Abstract
Background and Aims
The ability of individuals to change sex during their lifetime is known as environmental sex determination (ESD). This represents a unique life history trait, allowing plants to allocate resources differentially to male and female functions across lifetimes, potentially maximizing fitness in response to changing environmental or internal cues. In this study, Acer pensylvanicum, a species with an unconfirmed sex determination system, was investigated to see what patterns in sex expression existed across multiple years, if there were sex-based differences in growth and mortality, and whether this species conformed to theoretical predictions that females are larger and in better condition.
Methods
Patterns of sex expression were documented over 4 years in a phenotypically subdioecious A. pensylvanicum population located in New Jersey, USA, and data on size, mortality, health and growth were collected. A machine-learning algorithm known as a boosted classification tree was used to develop a model to predict the sex of a tree based on its condition, size and previous sex.
Results
In this study, 54 % of the trees switched sex expression during a 4-year period, with 26 % of those trees switching sex at least twice. Consistently monoecious trees could change relative sex expression by as much as 95 %. Both size and condition were influential in predicting sex, with condition exerting three times more relative influence than size on expressed sex. Contrary to theoretical predictions, the model showed that full female sex expression did not increase with size. Healthy trees were more likely to be male; predicted female sex expression increased with deteriorating health. Growth rate negatively correlated with multiple years of female sex expression. Populations maintained similar male-skewed sex ratios across years and locations and may result from differential mortality: 75 % of dead trees flowered female immediately before death.
Conclusions
This study shows conclusively that A. pensylvanicum exhibits ESD and that femaleness correlates with decreased health, in contrast to prevailing theory. The mortality findings advance our understanding of puzzling non-equilibrium sex ratios and life history trade-offs resulting from male and female sex expression.
Keywords: Angiosperms, Acer pensylvanicum, environmental sex determination, health, mortality, Sapindaceae, sex expression, dioecy, hardwood forests, plastic sex determination, diphasy
INTRODUCTION
Environmental sex determination (ESD) is a form of ontogenic sex change in which individuals may change sex expression from year to year (Charnov and Bull, 1977; Schlessman, 1986; Korpelainen, 1998). Factors that might affect sex include individual variables such as size, growth rate and mortality, or landscape-level variables such as light and nutrient resources (Heslop-Harrison, 1957; Charnov and Bull, 1977; Freeman et al., 1980; Schlessman, 1986; Korpelainen, 1998; de Jong and Klinkhamer, 2005). In most cases sex change is unidirectional but sex may be reversed under experimental conditions (Maekawa, 1924; Bierzychudek, 1984). Studies have reported ESD in at least 198 animal species within 89 fish and reptile genera (The Tree of Sex Consortium et al., 2014) and ~250 plant species (Renner, 2014), eight of them within the genus Acer (Renner et al., 2007). However, the lack of long-term data has subsequently called into question the evidence for ESD in many plant species (Lloyd and Bawa, 1984; Schlessman, 1988).
Flowering plants, like many animals, may differ greatly in resource allocation to sexual reproduction in males and females (Case and Ashman, 2005). In contrast to the stamens of male plants which wither after anthesis (Case and Ashman, 2005), female plants often have a heavy resource investment in developing seeds and fruits. This imbalance has led to the theory that advantageous conditions such as large size or overall good health select for female reproduction, while maleness is more feasible under harsh conditions (Charnov and Bull, 1977; Freeman et al., 1980; Schlessman, 1986; Korpelainen, 1998; de Jong and Klinkhamer, 2005).
The best documented examples of ESD in plants and animals fit the theory of size-dependent sex allocation, which predicts that the sex that gains the most fitness with size will be expressed by larger individuals (Ghiselin, 1969; Charnov, 1982; Warner, 1988; Day and Aarssen, 1997; Cadet et al., 2004; de Jong and Klinkhamer, 2005). For example, in Arisaema spp., increasing size (particularly of the underground storage corms and leaf area) manifests as increasing female sex expression (Schaffner, 1922; Maekawa, 1924; Bierzychudek, 1984). Other examples in which females manifest a larger size include Lilium apertum (Zhang et al., 2014), Coptis laciniata (Lindh, 2017) and Panax trifolium (Schlessman, 1991). Under size-dependent sex allocation, populations are expected to contain a larger proportion of smaller plants, resulting in male-skewed sex-ratios (Schlessman, 1988). Size, however, is not always correlated with sex expression, as studies have shown in Acer pensylvanicum (Hibbs and Fischer, 1979), A. japonicum (Sato, 2002) and A. rufinerve (Matsui, 1995).
The patchy environment model theorizes that ambient environmental factors may also affect allocation to male and female function (Charnov and Bull, 1989). In sexually stable species, environmental variables such as precipitation, light levels, general stress, trauma, soil nutrients, photoperiod, temperature or hormone application may impact relative sex expression (Heslop-Harrison, 1957; Freeman et al., 1980). Evidence regarding the impact of environmental variables in sexually plastic species is limited, but physical stress such as drought, lack of nutrients or low light levels may induce maleness (Heslop-Harrison, 1957; Freeman et al., 1980; Korpelainen, 1998). Examples of this include Atriplex canescens, in which some individuals exhibited changes to male following drought, extreme cold, and heavy fruiting, and Catasetum viridiflavum, which showed increased male flowering in response to reduced light levels (Freeman et al., 1984; Zimmerman, 1991). Conversely, in a study of 58 Acer rufinerve trees in Japan, female sex expression correlated with decreased health (Nanami et al., 2004). Although several reviews suggest that males are found in poor health (Heslop-Harrison, 1957; Freeman et al., 1980; Korpelainen, 1998), few studies have sought to quantify the relationship between decreased condition and sex expression. This may be due, in part, to the difficulty in quantifying poor health, which varies among species. In trees, the following features generally characterize poor condition: discoloured or dead foliage during the growing season, structural cracks, decay and dieback of branches, cankers or visible fungal fruiting bodies, or damaged and diseased roots (Lilly, 1999; Schwarze et al., 2000; Angwin et al., 2012). Healthy trees, on the other hand, exhibit bright and undamaged foliage, vigorous growth and a high proportion of live branches in the crown (Bond, 2010).
