M). Image of differentiation transplanted cell into GFAP-positive astrocytes (L1, the white dashed line on L1 showing GFAP-negative human cells that have a neuron-like morphology), NF70- and NF200-positive neurons and mature neurons positive for calbindin (D-F and F1–higher magnification of F), TH-5 (G-I and H1, I1), serotonin (J) and ChAT (K). Reproduced with permission from Romanyuk et al.11 iPSC, induced pluripotent stem cell; NPs, neural precursors.
Some researchers showed that implanted iPS-derived oligodendrocyte progenitors into the rat model of spinal cord moderate contusion showed reduced cavity formation, scarring, and microglial proliferation compared with the control group.20 Furthermore, NSCs derived from iPSCs were reported to exhibit capacity for remyelination and significantly improved neurobehavioral function in laminectomy models of mice spinal cord.89 One study showed that human-iPSC-derived neurospheres (hiPSCs-NSs) were implanted into the spinal cord of nonobese diabetic-severe combined immunodeficient injury site 9 days after T10 contusive in mice and promoted functional recovery by differentiating into neurons, oligodendrocytes, and astrocytes. Subsequent studies showed that hiPSCs-NSs secreted neurotrophic factors, promoted axon regeneration, angiogenesis, myelination, and reconstructed neural pathways at the site of injury, and no tumor formation was observed for a long time.90 Importantly, however, there are limitations to iPSCs, such as genetic/epigenetic abnormalities and tumor formation due to artificial induction genes, which need to be addressed before they can be used clinically.91
Nerve regeneration factors for cell therapeutics
The neurotrophic factor has been taken into account as the main or supplementary mechanism of many transplanted cells and can still play a neuroprotective role after the death of transplanted cells.92,93 Alternatively, the expression of neurotrophic factors by transplantation through cell modification, or neurotrophic factors produced by the response of the injured site to transplant cells, helps enhance the functional benefits of cell transplantation.94 See Table 2 and Fig. 11 for neurotrophic factors on SCI therapy and signaling pathway.
Table 2.
Effect of nutrition factors on spinal cord injury therapy
| Nutritional factor | Effect | Adjustment | Source |
|---|---|---|---|
| NEG | Promotes neuronal survival, growth, and axonal regeneration | Widely expressed throughout the body, high expression of SCI | First found in mouse sarcomas95,123 |
| BDNF | Axonal growth that promotes neuronal survival | Wide expression throughout the body | First discovered in the pig brain102 |
| NT-3 | Promotes the survival of sympathetic and sensory neurons | Widely distributed in the body | Cell gene regulation can be secreted123 |
| CNTF | Promotes neuronal survival, growing, and sprouting | High expression of SCI | First discovered in the ciliary ganglia of chicken embryonic eye tissue110,111 |
| FGF | Inducing angiogenesis at the injury site | Wide expression throughout the body | Secreted by the pituitary and hypothalamus115 |
| GDNF | Maintaining neuronal survival, reduces the production of glial scars | Wide expression of the nervous system | SC secretion117,124 |
CNTF, ciliary neurotrophic factor; FGF, fibroblast growth factor; GDNF, glial cell-derived neurotrophic factor.
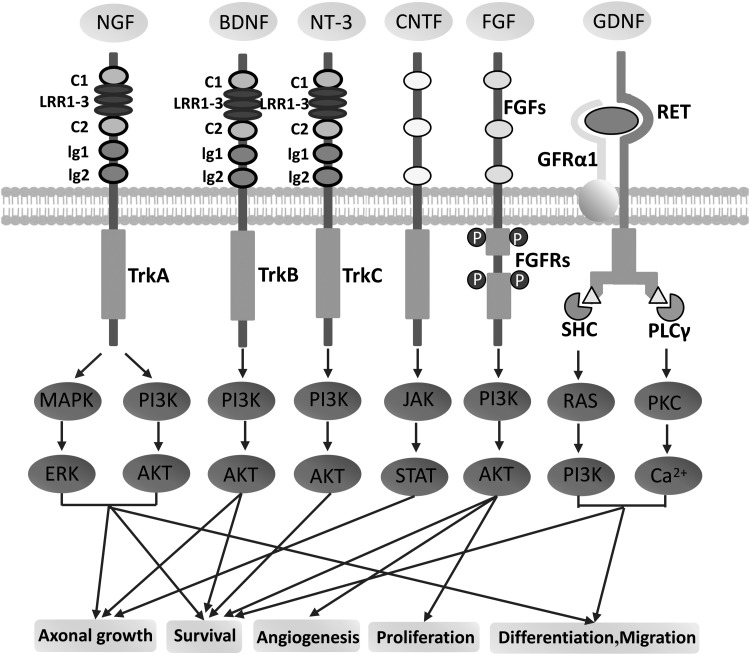
Figure 11.
