Abstract
Background
Characterizing the mutations selected by the integrase strand transfer inhibitor (INSTI) dolutegravir and their effects on susceptibility is essential for identifying viruses less likely to respond to dolutegravir therapy and for monitoring persons with virological failure (VF) on dolutegravir therapy.
Methods
We systematically reviewed dolutegravir resistance studies to identify mutations emerging under dolutegravir selection pressure, the effect of INSTI resistance mutations on in vitro dolutegravir susceptibility, and the virological efficacy of dolutegravir in antiretroviral-experienced persons.
Results and conclusions
We analysed 14 studies describing 84 in vitro passage experiments, 26 studies describing 63 persons developing VF plus INSTI resistance mutations on a dolutegravir-containing regimen, 41 studies describing dolutegravir susceptibility results, and 22 clinical trials and 16 cohort studies of dolutegravir-containing regimens. The most common INSTI resistance mutations in persons with VF on a dolutegravir-containing regimen were R263K, G118R, N155H and Q148H/R, with R263K and G118R predominating in previously INSTI-naive persons. R263K reduced dolutegravir susceptibility ∼2-fold. G118R generally reduced dolutegravir susceptibility >5-fold. The highest levels of reduced susceptibility occurred in viruses containing Q148 mutations in combination with G140 and/or E138 mutations. Dolutegravir two-drug regimens were highly effective for first-line therapy and for virologically suppressed persons provided dolutegravir’s companion drug was fully active. Dolutegravir three-drug regimens were highly effective for salvage therapy in INSTI-naive persons provided one or more of dolutegravir’s companion drugs was fully active. However, dolutegravir monotherapy in virologically suppressed persons and functional dolutegravir monotherapy in persons with active viral replication were associated with a non-trivial risk of VF plus INSTI resistance mutations.
Introduction
The integrase strand transfer inhibitor (INSTI) dolutegravir has an improved safety profile, greater efficacy and lower cost compared with efavirenz.1–3 Dolutegravir will play a dominant role in first-line therapy in many countries, including those with and without high levels of pre-treatment drug resistance. Dolutegravir has also recently been recommended by the WHO as a preferred component for second-line therapy.4
Characterizing the mutations selected by dolutegravir and their effects on dolutegravir susceptibility are essential for identifying viruses less likely to respond to dolutegravir therapy and for monitoring persons with virological failure (VF) on a dolutegravir-containing regimen. The efficacy of dolutegravir in ART-experienced persons and in monotherapy and dual-therapy regimens informs the risk of VF and emergent dolutegravir resistance and the optimal antiretrovirals (ARVs) to be used in combination with dolutegravir.
Here, we systematically review published studies and meeting presentations on dolutegravir resistance. The review maps key dolutegravir resistance concepts and analyses: the mutations emerging in vitro and in vivo under dolutegravir selection pressure; the effect of INSTI resistance mutations on in vitro dolutegravir susceptibility; and the virological efficacy of dolutegravir in persons at increased risk of VF and drug resistance.
Methods
Literature review
A systematic search of the NCBI PubMed database for all English language papers on dolutegravir resistance using the search string ‘Dolutegravir or GSK1349572’ was last updated on 24 January 2019. A list of the titles and abstracts presented at scientific meetings during 2017 and 2018 that contained the drug name ‘Dolutegravir’ was also compiled. The scientific meetings included the Conference on Retroviruses and Opportunistic Infections (CROI), IAS Conference on HIV Science, International AIDS Conference, International Workshop on HIV Drug Resistance and Treatment Strategies (HIVDRW), IDWeek, InterScience Conference on Antimicrobial Agents and Chemotherapy, European AIDS Conference, European Meeting on HIV & Hepatitis, and Glasgow HIV Drug Therapy. Additional publications and meeting presentations were identified from the reference lists of identified papers.