Here we examine the patterns and factors influencing sex expression in striped maple, Acer pensylvanicum (Sapindaceae). A 2-year study of A. pensylvanicum suggested that this species exhibits ESD (Hibbs and Fischer, 1979), but later analyses of the same data set called this conclusion into question, citing the need for longer-term quantitative data collection across multiple populations (Lloyd and Bawa, 1984; Schlessman, 1988). Acer pensylvanicum grows in rocky, mesic soils at higher elevations in the Appalachian mountain range from northern Georgia, USA, to south-eastern Canada, including areas north of the Great Lakes (Hibbs et al., 1980). As an understorey tree, it is well adapted to shaded environments, and its relatively low stature means that it incurs damage from white-tailed deer (through browsing and antler rubbing) and from falling canopy trees and their branches. It is an insect-pollinated, subdioecious species and most individuals bear either staminate or pistillate inflorescences in a given flowering season with male trees outnumbering females (Hibbs and Fischer, 1979). Individuals expressing both male and female inflorescences on the same tree represent less than 5 % of a population (Hibbs and Fischer, 1979). Within monoecious trees, the flowers within an inflorescence are the same sex; rarely male and female flowers can be found in the same inflorescence (de Jong, 1976). Interestingly, and importantly, the final sex determination in this species may occur as late as 3 weeks prior to anthesis, potentially allowing for environmental cues to impact sex the spring immediately prior to flowering (Blake-Mahmud and Struwe, 2018).
We investigated hypotheses derived from the following research questions on (1) the patterns and (2) the predictors for sex expression in A. pensylvanicum: (1a) Do trees change sex expression? (1b) If so, how frequently do individual trees change sex? (1c) What patterns of sex expression exist across years? (1d) Are there sex-based differences in growth rate? (1e) Are there sex-based differences in mortality rate? (2a) To what extent do tree size, tree health, previous sex and growing location influence expressed sex the following year? (2b) Are theoretical predictions that females would be larger and in better health supported?
MATERIALS AND METHODS
Study sites were located in the following state forests and state park lands in New Jersey, USA, that had extensive and accessible striped maple populations: Jenny Jump State Forest (40.913, −74.922, Warren County), Stokes State Forest (41.218, −74.720, Sussex County), High Point State Park (41.321, −74.662, Sussex County) and Wawayanda State Park (41.217, −74.451, Passaic and Sussex Counties). Study trees were those flowering trees that fell within the boundaries of the randomly delimited plots and were tagged to facilitate tracking over multiple years. Sexes grow intermixed at all sites; no spatial segregation of sexes was observed. Following high mortality during summer 2014 due to unexpected tree thinning by the park service resulting in 241 remaining study trees, we increased the plot areas in 2015 to include additional A. pensylvanicum trees. Final plot sizes were ~1000 m2. From 2015 to 2017, all trees added to the study were due to transition events, i.e. previously non-flowering trees flowered and consequently became study trees. In 2017, there were 457 live trees within study plots across all sites. Acer pensylvanicum is known to sprout from stumps or roots, and thus we considered multi-stemmed clumps to be genets, i.e. branches of a single individual (Stalter et al., 1997). We removed soil from bases of tree trunks growing in close proximity (within 6 in) to confirm whether they were joined by roots. Due to the presumed importance of hormonal signals in affecting sex expression, which may proceed either from the bud or the root, plants with common vascular systems were classified as one individual.
We collected the following data from 2014 to 2017 on all study trees within plots: size (measured as diameter 1.35 m from the ground; i.e. at breast height dbh), condition and flowering sex. There is not yet a discipline-wide consensus on methods for quantifying the size of multi-stemmed trees; dbh of the largest stem, as recommended by the U.S. Forest Service (Powell, 2005), was therefore used. We assessed condition, i.e. tree health, by looking for external physical damage to the tree such as broken or dead branches, open cankers, discoloration of the green-photosynthetic bark, or split trunk bark due to rapid spring warming, impact damage or antler-rubbing by deer. The U.S. Forest Service uses a visual assessment of street tree condition to allocate trees to numbered categories according to health (or damage condition based on risk analysis; Angwin et al., 2012). We modified this protocol to measure condition on a scale of 0–5 and adjusted for smaller tree sizes (e.g. so that a 1-m-tall tree missing three of four branches was scored as being in worse condition than a 5 m tree missing three of ten branches). The full condition assessment protocol is available in Supplementary Data Table S1. Trees receiving scores of 0–1 were considered to be in excellent condition, scores of 2 were in good condition, scores of 3 were in fair condition and scores of 4 were in poor condition. Trees receiving scores of 5 were in very poor condition and were 50 % or more dead. Trees were considered to be fully dead when their bark turned black and they failed to produce and expand leaves in the spring or when their bark turned black, leaves shrivelled and turned brown on the tree, and all twigs and branches became dry and brittle prior to leaf drop in autumn.
In 2014, we evaluated flowering and sex by visually scoring the sex of a minimum of 20 inflorescences per tree. The inflorescences were distributed from the tip of branches to the trunk on multiple branches and at multiple heights. We recorded the sex of each tree as male, female or monoecious. Although A. pensylvanicum is a small understorey tree, the sexual structures of inflorescences growing high in large specimens are not always visible to the unaided eye. In these cases, ~20 inflorescences from various regions on a tree were then spot-checked for sex expression with binoculars (Pentax Papilio 8.5×21 mm). Assessment of 27 trees of dbh 1.50–5.25 cm had indicated that our visual assessment of 15 inflorescences from multiple areas of different branches at different heights achieved >95 % accuracy in sex assessment. Following counts of all inflorescences, accuracy reached 100 %. For large monoecious trees, we viewed all inflorescences with binoculars (where necessary) and tallied them as male or female to compute relative percentage female sex expression. Trees that did not flower in a given year were included in analyses if they flowered at least once over the course of the study. We did not include consistently non-flowering trees during 2014–2017 in the analysis.
We assessed growth rate on trees that consistently exhibited unisexual sex expression during the years of this study by measuring shoot extension, a method often used to approximate the local cost of reproduction (Thomas, 2011). We selected the lowest branch in the south, south-west or west exposures and scored the growth rate by measuring the distance between terminal bud scars moving distally from branch tip to capture at least 3 years of shoot extension. Growth rate was averaged across years, yielding one mean rate per individual.
Statistics
To address the relative influence of environmental predictors on patterns of sex expression, we analysed the data using a combined classification and machine learning approach known as boosted classification trees (BCTs). A BCT modelling approach looks at which measured variables may be used to most accurately predict classification of specific outcomes. The variables that are more predictive are said to have higher ‘relative influence’ on the outcome. Following the development of a BCT, we apply the same model to new data to evaluate the extent to which the model can predict the observed outcomes.
Compared to other analysis methods such as generalized linear models, BCTs have several benefits. Boosted classification trees can be used easily for categorical variables, have no assumptions regarding observation independence, and have superior predictive power compared to other modelling methods (see Elith et al., 2008 for a full discussion). Unlike generalized linear mixed effects models (GLMMs) for categorical outcomes which may fail to converge for categories with zero observations, a common occurrence in ecological data, BCTs can better deal with these observations. We performed the BCT analysis in R (version 3.4.1, R Core Team, 2015) using the caret (Kuhn, 2008) and gbm packages (Ridgeway, 2016).