Neurotrophic factor signaling pathway. NGF has a high affinity with TrkA and facilitates differentiation and survival of neurons.126 BDNF has a high affinity with TrkB and enhances axonal growth and survival of neurons.102,127 NT-3 has a high affinity with TrkC and promotes the survival of neurons.126 CNTF signals, via the JAK/STAT pathway, have been widely and successfully used to facilitate the regeneration of retinal ganglion cell axons in adult rats.128 FGF signals through the PI3K/AKT pathway and stimulates proliferation, angiogenesis, and survival of neurons.129 GDNF can send signals through RET-independent or RET-dependent pathways. The latter can promote neuronal differentiation, survival, and migration.124,130 CNTF, ciliary neurotrophic factor; FGF, fibroblast growth factor; GDNF, glial cell-derived neurotrophic factor; JAK/STAT, Janus kinase/signal transducers and activators of transcription; NGF, nerve growth factor; NT-3, neurotrophin-3; PI3K/AKT, phosphoinositide 3 kinase/serine–threonine kinase; TrkA, tropomyosin receptor kinase A; TrkB, tropomyosin receptor kinase B; TrkC, tropomyosin receptor kinase C.
Nerve growth factor
NGF was demonstrated to have many characteristics, such as broad application prospect in the treatment of SCI. Nevertheless, NGF also has disadvantages, such as unstable physicochemical properties, and its ability to cross the blood/spinal cord barrier is low.95 Some studies demonstrated that NGF played an important role in the survival and maturation of developing neurons in PNS, and NGF was reported to change glial phenotype, enhance the survival of neurons, and promote axonal regeneration.95,96 In addition, NGF has limitations, and the expression of NGF in the spinal cord induces nociceptive axons, resulting in hyperalgesia that can lead to severe pain.95 A recent study reported that lentiviral-mediated aquaporin-4 inhibition can increase the expression of NGF and ultimately led to motor improvements of SCI rats.97 Furthermore, it is also a promising strategy to combine delivery of NGF genes or NGF with cell transplantation to injury site to achieve long-term expression of NGF.
Brain-derived neurotrophic factor
BDNF has the functions of axon regeneration, neurogenesis, remyelin, neuroprotection, adaptive synaptic plasticity, and synaptic transmission in various groups of neurons after SCI.98,99 Studies have demonstrated that delivery of BDNF and NT-3 genes into SCI promoted axonal growth at local sites and significantly reduced the axotomy-induced atrophy of large pyramidal neurons at the remote effects.100 Further studies in rodent models suggest that BDNF-secreting MSCs will further promote functional recovery after SCI.38,101,102 However, several studies have shown that cortical regions increase the survival of spinal motor neurons but do not promote the growth of CST axons.103
Neurotrophin-3
A promising tool is NT-3, and its expression in the developing spinal cord motor neurons has increased significantly. However, NT-3 is more challenging to identify because of its lower protein abundance.104 Furthermore, NT-3 facilitates survival of neurons and improves peripheral nerve regeneration.105,106 Alternatively, transplantation of NT-3 expressing cells to the site of injury showed that CST grew over short distances.107 Another advantage of using NT-3 is that it circumvents the target area associated with pain, so it does not cause side effects such as pain or spasticity.
Ciliary neurotrophic factor
It was demonstrated that transplantation of ciliary neurotrophic factor (CNTF) in mouse models has a neuroprotective and reparative effect in the central and PNSs.108 Furthermore, CNTF can improve the survival rate of neurons after development and axonal fracture.109 In addition, several studies have demonstrated that CNTF combined with multiple cell therapies is more effective than using CNTF alone.100,111 Although the treatment of damaged neurons by CNTF may require the involvement of other cell types, this cytokine is not completely unaffected in cultured SCs. OPC transplantation combined with CNTF expression can promote myelination and functional recovery after traumatic SCI.112
Fibroblast growth factor
Fibroblast growth factor (FGF) has a presence throughout the development of central and PNSs.113 FGF is expressed in both astrocytes and neurons and is involved in stimulating axon regeneration, promoting vascularization, exerting anti-inflammatory functions of inflammatory cells, and neuroprotection.114 Le's laboratory seeded basic FGF into collagen/gelatin sponge scaffolds before implanting them into inactivated skin and accelerated angiogenesis was observed.115
Glial cell-derived neurotrophic factor
Glial cell-derived neurotrophic factor (GDNF), a member of the transforming growth factor family, is crucial for motor neurons, dopaminergic neurons, and peripheral neurons.116 NGF plays an important part in sensory neurons. It increases the number of sprouting neurons and reduces lesion size at the injury site during inflammation. In addition, it also facilitates axonal regeneration in the central and PNSs after SCI.116,117 Furthermore, research groups have fabricated silk fibroin/alginates/GDNF scaffolds seeded with human umbilical cord mesenchymal stem cells to engineer neural tissue. It was reported to significantly enhance neuron survival and increase the number of surviving neurons.117
Conclusions and Future Directions
-
(1)
Cell therapeutics is a very promising approach to treat nerve regeneration, which has become a hot topic of extensive research. Cells play a role in the replacement of lost neurons and glial cells, secretion of neurotrophic factors and anti-inflammatory cytokines, stimulation of tissue retention and angiogenesis, reconstruction of neural pathways, filling the lesions of the cystic cavity, and stimulating axonal regeneration and remyelination at various levels, from molecules to tissues.