Retrieved studies were reviewed in three stages. First, titles and/or abstracts were reviewed to identify studies relevant to dolutegravir resistance. Following the title/abstract review, complete publications or posters (in the case of meeting presentations) were reviewed to determine which studies contained data relevant to the three main areas of focus: (i) mutations emerging under dolutegravir selection pressure in vitro and in vivo; (ii) in vitro dolutegravir susceptibility data; and (iii) virological efficacy of dolutegravir. In (iii), we examined the virological efficacy of dolutegravir when used with a reduced number of companion ARVs or when used to treat persons with viruses containing either INSTI resistance mutations or mutations that reduce the activity of the ARVs used in combination with dolutegravir. In the third stage, studies were reviewed further to determine whether they merited inclusion in a table, a figure or the text. The complete list of studies meeting the first review stage are available in a publicly available companion Zotero reference database (https://www.zotero.org/groups/2262131/dtg_stanfordhivdb).
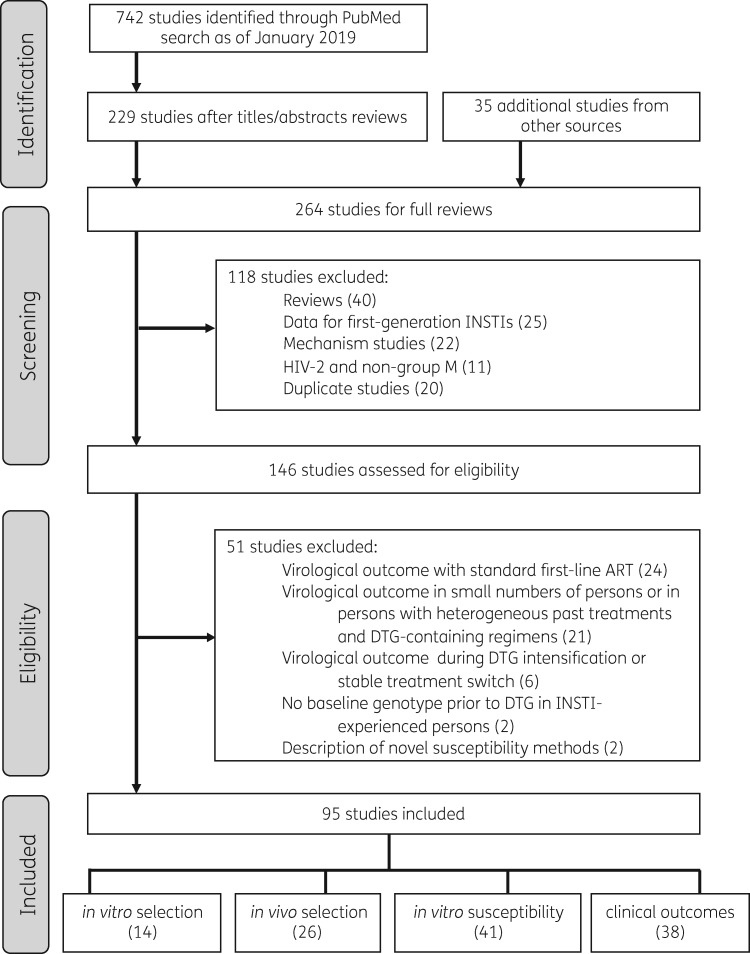
The following types of studies passing the first review stage were excluded: (i) review papers lacking primary data; (ii) studies containing drug resistance data for the INSTIs raltegravir or elvitegravir but not dolutegravir; (iii) in vitro experiments designed to understand the cellular, biochemical or biophysical (rather than genetic) mechanisms of dolutegravir resistance; (iv) studies of HIV-2 or non-group M viruses; and (v) studies containing redundant analyses of a clinical trial or cohort (Figure 1).
Figure 1.