Using the model parameters below, we trained a model for a categorical outcome (sex) and both categorical (sex the previous year, site) and continuous (size, condition) predictors. Because BCTs do not provide P-values, we included random continuous and categorical variables against which to compare the relative influence of predictors. Higher relative influences indicate that with this model, the predictor provides more information than random for correctly categorizing observations and is therefore more strongly correlated with the predicted variable (in this case, sex in year 2). The relative influence of the random variables provides a baseline by answering the following question: how well does a randomly generated set of data help us predict sex?
For building a model using the BCT method, we combined data across 4 years for all living study trees. Trees that died were included in a separate analysis for the sake of clarity. Trees that were initially tagged in 2014 and remained alive throughout the study period are represented three times in the data set, while trees that started to flower in 2017 are only represented once in the analysis. The resulting 1082 records were randomly divided into training (75 % of the data, n = 813 flowering bouts) and testing (25 % of the data, n = 269 flowering bouts) data sets. To ensure that stochasticity arising from random data partitioning did not influence the model training and testing, we compared the frequencies of sexual transitions between training and testing data sets and found them to be within 1.5 % of each other.
The BCT model was fit using the training data set, while the testing data set provided data unseen during model construction for an independent evaluation of the model’s predictive capacity. The algorithm searches for model parameters that maximize predictive ability (as measured by Cohen’s kappa) using a ten-fold cross-validation approach of the training data. We found the following parameters to produce the optimal classification tree: learning rate = 0.001, interaction depth = 3, number of trees = 1000, and bag fraction = 0.5 with multinomial error. The full range of tested model parameters are available online (Supplementary Data Table S2).
Model predictive performance may be assessed for accuracy using Cohen’s kappa. In situations where outcome categories are unevenly distributed, kappa is preferred because it compares predicted outcomes to real outcomes by controlling for expected accuracy. For example, in A. pensylvanicum, populations are male skewed. Kappa accounts for the fact that if the model simply predicted ‘male’ every time, it would have relatively good accuracy in predicting the future sex of a tree without any biological understanding of what is going on via other predictors. Because of this, kappa values are preferred for assessing a model’s predictive capacity, although they are often lower than measures of accuracy.
We also used a classification matrix to assess model fit. This matrix provides information regarding correct and incorrect classifications of the testing data set by the model by comparing the predicted and actual sexual states of trees. The effects of relatively influential variables can be examined using marginal effect plots. These graphs show the effect of changing a single variable on the predicted probability of an outcome (in this case, expressed sex). All other variables are held constant, allowing the impact of one predictor to be examined.
We trained the BCT model to allow for an interaction depth of 2–3, which means that both two- and three-way interactions were possible. In generalized linear model approaches, the presence of an interaction reduces the interpretability of main effects to simple effects for involved predictors. An added benefit of BCTs is that the relative importance of predictors and their marginal effects remain easily interpretable in the presence of interactions. Like other BCT model outcomes, P-values are not produced. Instead, interactions are quantified via Friedman’s H statistics, which vary from 0 (weak interaction) to 1 (strong interaction).
To address questions regarding differential mortality between sexes, we used Fisher’s exact test to compare observed and expected outcomes. Fisher’s exact test extends a chi-squared test to multiple categories and works well for small sample sizes (Fisher, 1935). We compared differences in growth (via stem elongation) for males and females using a t-test for unequal variances. We carried out t-tests and Fisher’s exact tests in JMP Pro (version 13) and created figures using ggplot2 (Wickham, 2009) and SankeyMatic (Bogart, 2016).
RESULTS
Male-dominated sex ratios occurred consistently across study sites and the 4 years that sex expression was monitored (Fig. 1). In any single 2-year period, trees were most likely to remain the same sex. Approximately one-third of trees changed during any 2-year period (2014–2015, 25.7 %; 2015–2016, 31.5 %; 2016–2017, 43.9 %). The five most common transitions were, in descending order of frequency: from non-reproductive to male, male to full or partial female flowering, female to dead, and from partial to full female flowering (Fig. 1).
Fig. 1.
Sankey diagram of sexual transition in Acer pensylvanicum showing male trees in blue, female in pink, monoecious in yellow, non-reproductive in green and dead in grey. Flowering years proceed left to right from 2014 to 2017. Darker coloured vertical bars under the years represent sex expression at that time, while numbers in black correspond to number of individuals expressing that sex at that time. Curves between bars of the same colour represent the fraction of trees that maintained that same sex expression. Curves take on the colour of the second bar representing the sexual state that subset of trees are changing to. Sample size grows from 2014 to 2015 due to increases in study area. Sample size grows between 2015 and 2017 due to transitions to the reproductive phase from previously non-flowering trees. Trees that did not flower during 2014–2017 are excluded from this analysis.
During the study period, 54 % of trees changed sex at least once. Of those that changed expression, 74 % switched sex once, 25 % switched sex twice and 1 % switched sex expression every year. Among trees exhibiting one change, over half of them (39 % of changing trees) switched from male, female or monoecious to a different flowering or non-flowering state. The others (34 % of changing trees) switched from non-flowering to flowering. While many of these may have simply reached sexual maturity for the first time, others resumed flowering after several years of not reproducing. Given the time limits of the study, it is not possible to say, with certainty, which trees belong to which reproductive schedule.
Of the trees classified as monoecious in consecutive years, many individual trees still changed their relative sex expression. Some trees changed the relative percentage of male and female flowers by as much as 95 % (essentially changing from an almost completely male to an almost completely female tree) while others maintained their within-tree flower sex ratio, changing by as little as 1 %. The mean change in within-tree sex expression for monoecious individuals was 38 %. In a 2-year period, monoecious trees might increase or decrease their percentage of female flowers. For example, of trees expressing both sexes at least once during 2015–2016, 61 % of individuals increased female flowering, while 39 % increased male flowering. In 2016–2017, the percentage of monoecious trees increasing allocation to femaleness was 83 %.
Mortality correlated strongly with female sex expression in the previous year. The majority of deaths (53 of the 71) occurred in trees that flowered fully female in the year prior to dying (Supplementary Data Table S3). If all recorded deaths were evenly distributed among flowering states (male, female, monoecious or non-reproductive), we would expect ~18 deaths per state. Even with this highly conservative expectation (given that populations contain more males) Fisher’s exact test yields P values of <0.0001 for the probability of getting these observed mortality data or more extreme data by chance alone. Only about 1 % of male-flowering trees died on average. Between 2and 5 % of monoecious trees died during any given year; a similarly small percentage (0–10 %) of non-flowering trees died. Females experienced 13 % mortality in 2014–2015, 15 % mortality in 2015–2016 and 39 % mortality in 2016–2017, following a dry, hot 2016 summer in the north-east and a generally mild 2016–2017 winter with several hard freezes.