-
(2)
There are noteworthy differences between similar cells depending on the species, age, culture conditions and delivery patterns of the donor. On the contrary, with regard to the timing of the intervention, almost all the studies about transplantation were conducted in subacute and acute conditions, while chronic treatment was rare. The late transplant time point would help to reduce the number of subjects required for clinical trials, as their outcome trajectory was more predictable.
-
(3)
Each transplant candidate has a specific risk in the translation process. The formation of tumors is a major risk that can be assessed over a long period of time through transplantation to large animals with longer life spans. Another risk of transplantation is an increased chance of infection. In cervical spine injury after SCI, systemic immune function is significantly reduced and patients may have severe immunosuppression.118,119 Therefore, cell therapy should be thoroughly investigated to ensure that there is no increase in the likelihood of tumors and infections.
-
(4)
Rehabilitation is generally assessed through rodent models of SCI before it is applied to clinical practice. However, most of the experimental data are based on mild or moderate chest injury models, the severity of which is still far from adequate compared with clinical patients. The most promising strategy is the study with primate models, because the species' spinal cord is the closest to the human spinal cord in terms of size and function, and the mechanism of SCI is more similar to that of humans.120
-
(5)
The future application of SCI therapy in clinical practice may include the combination of multiple strategies. The comprehensive treatment of injury of spinal cord is the focus of the present research. For example, two or more cells can be transplanted at the same time as treatment for SCI. One of these cells secretes nutritional factors that can provide nutritional support for another cell. In addition to cells, drug delivery, gene therapy, and biomaterials can also help promote regeneration after SCI.121,122 With the combination of different cell therapy strategies, future experiments will achieve more dramatic success in spinal cord repair.
Take-Home Messages
Great progress has been made in cell-based therapies for SCI, which results in impaired sensory and motor function below the injury level through initial and secondary injury.
There have been numerous articles in recent years on experimental cell therapy for SCI, involving SCs, olfactory ensheathing cells, activated macrophages, fibroblasts, NSCs, embryonic stem cells, BMSCs, and iPSCs.
Challenges after SCI include cystic cavities, glial scar, myelin inhibitor, and inflammation.
SCs can produce a variety of neurotrophic factors and reduce cyst and glial scar formation.
OECs obtained from olfactory mucosa and olfactory bulb can regulate glial scar formation, axon remyelination, and nerve tissue reconstruction.
Macrophages are classified into the classic M1 type with proinflammatory effect and the M2 type with anti-inflammatory effect.
NSCs can differentiate into neurons, astrocytes, and oligodendrocytes.
iPSCs are cell types obtained by reprogramming a combination of four transcription factors (SOX2, OCT3/4, KLF4, and c-MYC) into mouse or human fibroblasts using viral vectors.
Multiple neurotrophic factors, such as NGF, BDNF, NT-3, CNTF, FGF, and GDNF, enhance the functional benefits of SCI cell transplantation.
Acknowledgments and Funding Sources
This study was supported by grants from the National Natural Science Foundation of China (31700854), the Key Program of Anhui Educational Committee (No. KJ2018A1011 and KJ2019A0392), the Scientific Research Foundation of Bengbu Medical College (BYKY1848ZD, BYKY17118, and BYKY18108), and the Shanghai Municipal Health and Family Planning Commission (201840027).