Flow chart of study selection process. Of 742 studies identified through a PubMed search performed in January 2019 using the search string ‘Dolutegravir or GSK1349572’, 229 were read in their entirety following an initial review of titles and abstracts. Following a full-text review of these 229 studies and of 35 additional studies from meeting presentations, 95 studies met our inclusion criteria, containing data on mutations emerging under dolutegravir selection pressure in vitro and in vivo; in vitro dolutegravir susceptibility; and the risk of virological failure and drug resistance in clinical trials and cohorts.
Studies passing the first two review stages were subjected to additional exclusion criteria: (i) clinical trials and cohort studies of persons receiving a standard three-drug first-line dolutegravir-containing regimen were excluded from summary tables as it has already been established from previous reviews that such persons are at extremely low risk of VF and emergent resistance;1,5 (ii) clinical trials and cohort studies of dolutegravir intensification or switches to dolutegravir-containing three-drug regimens in virologically suppressed persons as persons in these studies would be expected to be at extremely low risk of VF; (iii) cohort studies containing <20 persons that did not yield findings that were not also observed in larger studies or containing persons with highly heterogeneous past treatment histories or dolutegravir-containing regimens; (iv) cohort studies and case reports of INSTI-experienced persons developing INSTI resistance mutations, if there was no baseline integrase genotype prior to dolutegravir therapy; and (v) studies describing novel in vitro susceptibility testing assays.
Data extraction
The data in the sections that follow were extracted independently by three reviewers (R. W. S., P. M. G. and S. Y. R.) using standardized spreadsheets for in vitro selection data, in vivo selection data, drug susceptibility data and virological outcome data. The extracted data in these spreadsheets were then used to annotate the Zotero reference database. Discrepancies were handled by jointly reviewing the full text of studies with discrepancies.
Mutations emerging under dolutegravir selection pressure
For studies in which viruses were cultured in the presence of increasing dolutegravir concentrations (in vitro passage experiments), we recorded data about the pre-passage virus, including its subtype, whether it was a laboratory strain or a clinical isolate, and whether it contained pre-existing known or suspected INSTI resistance mutations; and the integrase mutations that developed during in vitro passage.
For studies of persons whose viruses developed established or suspected INSTI resistance mutations while receiving dolutegravir we recorded: (i) the extent of ART prior to dolutegravir therapy, including whether the person was ART naive, ART experienced but INSTI naive, or INSTI experienced but dolutegravir naive; (ii) whether the person was stably virologically suppressed, defined as having a plasma HIV-1 RNA virus level <50 copies/mL for ≥6 months on unchanged ART; (iii) the ARVs used in combination with dolutegravir, specifically, whether the person received dolutegravir monotherapy or dual therapy, dolutegravir plus two NRTIs, or dolutegravir plus an optimized background regimen; and (iv) the integrase mutations reported to develop during therapy.
INSTI resistance mutations were defined as mutations previously reported to be selected by raltegravir or elvitegravir and associated with reduced raltegravir or elvitegravir susceptibility. Non-polymorphic INSTI resistance mutations were defined as those occurring in <1% of INSTI-naive persons and included H51Y, T66A/I/K, E92Q, G118R, F121Y, G140A/S/C, Y143C/G/H/K/R/S, S147G, Q148H/K/R, S153Y/F, N155H, S230R and R263K.6,7 Polymorphic accessory INSTI-associated mutations were defined as those occurring at a prevalence ≥1% of INSTI-naive persons in one or more subtypes and included L74I/M, Q95K, T97A, V151I, E157Q, G163K/R and D232N.6,8
In vitro susceptibility data
For studies containing in vitro susceptibility data, we recorded: (i) whether the virus was a clinical or laboratory isolate; (ii) the integrase mutations in the virus and, for laboratory isolates, whether the virus contained one or more mutations placed by site-directed mutagenesis; (iii) the virus subtype; (iv) the method of susceptibility testing; and (v) the fold reduction in susceptibility compared with WT. Duplicate results, defined as identical results on the same site-directed mutant performed by the same laboratory method, were excluded.