Growth, as measured by branch elongation per year, was different between the sexes. Trees flowering consistently as females over a 3- to 4-year period had lower mean rates of stem elongation (18.5 ± 9.7 mm per year) compared with trees flowering consistently male (51.5 ± 29.4 mm per year) during this time period [t-test for unequal variances n = 14 (7 male, 7 female), d.f. = 7, P = 0.0257).
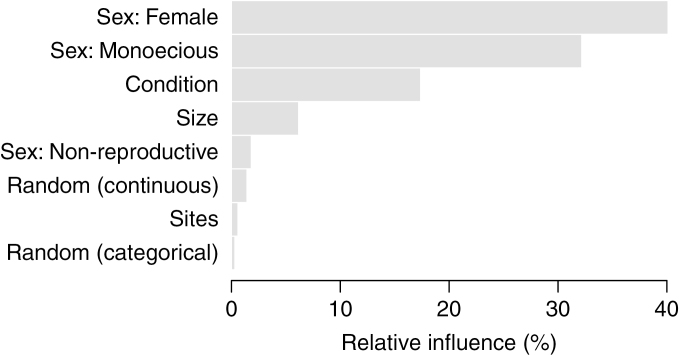
We evaluated a BCT model that looked at the influence of starting sex, condition, size and site on sex the following year. The relative influences of the predictors were compared against a random continuous and random categorical variable (shown in Fig. 2, and given in numerical format in Supplementary Data Table S4). In addition to comparisons against random predictors, model fit may also be assessed using other means; the categorization efficiency of the model is indicated by accuracy and kappa values. There are no hard and fast guidelines for interpreting predictive capacity via Cohen’s kappa values, although an often-cited rule of thumb in the field of medicine indicates that agreement values of 0.4–0.6 signify moderate to good agreement (Landis and Koch, 1977). The accuracy of this model is 0.758 (where 1.0 is perfect predictability), indicating that the predictive ability of the model is good (Landis and Koch, 1977). Cohen’s kappa is 0.452 and indicates moderate agreement in model predictions and actual data (Landis and Koch, 1977). A classification matrix comparing the model-predicted data values with the actual observed values is available online (Table S6).
Fig. 2.
Bar chart showing the relative influence of predictors in the model for expressed sex in Acer pensylvanicum. Sexual state in year 1 has high influence in determining sex the following year. Reproductive states (female, monoecious) are more influential than the non-reproductive state (i.e. non-flowering). Condition is approximately three times more influential than size, both of which are more influential than a random predictor. Site is not influential.
The BCT model showed that the flowering sex in the previous year heavily influences the flowering sex the next year. This is expected, as roughly two-thirds of trees do not change sex in any one year. Among flowering trees, femaleness has the most influence on sex the next year, followed by monoecy (Fig. 2, Supplementary Data Table S5). The non-flowering state exerts a slight influence on sex the following year and is more informative than a random categorical variable. As in other types of analyses such as logistic regression, one group is necessarily withheld as the comparison group; in this BCT model, males are the group to which others are compared.
Condition is approximately three times as influential (17.4 %) as size (6.2 %) in determining sex (Fig. 2); both variables are better predictors of sex than a randomly generated continuous predictor (1.4 %). Site is relatively uninfluential in determining sex the following year (0.6 %, all sites combined), approximately as informative as a random categorical variable (0.3 %; Fig. 2).
By holding other variables constant, we can examine the marginal effect of changing one predictor, such as initial sexual state or ‘starting sex’. Based on our model, flowering trees are most likely to remain their current flowering sex in the following year (Fig. 3). Of non-reproducing trees that flowered at least once during the study period, most were predicted to switch to male the following year (Fig. 3). When condition and size parameters are held constant, the model predicts that males and females have approximately equal baseline probabilities of changing to a different sexual state (Fig. 3, Supplementary Data Table S5). Males and females have high predicted probabilities (58 % and 61 %, respectively) of remaining in that sexual state from year to year. Monoecious trees have approximately equal probabilities (37 % and 35 %, respectively) of remaining monoecious or changing to female and a lower probability (18 %) of changing to male (Fig. 3, Table S5).
Fig. 3.
Stacked bar chart showing the predicted probability of sex (y-axis) in Acer pensylvanicum, by considering starting sex alone (x-axis); that is, by holding constant the parameters of size, condition, and site. Sexual state in the initial year is on the x-axis and marginal predicted probability of a particular sex change is on the y-axis. Colours represent the sex changed to. Females are represented in pink, males in blue, monoecious individuals in yellow and non-reproductive trees in green. Flowering sex in one year strongly influences flowering sex in the next year.
We examined the effect of tree health on flowering sex and found that health substantially influences sex the following year (Fig. 4) when other variables are held constant. The marginal effect plot indicates that healthy trees are most likely to be male (blue line, Fig. 4). For trees in generally good health (condition levels 0–2; Supplementary Data Table S1) the worsening of condition has little influence on the marginal probability of various expressed sexes, indicated by the relatively flat slopes of lines over these condition levels (condition levels 0–2; Table S1). Worsening condition for trees in moderate to poor health (levels 3–5) changes the probability of being male or female substantially. For trees flowering female, individuals are more likely to be in poor health than good health; the reverse is true for males. Specifically, the predicted probability of being male decreases from ~0.65 to 0.35 with deteriorating condition, while the probability of being female increases from 0.14 to 0.35. At the worst levels of condition where trees have multiple kinds of damage to vascular tissue and branches, including sections of crown dieback, a tree is equally likely to be male or female (far right in Fig. 4). The probability of being non-flowering increases slightly with poorer condition from 0.09 to 0.13. The probability of monoecious flowering starts at 0.13 for healthy trees and peaks at 0.2 for intermediate levels of condition (Fig. 4). Because overall population sex ratios are male-skewed in striped maple populations, we would expect the predicted probability of male sex expression in year 2 to always be higher. The fact that female sex expression has an equal predicted probability at the worst levels of health indicates that females are more over-represented in trees of poor condition than would be expected (Fig. 4). The correlation between size and condition was low. High correlations would have resulted if condition scores were assigned by assessing absolute tree damage so that only very large trees would have high condition scores.
Fig. 4.
The effect of poor condition (x-axis) on the predicted probability of sex in the second year (y-axis) in Acer pensylvanicum with size, site and starting sex held constant. Colours indicate predicted sexual state in year 2: females are represented in pink, males in blue, monoecious individuals in yellow and non-reproductive trees in green. In generally healthy trees (condition levels 0–2), males are predicted to constitute ~70 % of the populations, with females and monoecious trees each representing ~10 % of the population. In generally unhealthy trees (condition levels 3–5), the proportion of predicted males decreases and the predicted probability of female sex expression rises. Female trees (represented by the pink line) are more likely to be in poor health than good health.