Abbreviations and Acronyms
- BDNF
brain-derived neurotrophic factor
- BMSCs
bone marrow mesenchymal stem cells
- CDNF
cerebral dopamine neurotrophic factor
- ChABC
chondroitinase ABC
- CNS
central nervous system
- CNTF
ciliary neurotrophic factor
- CSPG
chondroitin sulfate proteoglycan
- CST
corticospinal tract
- ECM
extracellular matrix
- ESC-NS/PCs
ESC-derived neural stem/progenitor cells
- ESCs
embryonic stem cells
- FGF
fibroblast growth factor
- GDNF
glial cell-derived neurotrophic factor
- GLD
globoid cell leukodystrophy
- GRP
glial restricted precursors
- iPSCs
induced pluripotent stem cells
- JAK/STAT
Janus kinase/signal transducers and activators of transcription
- KLF4
Kruppel-like factor 4
- MAG
myelin-associated glycoprotein
- mESCs
mouse ESCs
- NGF
nerve growth factor
- Nogo
neurite outgrowth inhibitor
- NPs
neural precursors
- NRP
neuronal restricted precursors
- NSCs
neural stem cells
- NT-3
neurotrophin-3
- OECs
olfactory ensheathing cells
- OMgp
oligodendrocyte myelin glycoprotein
- OPCs
oligodendrocyte precursor cells
- PI3K/AKT
phosphoinositide 3 kinase/serine–threonine kinase
- PNS
peripheral nervous system
- SCI
spinal cord injury
- SCs
Schwann cells
- TrkA
tropomyosin receptor kinase A
- TrkB
tropomyosin receptor kinase B
- TrkC
tropomyosin receptor kinase C
Author Disclosure and Ghostwriting Statement
No competing financial interests exist. The content of this article was expressly written by the authors listed.
About the Authors
Wenguo Cui, PhD, is a full professor at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. He is at present the group leader of Regenerative Biomaterials. His scientific interests are focused on the development of novel biomaterials and nanomaterials for tissue regeneration, drug delivery, and disease treatment. He has published more than 130 SCI articles (H = 37, citation >5,000, including an over 300-citation article), 1 chief editor of 1 book and 9 book chapters as first or corresponding author, and more than 30 patents, many of which are licensed. He worked as PI for three projects of the National Natural Science Foundation of China and more than 10 other grants in Shanghai. Pinghui Zhou, MD, and Jingjing Guan, BS, are attending physicians of the Department of Orthopedics, First Affiliated Hospital of Bengbu Medical College. Panpan Xu is a graduate student at the Department of Orthopedics, First Affiliated Hospital of Bengbu Medical College. Yingji Mao, PhD, is a lecturer at the School of Life Science, Bengbu Medical College.
References
- 1. Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng 2003;5:293–347 [DOI] [PubMed] [Google Scholar]
- 2. Silva NA, Sousa N, Reis RL, et al. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol 2014;114:25–57 [DOI] [PubMed] [Google Scholar]
- 3. Kaur C, Rathnasamy G, Ling EA. Biology of microglia in the developing brain. J Neuropathol Exp Neurol 2017;76:736–753 [DOI] [PubMed] [Google Scholar]
- 4. Matcovitch-Natan O, Winter DR, Giladi A, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016;353:aad8670. [DOI] [PubMed] [Google Scholar]
- 5. Tian L, Prabhakaran MP, Ramakrishna S. Strategies for regeneration of components of nervous system: scaffolds, cells and biomolecules. Regen Biomater 2015;2:31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miron VE, Boyd A, Zhao J, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 2013;16:1211–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu B, Zhao Y, Xiao Z, et al. A dual functional scaffold tethered with EGFR antibody promotes neural stem cell retention and neuronal differentiation for spinal cord injury repair. Adv Healthc Mater 2017;6:160–279 [DOI] [PubMed] [Google Scholar]
- 8. Zörner B, Filli L, Starkey ML, et al. Profiling locomotor recovery: comprehensive quantification of impairments after CNS damage in rodents. Nat Methods 2010;7:701–708 [DOI] [PubMed] [Google Scholar]
- 9. Batbayar K. Conversion equation between the drop height in the New York University impactor and the impact force in the Infinite Horizon impactor in the contusion spinal cord injury model. J Neurotrauma 2015;32:1987–1993 [DOI] [PubMed] [Google Scholar]
- 10. Richard B, Riyi S. Immediate recovery from spinal cord injury through molecular repair of nerve membranes with polyethylene glycol. FASEB J 2018;14:27–35 [DOI] [PubMed] [Google Scholar]
- 11. Romanyuk N, Amemori T, Turnovcova K, et al. Beneficial effect of human induced pluripotent stem cell-derived neural precursors in spinal cord injury repair. Cell Transplant 2015;24:1781–1797 [DOI] [PubMed] [Google Scholar]
- 12. Kjell J, Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech 2016;9:1125–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han S, Li X, Xiao Z, et al. Complete canine spinal cord transection model: a large animal model for the translational research of spinal cord regeneration. Sci China Life Sci 2018;61:115–117 [DOI] [PubMed] [Google Scholar]
- 14. Bechara SL, Judson A, Popat KC. Template synthesized poly(epsilon-caprolactone) nanowire surfaces for neural tissue engineering. Biomaterials 2010;31:3492–3501 [DOI] [PubMed] [Google Scholar]
- 15. Burdick JA, Ward M, Liang E, et al. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials 2006;27:452–459 [DOI] [PubMed] [Google Scholar]