Virological outcome studies
In both clinical trials and cohort studies, study subjects were characterized according to: (i) their past ART history as either ART naive, ART experienced but INSTI naive, or INSTI experienced; (ii) whether they were stably virologically suppressed, defined as having a plasma HIV-1 RNA level <50 copies/mL on two or more occasions for a period of ≥6 months on unchanged ART; and (iii) the components of their dolutegravir-containing regimen: dolutegravir monotherapy or dual therapy, dolutegravir plus two NRTIs, or dolutegravir plus an optimized background defined as ARVs selected by a clinical trial subject’s care provider to be used in combination with dolutegravir. For clinical trial results reporting data at multiple timepoints, we extracted data from the 24 and 48 week timepoints.
Analysis
Mutations emerging under dolutegravir selection pressure
In vitro passage experiments included those performed in cell culture and those performed in humanized mice. The analysis of these experiments focused on established non-polymorphic INSTI resistance mutations as no novel non-polymorphic mutations were observed in multiple studies.
The analysis of emergent INSTI resistance mutations in persons receiving dolutegravir compiled all reported established non-polymorphic and polymorphic INSTI resistance mutations and additional novel non-polymorphic integrase mutations. The analysis included reports of emergent INSTI resistance mutations from clinical trials, cohort studies and case reports to identify the full spectrum of dolutegravir-selected mutations in vivo.
In vitro susceptibility data
For site-directed mutants, the complete list of mutations was known. However, for most clinical isolates, neither the complete nucleotide sequence nor the complete list of integrase mutations was reported. Therefore, for clinical isolates, only those mutations provided by authors were reported. Of note, the INSTI-selected mutation V151I occurs in the NL43 laboratory isolate, the most common laboratory isolate used for creating site-directed mutants. Therefore, this mutation was excluded from our analyses. The Supplementary data (available at JAC Online) indicates which susceptibility tests were performed on NL43 site-directed mutants. The in vitro susceptibilities of site-directed mutants and clinical isolates containing the four most commonly selected INSTI resistance mutations among persons receiving dolutegravir—R263K, G118R, N155H and Q148H/R/K—were characterized.
Virological outcome studies
VF was defined across all studies using an ITT approach such that subjects discontinuing therapy for any reason, such as intolerance or non-adherence, were categorized as experiencing VF. This approach was adopted both for studies that reported their results in this manner and for those that employed a narrower definition of protocol-defined VF. The 95% Clopper–Pearson CIs for proportions of VF and VF plus emergent INSTI resistance were estimated for each individual study. Pooled proportion and the I2 statistic, a measure of heterogeneity among studies, were calculated using the random-effects model implemented in the R meta package for the proportions of VF and VF plus resistance at weeks 24 and 48.
Results and discussion
Dolutegravir-selected mutations
In vitro passage experiments
Fourteen studies described 84 in vitro passage experiments in which an HIV-1 group M virus was cultured with increasing dolutegravir concentrations (Table 1). In 62 experiments, mutations were selected after a median of 30 weeks (IQR 20–46 weeks). These 62 experiments used 37 clinically derived isolates and 25 laboratory isolates. Isolates lacking established INSTI-associated drug resistance mutations prior to dolutegravir passage were labelled as WT. Fifty-three of the viruses had a subtype B backbone; 4 had a subtype C backbone, 2 had a CRF02_AG backbone, 2 had a subtype D backbone and 1 had a CRF01_AE backbone.
Table 1.