We did find that size was a better-than-random predictor of sexual state (Fig. 2); specifically, the probability of flowering monoeciously increased with size, while non-flowering plants were more likely to be small. However, the marginal effect plot depicts a relatively constant relationship with size for trees flowering exclusively male or female (Fig. 5; slope of blue and pink lines). Because populations have a male-biased sex ratio, at any given size a tree has a higher overall marginal probability of being male, as indicated by the higher placement of the blue line. The slope of the line demonstrates that predicted male flowering peaks at sizes of 2 cm dbh and then decreases slightly with increasing size. The probability of being female remains constant over all documented size classes; the predicted probability of flowering monoeciously doubles from ~0.1 to 0.2. With increasing size, the predicted probabilities of being non-flowering are highest at very small sizes (<1 cm dbh), then remain low across size classes (Fig. 5).
Fig. 5.
The predicted relationship of increasing size (x-axis) on the predicted probability of sex in the second year (y-axis) in Acer pensylvanicum while holding the parameters of condition, site and starting sex constant. Colours indicate predicted sexual state in year 2: females are represented in pink, males in blue, monoecious individuals in yellow and non-reproductive trees in green. At the smallest sizes (dbh) the model predicts ~55 % male, 13 % monoecious, 16 % female and 16 % non-flowering trees, while at the largest dbh, the model predicts 50 % male, 18 % female, 22 % monoecious and 10 % non-flowering. Notably, when all other variables are held constant (sex in year 1, health of the tree, site) and size alone changes, the probability of female sex expression is predicted to be equal (i.e. the slope of the line is flat) across all size classes.
Tests of the strength of two and three-way interactions were conducted using Friedman’s H statistic (varying from 0, weak interaction, to 1, strong interaction; Table 1). Interactions with condition are strongest for males (0.420), followed by females (0.181) and monoecious trees (0.115). Healthy trees are proportionally over-represented in trees flowering male, while unhealthy trees are over-represented in the female state. Non-flowering A. pensylvanicum trees or trees that contained both male and female inflorescences exhibit a slightly different response to size. Smaller trees are over-represented in the non-flowering state, while larger trees are over-represented in the monoecious state (Fig. 5; green and yellow lines). This is quantified in the interactions between starting sex and size for both monoecious and non-reproductive trees (0.296 and 0.286, respectively; Table 1). Although our best-fit model indicated a tree depth of 3 (Table S2), allowing for three-way interactions, the interaction between starting sex, size and condition is negligible.
Table 1.
Interaction strengths, measured as Friedman’s H statistics, of two and three-way interactions on flowering state in Acer pensylvanicum. Friedman’s H statistics vary from 0, indicating a weak interaction, to 1, which indicates a strong interaction. Largest interactions exist between male sex and condition, female sex and condition, monoecious sex and size, and non-reproductive trees and size
| Male | Monoecious | Female | Non-reproductive | |
|---|---|---|---|---|
| Sex × Condition | 0.420 | 0.115 | 0.181 | 0.052 |
| Sex × Size | 0.059 | 0.296 | 0.037 | 0.286 |
| Condition × Size | 0.023 | 0.093 | 0.014 | 0.165 |
| Condition × Size × Sex | 0.009 | 0.008 | 0.001 | 0.008 |
DISCUSSION
Here we show for the first time that A. pensylvanicum individuals may repeatedly change sex over time (Fig. 1). Furthermore, monoecy is not merely an expression of male inconstancy as previously hypothesized (Schlessman, 1986). Numerous trees enter monoecy from both male and female flowering states. Trees may flower monoeciously for consecutive years or may subsequently become full males or females. (Figs 1 and 3). Contrary to the theoretical predictions of size-dependent sex allocation and patchy environmental models, our data-informed model found that females are proportionally over-represented in trees of poor condition (Fig. 4) and are not predicted to be larger (Fig. 5). Model predictions indicate that deteriorating health shows a moderate threshold effect, with predicted sex ratios changing drastically for trees in the worst condition (Fig. 4). Mortality is highest among females (Table S3), confirming a previous observation of a natural population (Hibbs and Fischer, 1979).
In other plant species exhibiting ESD, it appears that males are typically individuals found in poorer health, or that have experienced environmental stress, as in Atriplex canescens (Freeman et al., 1980, 1984; Korpelainen, 1998). However, in Acer pensylvanicum as individual health deteriorates and trees incur more damage, the incidence of partial and full female flowering increases. Amongst the least healthy trees, population sex ratios are ~1: 1, compared to overall sex ratios of ~3.5 males to one female (Figs 1 and 4). Paralleling the findings of this study, Nanami et al. (2004) showed that unhealthy Acer rufinerve trees were more likely to be female. Our work builds on this finding by quantifying the relationship with health. We show that it is not simply the presence of any mild damage or the gradual accumulation of minor injuries associated with general ageing that correlates with changing sex expression. Rather, it appears that a threshold exists after which the occurrence of multiple kinds of severe damage drastically increases the probability of full female sex expression.
Poor physical condition in trees due to dead or lost branches, open cankers, infection or bark injuries can affect various physiological processes. Wounds and infections are sealed off from surrounding tissues by the deposition of chemical and anatomical barriers (Shigo, 1984; Dujessiefken and Liese, 2015). The resulting compartmentalization limits the direct extent of injuries and infections and allows the tree to continue to live and grow. The tree-produced boundaries, while protective, are common starting points for structural failures (Shigo, 1984; Schwarze et al., 2000). Over time, one might expect the repeated compartmentalization to impede the vascular connections between leaves and roots that are necessary for transportation of water, nutrients, non-structural carbohydrates and regulatory hormones (Franklin et al., 1987). This could both reduce the health of individuals and change the hormonal balance responsible for sex-specific flowering. The mechanistic links between wounding, decreased health and plastic sex expression in plants have yet to be uncovered.
Under size-dependent sex-allocation, females are often larger (Heslop-Harrison, 1957; Freeman et al., 1984; Korpelainen, 1998), particularly in insect-pollinated species (de Jong and Klinkhamer, 2005). However, in Acer, size is often less informative, with individuals of A. japonicum (Sato, 2002) and A. rufinerve (Matsui, 1995) showing no correlation between sex expression and size. While previous work on A. pensylvanicum had suggested that sex expression was not correlated with size (Hibbs and Fischer, 1979), our results indicate a more nuanced relationship of sex and size. Similarly to A. saccharinum, in which monoecious trees are larger than either single-sexed females or males (Sakai and Oden, 1983), we found that the predicted probability of monoecious flowering in A. pensylvanicum increased with size.