- 16. Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014;81:229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prang P, Muller R, Eljaouhari A, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials 2006;27:3560–3569 [DOI] [PubMed] [Google Scholar]
- 18. Milhorat T, Capocelli AL, Anzil AP. Pathological basis of spinal cord cavitation in syringomyelia:analysis of 105 autopsy cases. J Neurosurg 1995;82:802–812 [DOI] [PubMed] [Google Scholar]
- 19. Kanno H, Pressman Y, Moody A, et al. Combination of engineered schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. J Neurosci 2014;34:1838–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. All AH, Gharibani P, Gupta S, et al. Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. PLoS One 2015;10:e0116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schachtrup C, Ryu JK, Helmrick MJ, et al. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci 2010;30:5843–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faulkner JR, Herrmann JE, Woo MJ, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 2004;24:2143–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery afterspinal cord injury. Lett Nat 2002;416:6461–6474 [DOI] [PubMed] [Google Scholar]
- 24. Fisher D, Xing B, Dill J, et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci 2011;31:14051–14066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen YQ, Tenney AP, Busch SA, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009;326:592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kottis V, Thibault P, Mikol D. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J Neurochem 2002;82:1566–1569 [DOI] [PubMed] [Google Scholar]
- 27. Lee JK, Chan AF, Luu SM, et al. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J Neurosci 2009;29:8649–8654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cafferty WB, Duffy P, Huebner E, et al. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci 2010;30:6825–6837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen MS, Huber AB, Frank M. Nogo-aisamyelin-associatedneurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Lett Nat 2000;403:443–459 [DOI] [PubMed] [Google Scholar]
- 30. Lee JK, Geoffroy CG, Chan AF, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron 2010;66:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol 2017;79:619–643 [DOI] [PubMed] [Google Scholar]
- 32. Papa S, Caron I, Erba E, et al. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials 2016;75:13–24 [DOI] [PubMed] [Google Scholar]
- 33. Kigerl KA, Gensel JC, Ankeny DP, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 2009;29:13435–13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vismara I, Papa S, Rossi F, et al. Current options for cell therapy in spinal cord injury. Trends Mol Med 2017;23:831–849 [DOI] [PubMed] [Google Scholar]
- 35. Hunter G, Powis RA, Jones RA, et al. Restoration of SMN in Schwann cells reverses myelination defects and improves neuromuscular function in spinal muscular atrophy. Hum Mol Genet 2016;25:2853–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lavdas AA, Chen J, Papastefanaki F, et al. Schwann cells engineered to express the cell adhesion molecule L1 accelerate myelination and motor recovery after spinal cord injury. Exp Neurol 2010;221:206–216 [DOI] [PubMed] [Google Scholar]
- 37. Bunge MB, Wood PM. Realizing the maximum potential of schwann cells to promote recovery from spinal cord injury. Handb Clin Neurol 2012;109:523–540 [DOI] [PubMed] [Google Scholar]
- 38. Liu S, Sandner B, Schackel T, et al. Regulated viral BDNF delivery in combination with schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater 2017;60:167–180 [DOI] [PubMed] [Google Scholar]
- 39. Sparling JS, Bretzner F, Biernaskie J, et al. Schwann cells generated from neonatal skin-derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat. J Neurosci 2015;35:6714–6730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hill CE, Hurtado A, Blits B, et al. Early necrosis and apoptosis of Schwann cells transplanted into the injured rat spinal cord. Eur J Neurosci 2007;26:1433–1445 [DOI] [PubMed] [Google Scholar]
- 41. Pearse DD, Sanchez AR, Pereira FC, et al. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: survival, migration, axon association, and functional recovery. Glia 2007;55:976–1000 [DOI] [PubMed] [Google Scholar]
- 42. Moradi F, Bahktiari M, Joghataei MT, et al. BD PuraMatrix peptide hydrogel as a culture system for human fetal Schwann cells in spinal cord regeneration. J Neurosci Res 2012;90:2335–2348 [DOI] [PubMed] [Google Scholar]
- 43. Chau CH, Shum DK, Li H, et al. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J 2004;18:194–196 [DOI] [PubMed] [Google Scholar]
- 44. Houle JD, Tom VJ, Mayes D, et al. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci 2006;26:7405–7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deng LX, Deng P, Ruan Y, et al. A novel growth-promoting pathway formed by GDNF-overexpressing schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J Neurosci 2013;33:5655–5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dombrowski MA, Sasaki M, Lankford KL, et al. Myelination and nodal formation of regenerated peripheral nerve fibers following transplantation of acutely prepared olfactory ensheathing cells. Brain Res 2006;1125:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Radtke C, Aizer AA, Agulian SK, et al. Transplantation of olfactory ensheathing cells enhances peripheral nerve regeneration after microsurgical nerve repair. Brain Res 2009;1254:10–17 [DOI] [PubMed] [Google Scholar]