HIV-1 group M viruses developing integrase mutations during in vitro passage experimentsa
| Position (Cons) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 51 | 66 | 92 | 118 | 138 | 140 | 147 | 148 | 153 | 263 | ||
| AuthorYr | Parent virusb | (H) | (T) | (E) | (G) | (E) | (G) | (S) | (Q) | (S) | (R) |
| Kobayashi11 | NL43 | F/Y | |||||||||
| Quashie12 | clinical (n = 3) | K | |||||||||
| clinical | K | K | |||||||||
| clinical | Y | K | |||||||||
| clinical (C) | Y | R | |||||||||
| clinical (C) | Y | T | |||||||||
| clinical (02) | R | ||||||||||
| clinical (02) | Y | K | |||||||||
| Oliveira14 | NL43 | K | |||||||||
| NL43-118R | I | K | |||||||||
| NL43-51Y/263K | K | ||||||||||
| Anstett15 | NL43 | K | |||||||||
| NL43-92Q | K | ||||||||||
| NL43-140S | R | ||||||||||
| NL43-140S/148R | Y | ||||||||||
| NL43-155H | K | ||||||||||
| Departureaux15 | NL43 | Y | |||||||||
| Seki15 | NL43 | Q | |||||||||
| NL43-148H | K | S | |||||||||
| NL43-148K | K | ||||||||||
| NL43-148R | K | S | |||||||||
| Brenner16 | NL43-118R | I | K | ||||||||
| clinical-118R (C) | A | K | |||||||||
| Oliveira16 | NL43 | F | K | ||||||||
| Brenner17 | clinical | Y | |||||||||
| clinical (n = 6) | K | ||||||||||
| clinical | F | K | |||||||||
| clinical (n = 2) | Y | ||||||||||
| Andreatta18 | LAI (n = 2) | Y | |||||||||
| LAI-155H | N | ||||||||||
| LAI-148R | K | ||||||||||
| Oliveira18 | NL43 | K | |||||||||
| NL43-157Q | K | ||||||||||
| clinical | Y | ||||||||||
| clinical (n = 6) | K | ||||||||||
| clinical | Y | G | |||||||||
| clinical | F | ||||||||||
| clinical (C) | K | ||||||||||
| clinical (01) | K | ||||||||||
| clinical (02) | K | ||||||||||
| clinical-157Q (n = 3) | K | ||||||||||
| clinical-157Q (D) | K | ||||||||||
| clinical-157Q (D) | F | ||||||||||
Cons, consensus subtype B amino acid.
Columns contain established non-polymorphic INSTI resistance mutations. Mutations not shown include: M50I in two non-mutated viruses (Quashie12 and Oliveria14); E157Q in two viruses (Oliveira18); 101I/124A (Kobayashi11); 262K with NL43-51Y (Oliveira14); 75I/97A/154I in addition to 138K/140S in NL43-148H (Seki15); 193E with 51Y/153T in Clinical (C) (Quashie12) and with 92Q in NL43 (Seki15); 234F with 153Y in LAI (Andreatta18); 144D in LAI with the RT mutation 184V; 95K/146R in Clinical (Oliveira18).
Clinical: virus isolates obtained from INSTI-naive individuals lacking known INSTI resistance mutations. NL43 and LAI are WT laboratory strains. Drug-resistance mutations in laboratory strains were placed by site-directed mutagenesis. Non-B subtypes are indicated in parentheses: C, D, CRF01_AE (01), and CRF02_AG (02). Replicate experiments yielding the same results are followed by the number of experiments (n) in parentheses. Notes: 22 subtype B are not shown including one developing polypurine tract mutations and 21 that did not develop mutations, including those with parent viruses 92Q (2), 92Q/155H (1), 51Y/118R (1), 143C (1), 143R (1), 148R (1), 155H (1), 263K (2), RT-65R (1), RT-184V/I (2) and 8 without mutations.
R263K was the most commonly selected INSTI resistance mutation, developing in 33 experiments from six studies (Table 1). In 31 of these experiments, the baseline virus was WT (including five with the pre-existing polymorphic accessory resistance mutation, E157Q); the other two baseline viruses contained E92Q or N155H. Other commonly selected INSTI resistance mutations included S153Y/F (11 isolates, six studies), E138K (9 isolates, four studies), H51Y (7 isolates, four studies), T66A/I (3 isolates, two studies) and G118R (2 isolates, one study). In 21 experiments using 11 WT and 10 viruses containing INSTI resistance mutations, no mutations emerged, consistent with the difficulty in selecting dolutegravir resistance mutations in vitro. There were insufficient data to determine whether a particular subtype predisposed to the emergence of specific mutations.