Although most hypotheses for ESD in plants conform to the size-dependent or patchy-environment models, other theories in the animal literature exist explaining when and why organisms could change sex. Iwasa (1991) developed two complementary models based on the importance of growth rate and mortality. In the mortality-advantage and growth-rate advantage models, an individual may have multiple viable reproductive states, including a resting (or non-reproductive) option. In these models, costliness is related to slower growth or higher mortality; larger organisms are assumed to have higher fitness. The model-predicted, evolutionarily stable strategies for individuals are to minimize mortality risk and maximize growth rate by switching amongst reproductive states. While the specific reproductive schedule will vary within and among species based on numerous factors, these models potentially support repeated switching between sexes and prolonged or iterative bouts of non-reproduction (Iwasa, 1991). Nanami et al (2004) found evidence of differential mortality and growth rates among the subdioecious, sexually plastic A. rufinerve, with female and monoecious trees having lower rates of growth and higher rates of mortality than males.
While we did not find that larger size increased the predicted probability of full female sex expression, we did find evidence of other trade-offs and support for the growth rate/mortality advantage model (Iwasa, 1991), which hypothesizes that when growth rate and mortality risk vary between sexes, sexual plasticity may be selected for. The evolutionarily stable strategy is to express the less-costly sex first (as measured by higher growth rate or lower mortality), perhaps followed by periods of non-reproduction, before expressing the more-costly sex (Iwasa, 1991). Our findings of decreased growth and increased mortality among females support this model. Large long-term, multi-population studies on sex-differential mortality are rare, particularly in species exhibiting ESD. Our results are in line with a smaller-scale study done on A. rufinerve in which females experienced significantly higher mortality than males (Nanami et al., 2004), but contrast with studies of A. rubrum in which no differential mortality was observed (Sakai, 1990).
Evidence of reduced growth rates in females is also consistent with increased sex-specific costs of reproduction (e.g. devoting energy to provisioning seeds rather than growth; Obeso, 2002) for females. Previous research on sex-specific costs of reproduction has yielded mixed results. Work on perennial plants with stable sex expression, such as Populus or Asparagus species, indicated that males often exhibit increased growth rates (reviewed by Lloyd and Webb, 1977). Other work has found the opposite, with growth of female clones of Populus tremuloides exceeding that of males (Sakai and Burris, 1985). Dioecious Acer negundo showed no sex-based differences in shoot elongation (Willson, 1986). The growth rate/mortality advantage model seems to describe the observed dynamics of A. pensylvanicum and A. rufinerve, both members of the Macrantha clade. The extent to which these models accurately characterize sex expression in other plants exhibiting ESD, including Acer species, is inconclusive.
Our model indicates that sex the previous year is the most influential predictor of sex the following year (Fig. 2), a finding probably driven by the large percentage of trees in any two-year period that maintain the same sex expression (Figs 1 and 3). Work done on sex expression in Acer rubrum and A. grandidentatum indicated similar frequencies of sex change, of between 15 and 26 % (Barker et al., 1982; Primack and McCall, 1986). After accounting for size, condition and site, the marginal effect plot indicates that female to male transitions and male to female transitions are equally probable (Fig. 3). This suggests that the observed larger numbers of male to female transitions are influenced by other variables or by skewed sex ratios. Combining the predictions that monoecious trees exhibit increased size (Fig. 5), moderate health (Fig. 4) and are the least likely to maintain their flowering status (Fig. 3) suggests that monoecy may be a transitional state for healthier growing trees. Iwasa’s (1991) models suggest that the sex expressing the lowest mortality and higher growth should be expressed first, a prediction supported by the observation that most non-reproducing trees in year 1 flowered male in year 2, probably reflecting the onset of sexual maturity (Fig. 3). More data are needed to explicitly test the relationship between transitions in and out of the non-reproductive state and its correlation with size and health.
Unlike other studies investigating ESD, our machine learning approach creates a model that may be used to predict sex expression in the future. The categorization efficiency of the model is indicated by accuracy and kappa values. The use of kappa is not widespread in ecology, although a meta-analysis of 34 studies indicated that the majority of ecological research with presence–absence data had kappa values of <0.4 for their explanatory models [Manel et al. (2001); but see recent work in ecological fisheries with kappa values as high as 0.55 (Free et al., 2017)]. The interpretation guidelines are ‘clearly arbitrary’ (Landis and Koch, 1977) and certainly context-dependent. Due to the complexity and environmental stochasticity inherent in ecological studies, revised benchmarks and ecologically based test statistics could be useful (Manel et al., 2001). The current guidelines, coupled with comparisons with other ecological studies, indicate that our model sufficiently encapsulates the effects of the independent variables to adequately predict future flowering behaviour. In particular, for forest managers concerned with the population size and persistence of striped maple, particular attention should be paid to the role of tree health in maintaining historical male-biased sex ratios.
While fully untangling the underlying ecological controls of sex expression in this species will take decades of monitoring, complex environmental data sets and sophisticated statistical analyses, the generalized pathway we suggest in Fig. 6 is consistent with observations and results from our 4-year study. In this data-informed hypothesized schematic (Fig. 6), increases in body size generally cause individuals to move from non-reproductive to male-flowering phases. Trees leaving the male phase often do so by one or two primary paths. They may begin flowering monoeciously, apparently due to increased size. Males may also skip the in-between stage and transition directly to complete female flowering if their health is deteriorating. Individuals may remain in any flowering state for years, with trees more likely to maintain female or male flowering than they are monoecious flowering. Most trees die after flowering female. While ‘reverse’ transitions occur in A. pensylvanicum, current data are not yet sufficient to hypothesize on the causes of these transitions.
Fig. 6.
A generalized pathway for sex expression in Acer pensylvanicum for the most common transitions in flowering. For specific changes, including reverse transitions, see Fig. 1.
This study clarifies the sexual expression of A. pensylvanicum and confirms its system of sex determination. Nevertheless, several outstanding questions remain. Is decreasing health a cause or consequence of female sex expression? To what extent can femaleness be cued by significant injury events? Why do female trees die more frequently? Is it because they have devoted a critical mass of carbohydrate resources to seed production, leaving insufficient non-structural carbohydrates for everyday functioning or for another reason such as drought or disease? Here we have demonstrated which variables are most influential for predicting sex expression; are these same variables equally influential in predicting all directions of sex changes? Further work will investigate these questions using manipulative experiments and longer-term data collection.
CONCLUSIONS
We have shown that in the sexually plastic tree A. pensylvanicum, a variety of factors influence expressed sex. Chief among them are previous sex and the health of an individual. Although the general theory regarding ESD in dioecious plants has indicated that females are often found in relatively better condition and at larger sizes, we find the opposite pattern in this species. Taken with findings on other species in the same genus (Renner et al., 2007), this supports the idea that Acer has evolved not only to be reproductively diverse, but also with unique pathways of sex determination. Furthermore, we show that mortality is disproportionately high in females, which potentially explains the overrepresentation of males in these populations. Studies like this are rare due to their long-term scale and intense field efforts, but are valuable to examine the viability of theoretical predictions in wild populations.