- 48. Richter MW, Fletcher PA, Liu J, et al. Lamina propria and olfactory bulb ensheathing cells exhibit differential integration and migration and promote differential axon sprouting in the lesioned spinal cord. J Neurosci 2005;25:10700–10711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kubasak MD, Jindrich DL, Zhong H, et al. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain 2008;131:264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li Y, Decherchi P, Raisman G. Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci 2003;23:727–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Franklin RJM, Barnett SC. Do olfactory glia have advantages over Schwann cells for CNS repair? J Neurosci Res 1997;50:665–672 [DOI] [PubMed] [Google Scholar]
- 52. Kawaja MD, Boyd G, Smithson LJ, et al. Technical strategies to isolate olfactory ensheathing cells for intraspinal implantation. J Neurotrauma 2009;26:155–177 [DOI] [PubMed] [Google Scholar]
- 53. Chen J, Wang Z, Zheng Z, et al. Neuron and microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent neuroinflammation to improve functional recovery after spinal cord injury. Cell Death Dis 2017;8:e3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. David S, Lopez-Vales R, Wee Yong V. Harmful and beneficial effects of inflammation after spinal cord injury: potential therapeutic implications. Handb Clin Neurol 2012;109:485–502 [DOI] [PubMed] [Google Scholar]
- 55. Cusimano M, Biziato D, Brambilla E, et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain 2012;135:447–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ruckh JM, Zhao JW, Shadrach JL, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 2012;10:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kondo Y, Adams JM, Vanier MT, et al. Macrophages counteract demyelination in a mouse model of globoid cell leukodystrophy. J Neurosci 2011;31:3610–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang W, Liu R, Su Y, et al. MicroRNA-21-5p mediates TGF-beta-regulated fibrogenic activation of spinal fibroblasts and the formation of fibrotic scars after spinal cord injury. Int J Biol Sci 2018;14:178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shearer MC, Fawcett JW. The astrocyte/meningeal cell interface—a barrier to successful nerve regeneration? Cell Tissue Res 2001;305:267–273 [DOI] [PubMed] [Google Scholar]
- 60. Krupka AJ, Fischer I, Lemay MA. Transplants of neurotrophin-producing autologous fibroblasts promote recovery of treadmill stepping in the acute, sub-chronic, and chronic spinal cat. J Neurotrauma 2017;34:1858–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Park JH, Min J, Baek SR, et al. Enhanced neuroregenerative effects by scaffold for the treatment of a rat spinal cord injury with Wnt3a-secreting fibroblasts. Acta Neurochir (Wien) 2013;155:809–816 [DOI] [PubMed] [Google Scholar]
- 62. Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci 2003;23:9276–9288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tobias CA, Shumsky JS, Shibata M, et al. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol 2003;184:97–113 [DOI] [PubMed] [Google Scholar]
- 64. Emgard M, Piao JH, Aineskog H, et al. Neuroprotective effects of human spinal cord-derived neural precursor cells after transplantation to the injured spinal cord. Exp Neurol 2014;253:138–145 [DOI] [PubMed] [Google Scholar]
- 65. Cheng ZJ, Bosco DB, Sun L, et al. Neural stem cell-conditioned medium suppresses inflammation and promotes spinal cord injury recovery. Cell Transplant 2017;26:469–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gregory WJ, Mothe A, Wang J, et al. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev 2012;21:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol 2005;194:230–242 [DOI] [PubMed] [Google Scholar]
- 68. Lu P, Wang Y, Graham L, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012;150:1264–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xie J, Willerth SM, Li X, et al. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 2009;30:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Salewski RP, Mitchell RA, Shen C, et al. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev 2015;24:36–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Najbauer J, Erceg S, Laínez S, et al. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium monditions. PLoS One 2008;3:e2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 2005;25:4694–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang YW, Denham J, Thies RS. Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem Cells Dev 2006;15:943–952 [DOI] [PubMed] [Google Scholar]
- 74. Faulkner J, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transpl Immunol 2005;15:131–142 [DOI] [PubMed] [Google Scholar]
- 75. Iwai H, Shimada H, Nishimura S, et al. Allogeneic neural stem/progenitor cells derived from embryonic stem cells promote functional recovery after transplantation into injured spinal cord of nonhuman primates. Stem Cells Transl Med 2015;4:708–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sun Y, Xu CC, Li J, et al. Transplantation of oligodendrocyte precursor cells improves locomotion deficits in rats with spinal cord irradiation injury. PLoS One 2013;8:e57534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Manley NC, Priest CA, Denham J. Human embryonic stem cell-derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl Med 2017;6:1917–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nakajima H, Uchida K, Guerrero AR, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma 2012;29:1614–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li LM, Han M, Jiang XC, et al. Peptide-tethered hydrogel scaffold promotes recovery from spinal cord transection via synergism with mesenchymal stem cells. ACS Appl Mater Interfaces 2017;9:3330–3342 [DOI] [PubMed] [Google Scholar]
- 80. Gunther MI, Weidner N, Muller R, et al. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater 2015;27:140–150 [DOI] [PubMed] [Google Scholar]
- 81. Wang LJ, Zhang RP, Li JD. Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochir (Wien) 2014;156:1409–1418 [DOI] [PubMed] [Google Scholar]
- 82. Zhao H, Cheng L, Du X, et al. Transplantation of cerebral dopamine neurotrophic factor transducted BMSCs in contusion spinal cord injury of rats: promotion of nerve regeneration by alleviating neuroinflammation. Mol Neurobiol 2014;53:187–199 [DOI] [PubMed] [Google Scholar]
- 83. Liu W, Wang Y, Gong F, et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma 2019;36:469–484 [DOI] [PubMed] [Google Scholar]
- 84. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 2007;448:313–317 [DOI] [PubMed] [Google Scholar]
- 85. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676 [DOI] [PubMed] [Google Scholar]
- 86. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872 [DOI] [PubMed] [Google Scholar]
- 87. Khazaei M, Ahuja CS, Fehlings MG. Induced pluripotent stem cells for traumatic spinal cord injury. Front Cell Dev Biol 2017;4:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Amemori T, Ruzicka J, Romanyuk N, et al. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res Ther 2015;6:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Salewski RP, Mitchell RA, Li L, et al. Transplantation of induced pluripotent stem cell-derived neural stem cells mediate functional recovery following thoracic spinal cord injury through remyelination of axons. Stem Cells Transl Med 2015;4:743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nori S, Okada Y, Yasuda A, et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A 2011;108:16825–16830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nori S, Okada Y, Nishimura S, et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep 2015;4:360–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Enzmann GU, Benton RL, Talbott JF, et al. Functional considerations of stem cell transplantation therapy for spinal cord repair. J Neurotrauma 2006;23:479–495 [DOI] [PubMed] [Google Scholar]
- 93. Koshizuka S, Okada S, Okawa A, et al. Transplanted hematopoietic stem cells from bone marrow differentiate into neural lineage cells and promote functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol 2004;63:64–72 [DOI] [PubMed] [Google Scholar]
- 94. Niekerk EA, Tuszynski MH, Lu P, et al. Molecular and cellular mechanisms of axonal regeneration after spinal cord injury. Am Soc Biochem Mol Biol 2005;74:541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhao YZ, Jiang X, Xiao J, et al. Using NGF heparin-poloxamer thermosensitive hydrogels to enhance the nerve regeneration for spinal cord injury. Acta Biomater 2016;29:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Harvey AR, Lovett SJ, Majda BT, et al. Neurotrophic factors for spinal cord repair: which, where, how and when to apply, and for what period of time? Brain Res 2015;1619:36–71 [DOI] [PubMed] [Google Scholar]
- 97. Chen J, Zeng X, Li S, et al. Lentivirus-mediated inhibition of AQP4 accelerates motor function recovery associated with NGF in spinal cord contusion rats. Brain Res 2017;1669:106–113 [DOI] [PubMed] [Google Scholar]
- 98. Leech KA, Hornby TG. High-intensity locomotor exercise increases brain-derived neurotrophic factor in individuals with incomplete spinal cord injury. J Neurotrauma 2017;34:1240–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kovalchuk Y, Holthoff K, Konnerth A. Neurotrophin action on a rapid timescale. Curr Opin Neurobiol 2004;14:558–563 [DOI] [PubMed] [Google Scholar]
- 100. Brock JH, Rosenzweig ES, Blesch A, et al. Local and remote growth factor effects after primate spinal cord injury. J Neurosci 2010;30:9728–9737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Uchida S, Hayakawa K, Ogata T, et al. Treatment of spinal cord injury by an advanced cell transplantation technology using brain-derived neurotrophic factor-transfected mesenchymal stem cell spheroids. Biomaterials 2016;109:1–11 [DOI] [PubMed] [Google Scholar]
- 102. Gransee HM, Zhan WZ, Sieck GC, et al. Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J Neurotrauma 2015;32:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lu P, Blesch A, Tuszynsk A. Neurotrophism without neurotropism:BDNF promotes survival but not growth of lesioned corticospinal neurons. J Comp Neurol 2001;436:456–470 [DOI] [PubMed] [Google Scholar]