The population- and forest-level implications of these findings could be substantial but are not yet fully clear. The climate is changing, with multiple effects, including warming, and an increased incidence of strong meteorological incidents (IPCC, 2014). We might expect the number of females in populations to increase due to reduced tree health following storm damage, increased severe drought, or increased prevalence of fungal or other infections – all risks associated with the effects of climate change in temperate regions (Barker et al., 2007; Moran et al., 2017). While an increased shift to femaleness could increase seed-set in the short term, the larger number of females will probably also result in increased mortality across populations. At its extreme, this scenario endangers the long-term recruitment and persistence of A. pensylvanicum populations. Further understanding of the correlated patterns and causative effects of body size, health, damage levels and other individual variation on sex expression is needed to fully evaluate potential interacting effects of emerging diseases, climate change and weather, and forest dynamics on species with plastic sex determination.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Tree condition as evaluated using a modified tree assessment based on the forest services’ hazard tree assessment protocol. Table S2: Standard parameters used in computing BCTs – interaction depth, learning rate, number of trees and bag fraction. Table S3: Sex of study trees prior to dying. Table S4: Relative influence of predictors on determining sex in year 2. Table S5: Model-predicted probability of sex in year 2, while holding the parameters of size, condition and site constant. Table S6: Classification matrix for predicted and observed data on sexual state of individual Acer pensylvanicum trees.
FUNDING
This work was supported by: Ecology and Evolution Graduate Program, Rutgers University; the Torrey Botanical Society; the Botanical Society of America; the Philadelphia Botanical Society, USDA-Hatch NJ17142; and the National Science Foundation NSF-DGE/IGERT 0903675.
ACKNOWLEDGEMENTS
We thank Greg Anderson, Peter Morin and Jason Grabosky for their helpful comments as well as Chris Free, Olaf Jensen, Rachael Winfree, Peter Morin and Peter Smouse for statistical and modelling help. Thanks to Pepe Bowman, Anny Marchioni, Gil and Diane Jeffer, Naved Mahmud and Kepler Mahmud for field assistance. Field research permits were obtained from the New Jersey Department of Environmental Protection.
LITERATURE CITED
- Angwin PA, Cluck DR, Zambino PJ, Oblinger BW, Woodruf WC. 2012. Hazard Tree Guidelines For Forest Service Facilities and Roads in the Pacific Southwest Region Available from: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/ stelprdb5332560.pdf.
- Barker P, Freeman D, Harper K. 1982. Variation in the breeding system of Acer grandidentatum. Forest Science 28: 563–572. [Google Scholar]
- Barker T, Bashmakov I, Bernstein L, et al. 2007. Technical summary. In: Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA, eds. Climate change 2007: mitigation. Contribution of working group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York: Cambridge University Press. [Google Scholar]
- Bierzychudek P. 1984. Determinants of gender in Jack-in-the-pulpit: the influence of plant size and reproductive history. Oecologia 65: 14–18. [DOI] [PubMed] [Google Scholar]
- Blake-Mahmud J, Struwe L. 2018. Down to the wire: late season changes in sex expression in a sexually labile tree species, Acer pensylvanicum (Sapindaceae). Trees - Structure and Function 32: 549–557. [Google Scholar]
- Bogart S. 2016. SankeyMatic http://sankeymatic.com.
- Bond BJ. 2010. Tree condition: health. Arborist News 34–38. [Google Scholar]
- Cadet C, Metz JAJ, Klinkhamer PGL. 2004. Size and the not‐so‐single sex: disentangling the effects of size and budget on sex allocation in hermaphrodites. The American Naturalist 164: 779–792. [DOI] [PubMed] [Google Scholar]
- Case AL, Ashman T-L. 2005. Sex specific physiology and its implications for the cost of reproduction. In: Reekie EG, Bazzaz FA, eds. Reproductive allocation in plants. Burlington: Elsevier Academic Press, 129–157. [Google Scholar]
- Charnov EL. 1982. The theory of sex allocation. Princeton: Princeton University Press. [PubMed] [Google Scholar]
- Charnov EL, Bull J. 1977. When is sex environmentally determined? Nature 266: 828–830. [DOI] [PubMed] [Google Scholar]
- Charnov EL, Bull JJ. 1989. The primary sex ratio under environmental sex determination. Journal of Theoretical Biology 139: 431–436. [DOI] [PubMed] [Google Scholar]
- Day T, Aarssen LW. 1997. A time commitment hypothesis for size-dependent gender allocation. Evolution 51: 988–993. [DOI] [PubMed] [Google Scholar]
- Dujessiefken D, Liese W. 2015. The CODIT principle and arboriculture: implications for best practices. Champaign: International Society of Arboriculture. [Google Scholar]
- Elith J, Leathwick JR, Hastie T. 2008. A working guide to boosted regression trees. Journal of Animal Ecology 77: 802–813. [DOI] [PubMed] [Google Scholar]
- Fisher RA. 1935. The logic of inductive inference. Journal of the Royal Statistical Society 1: 39–82. [Google Scholar]
- Franklin JF, Shugart HH, Harmon ME. 1987. Tree death as an ecological process. BioScience 37: 550–556. [Google Scholar]
- Free CM, Jensen OP, Wiedenmann J, Deroba JJ. 2017. The refined ORCS approach: a catch-based method for estimating stock status and catch limits for data-poor fish stocks. Fisheries Research 193: 60–70. [Google Scholar]
- Freeman DC, Harper KT, Charnov EL. 1980. Sex change in plants: old and new observations and new hypotheses. Oecologia 47: 222–232. [DOI] [PubMed] [Google Scholar]
- Freeman DC, McArthur ED, Harper KT. 1984. The adaptive significance of sexual lability in plants using Atriplex canescens as a principle example. Annals of the Missouri Botanical Garden 71: 265–277. [Google Scholar]
- Ghiselin MT. 1969. The evolution of hermaphroditism among animals. Quarterly Review of Biology 44: 189–208. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. 1957. The experimental modification of sex expression in flowering plants. Biological Reviews 32: 38–90. [Google Scholar]
- Hibbs DE, Fischer BC. 1979. Sexual and vegetative reproduction of striped maple (Acer pensylvanicum L.). Bulletin of the Torrey Botanical Club 106: 222–227. [Google Scholar]