- 104. Hohn A, Leibrock J, Bailey K, et al. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature 1990;344:339–341 [DOI] [PubMed] [Google Scholar]
- 105. Bloch J, Fine EG, Bouche N, et al. Nerve growth factor- and neurotrophin-3-releasing guidance channels promote regeneration of the transected rat dorsal root. Exp Neurol 2001;172:425–432 [DOI] [PubMed] [Google Scholar]
- 106. Piantino J, Burdick JA, Goldberg D, et al. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol 2006;201:359–367 [DOI] [PubMed] [Google Scholar]
- 107. Weishaupt N, Mason AL, Hurd C, et al. Vector-induced NT-3 expression in rats promotes collateral growth of injured corticospinal tract axons far rostral to a spinal cord injury. Neuroscience 2014;272:65–75 [DOI] [PubMed] [Google Scholar]
- 108. Leibinger M, Muller A, Andreadaki A, et al. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci 2009;29:14334–14341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Del Gaizo DJ, Conor MM MD.a R, et al. The effect of methylprednisolone intravenous infusion on the expression of ciliary neurotrophic factor in a rat spinal cord injury model. Spine J 2013;13:439–442 [DOI] [PubMed] [Google Scholar]
- 110. Rosenberg LJ, Lucas JH. The effects of ciliary neurotrophic factor on murine spinal cord neurons subjected to dendrite transection injury. Brain Res 1997;775:209–213 [DOI] [PubMed] [Google Scholar]
- 111. Abbaszadeh HA, TiraihiI T, Delshad AR. Human ciliary neurotrophic factoreoverexpressing stable bone marrow stromal cells in the treatment of a rat model of traumatic spinal cord injury. Cytotherapy 2015;17:912–921 [DOI] [PubMed] [Google Scholar]
- 112. Cao Q, He Q, Wang Y, et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci 2010;30:2989–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. DePaul MA, Lin CY, Silver J, et al. Peripheral nerve transplantation combined with acidic fibroblast growth factor and chondroitinase induces regeneration and improves urinary function in complete spinal cord transected adult mice. PLoS One 2015;10:157–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Li S, Bock E, Berezin V. Neuritogenic and neuroprotective properties of peptide agonists of the fibroblast growth factor receptor. Int J Mol Sci 2010;11:2291–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Le TM, Morimoto N, Mitsui T, et al. The sustained release of basic fibroblast growth factor accelerates angiogenesis and the engraftment of the inactivated dermis by high hydrostatic pressure. PLoS One 2019;14:e0208658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kao CH, Chen SH, Chio CC, et al. Exogenous administration of glial cell line-derived neurotrophic factor improves recovery after spinal cord injury. Resuscitation 2008;77:395–400 [DOI] [PubMed] [Google Scholar]
- 117. Jiao G, Lou G, Mo Y, et al. A combination of GDNF and hUCMSC transplantation loaded on SF/AGs composite scaffolds for spinal cord injury repair. Mater Sci Eng C Mater Biol Appl 2017;74:230–237 [DOI] [PubMed] [Google Scholar]
- 118. Meisel C, Schwab JM, Prass K, et al. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci 2005;6:775–786 [DOI] [PubMed] [Google Scholar]
- 119. Zhang Y, Guan Z, Reader B, et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J Neurosci 2013;33:12970–12981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Salegio EA, Bresnahan JC, Sparrey CJ, et al. A unilateral cervical spinal cord contusion injury model in non-human primates (Macaca mulatta). J Neurotrauma 2016;33:439–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. De Laporte L, Yan AL, Shea LD. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials 2009;30:2361–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pertici V, Trimaille T, Laurin J, et al. Repair of the injured spinal cord by implantation of a synthetic degradable block copolymer in rat. Biomaterials 2014;35:6248–6258 [DOI] [PubMed] [Google Scholar]
- 123. Keefe K, Sheikh I, Smith G. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci 2017;18:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rosich K, Hanna BF, Ibrahim RK, et al. The effects of glial cell line-derived neurotrophic factor after spinal cord injury. J Neurotrauma 2017;34:3311–3325 [DOI] [PubMed] [Google Scholar]
- 125. Assinck P, Duncan GJ, Hilton BJ, et al. Cell transplantation therapy for spinal cord injury. Nat Neurosci 2017;20:637–647 [DOI] [PubMed] [Google Scholar]
- 126. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001;24:677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Almeida RD, Manadas BJ, Melo CV, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 2005;12:1329–1343 [DOI] [PubMed] [Google Scholar]
- 128. Fischer D, Leibinger M. Promoting optic nerve regeneration. Prog Retin Eye Res 2012;31:688–701 [DOI] [PubMed] [Google Scholar]
- 129. Dailey L, Ambrosetti D, Mansukhani A, et al. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 2005;16:233–247 [DOI] [PubMed] [Google Scholar]
- 130. Diez del Corral R, Morales AV. The multiple roles of FGF signaling in the developing spinal cord. Front Cell Dev Biol 2017;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]