- Hibbs DE, Wilson BF, Fischer BC. 1980. Habitat requirements and growth of striped maple (Acer pensylvanicum L.). Ecology 61: 490–496. [Google Scholar]
- IPCC. 2014. Climate change 2014: synthesis report. In: Core Writing Team, Pachauri RK, Meyer LA, eds. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva: IPCC, 151. [Google Scholar]
- Iwasa Y. 1991. Sex change evolution and cost of reproduction. Behavioral Ecology 2: 56–68. [Google Scholar]
- de Jong PC. 1976. Flowering and sex expression in Acer L. Mededelingen Landbouwhogeschool Wageningen. [Google Scholar]
- de Jong T, Klinkhamer P. 2005. Evolutionary ecology of plant reproductive strategies. New York: Cambridge University Press. [Google Scholar]
- Korpelainen H. 1998. Labile sex expression in plants. Biological Reviews of the Cambridge Philosophical Society 73: 157–180. [Google Scholar]
- Kuhn M. 2008. The caret package. Journal of Statistical Software 28: 1–26.27774042 [Google Scholar]
- Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33: 159–174. [PubMed] [Google Scholar]
- Lilly S. 1999. Keeping trees healthy. In: Skiera J, ed. Golf Course Tree Management. Chelsea: Ann Arbor Press, 103–123. [Google Scholar]
- Lindh B. 2017. Gender switching in the clonal understory herb Coptis laciniata (Ranunculaceae). International Journal of Plant Sciences 178: 94–103. [Google Scholar]
- Lloyd DG, Bawa KS. 1984. Modification of the gender of seed plants in varying conditions. Evolutionary Biology 17: 255–339. [Google Scholar]
- Lloyd DG, Webb CJ. 1977. Secondary sex characters in plants. The Botanical Review 43: 177–216. [Google Scholar]
- Maekawa T. 1924. On the phenomena of sex transition in Arisaema japonica Bl. Journal of the College of Agriculture, Hokkaido Imperial University, Sapporo, Japan 13: 217–305. [Google Scholar]
- Manel S, Williams HC, Ormerod SJ. 2001. Evaluating presence absence models in ecology; the need to count for prevalence. Journal of Appied Ecology 38: 921–931. [Google Scholar]
- Matsui K. 1995. Sex expression, sex change and fruiting habit in an Acer rufinerve population. Ecological Research 10: 65–74. [Google Scholar]
- Moran D, Whytlaw J, Herb J, Kaplan M. 2017. New Jersey climate and health profile report. New Brunswick: New Jersey Climate Adaptation Alliance, Rutgers University. [Google Scholar]
- Nanami S, Kawaguchi H, Yamakura T. 2004. Sex change towards female in dying Acer rufinerve trees. Annals of Botany 93: 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JR. 2002. The costs of reproduction in plants. New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- Powell DC. 2005. How To Measure a Big Tree. U.S. Forest Service: Umatilla National Forest; Available from: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/ stelprdb5202838.pdf [Google Scholar]
- Primack RB, McCall C. 1986. Gender variation in a red maple population (Acer rubrum: Aceraceae): a seven-year study of a “polygamodioecious” species. American Journal of Botany 73: 1239–1248. [Google Scholar]
- R Core Team 2015. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.R-project.org [Google Scholar]
- Renner SS. 2014. The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. American Journal of Botany 101: 1588–1596. [DOI] [PubMed] [Google Scholar]
- Renner SS, Beenken L, Grimm GW, Kocyan A, Ricklefs RE. 2007. The evolution of dioecy, heterdichogamy, and labile sex expression in Acer. Evolution 61: 2701–2719. [DOI] [PubMed] [Google Scholar]
- Ridgeway G. 2016. Gbm package: Generalized boosted classification models, R version 2.1.1. https://github.com/gbm-developers/gbm.
- Sakai A. 1990. Sex ratios of red maple (Acer rubrum) populations in northern lower Michigan. Ecology 71: 571–580. [Google Scholar]
- Sakai AK, Burris TA. 1985. Growth in male and female aspen clones : a twenty-five-year longitudinal study. Ecology 66: 1921–1927. [Google Scholar]
- Sakai AK, Oden NL. 1983. Spatial pattern of sex expression in silver maple (Acer saccharinum L.): Morisita’s index and spatial autocorrelation. The American Naturalist 122: 489–508. [Google Scholar]
- Sato T. 2002. Phenology of sex expression and gender variation in a heterodichogamous maple, Acer japonicum. Ecology 83: 1226–1238. [Google Scholar]
- Schaffner JH. 1922. Control of the sexual state in Arisaema triphyllum and Arisaema dracontium. American Journal of Botany 9: 72–78. [Google Scholar]
- Schlessman MA. 1986. Interpretation of evidence for gender choice in plants. The American Naturalist 128: 416–420. [Google Scholar]
- Schlessman MA. 1988. Gender diphasy (“sex choice”). In: Doust JL, Doust LL, eds. Plant reproductive ecology: patterns and strategies. New York: Oxford University Press, 139–153. [Google Scholar]
- Schlessman M. 1991. Size, gender, and sex change in dwarf ginseng, Panax trifolium (Araliaceae). Oecologia 87: 588–595. [DOI] [PubMed] [Google Scholar]
- Schwarze F,, Engels J,, Mattheck C. 2000. Fungal strategies of wood decay in trees. Berlin: Springer. [Google Scholar]
- Shigo AL. 1984. Compartmentalization: a conceptual framework for understanding how trees grow and defend themselves. Annual Review of Phytopathology 22: 189–214. [Google Scholar]
- Stalter AM, Krasny ME, Fahey TJ. 1997. Sprouting and layering of Acer pensylvanicum L. in hardwood forests of central New York. Torrey Botanical Society 124: 246–253. [Google Scholar]
- The Tree of Sex Consortium, Ashman T-L, Bachtrog D, et al. 2014. Data from: Tree of sex: a database of sexual systems. Dryad Digital Repository. 10.5061/dryad.v1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SC. 2011. Age-related changes in tree growth and functional biology: the role of reproduction. In: Meinzer FC, ed. Size- and age- related changes in tree structure. Berlin: Springer,33– 64. [Google Scholar]
- Warner RR. 1988. Sex-change and the size advantage model. TREE 3: 133–136. [DOI] [PubMed] [Google Scholar]
- Wickham H. 2009. ggplot2: Elegant Graphics for Data Analysis http://ggplot2.tidyverse.org; https://github.com/tidyverse/ggplot2.
- Willson MF. 1986. On the costs of reproduction in plants: Acer negundo. The American Midland Naturalist 115: 204–207. [Google Scholar]
- Zhang ZQ, Zhu XF, Sun H, Yang YP, Barrett SCH. 2014. Size-dependent gender modification in Lilium apertum (Liliaceae): does this species exhibit gender diphasy? Annals of Botany 114: 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JK. 1991. Ecological correlates of labile sex expression in the orchid Catasetum viridiflavum. Ecology 72: 597–608. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.