There were two studies not shown in Table 1 of humanized mice infected with a laboratory HIV-1 strain and subsequently treated with dolutegravir monotherapy. In one study, one of five mice treated with dolutegravir monotherapy for 20 weeks developed the INSTI resistance mutations E138K, G140S, Q148H, H155H and S230R.9 In another study, two of four mice treated with an injectable long-acting dolutegravir formulation developed R263K and E157Q.10
In three additional studies, also not shown in Table 1, mutations outside of integrase were selected during dolutegravir in vitro passage and shown to reduce dolutegravir susceptibility, including (i) three nucleotide mutations and one nucleotide deletion (resulting in a downstream stop codon in nef) in the highly conserved six terminal nucleotides of the 3′ polypurine tract (GGGGGG→GCATG) and one nucleotide mutation just upstream of the 3′ polypurine tract;11 (ii) a nucleocapsid mutation, G19S, postulated to destabilize the integrase/viral DNA/dolutegravir complex;12 and (iii) gp41 envelope mutations A539V and A556T postulated to reduce susceptibility to multiple ARVs by enhancing cell-to-cell HIV-1 transmission and increasing intracellular HIV-1 inoculum size.13 In a fourth study, the second-generation INSTI cabotegravir was shown to select for HIV-1 LTR mutations, which were hypothesized to have a similar effect to 3′ polypurine tract mutations.14 Only the first of these sets of mutations has so far been reported in persons receiving dolutegravir.
Persons receiving dolutegravir
The studies in which persons receiving dolutegravir developed VF and one or more INSTI resistance mutations included: (i) 11 studies of INSTI-naive persons (Table 2); (ii) 8 studies of virologically suppressed persons receiving dolutegravir monotherapy for treatment simplification (Table 3); and (iii) 8 studies of persons who had a history of VF and INSTI resistance mutations on a raltegravir- or, less commonly, elvitegravir-containing regimen (Table 4). Emergent INSTI resistance mutations have not been reported in any of the trials of a first-line regimen comprising dolutegravir plus two NRTIs.1,5
Table 2.
Emergent INSTI-associated drug resistance mutations (DRMs) in INSTI-naive persons with active virus replication receiving a standard dolutegravir (DTG)-containing regimen
| AuthorYr | Population | Past ART | DTG ART (q24h) | Subjects | Subjects with DRMs | Emergent INSTI DRMsa |
|---|---|---|---|---|---|---|
| Underwood15 | RCT (SAILING) | ART experienced; INSTI naive; resistance to ≥2 classes but with 1 or 2 fully active drugs for OB | DTG/OB × 48W (Phase I); post 48W (Phase II) | 354 | 5 | (1) R263K |
| (2) R263K | ||||||
| (3) T97A, N155H (C) | ||||||
| (4) N155H (A) | ||||||
| (5) A49G, S230R, R263K | ||||||
| Vavro18 | trial (P1093) | ART experienced; INSTI naive | DTG/OB | 61 | 3 | (1) A49G, M50V, E138T, S147G, R263K |
| (2) L74M, G118R | ||||||
| (3) G118R | ||||||
| Taiwo18 | trial (A5353) | ART naive | DTG/3TC | 120 | 1 | (1) R263K |
| Wang18 | RCT (DAWNING) | ART experienced; INSTI naive; h/o VF on first-line ART | DTG + 2 NRTIs (48W) | 297 | 2 | (1) H51Y, G118R, E138K, R263K |
| (2) G118R | ||||||
| Lepik17 | cohort | INSTI naive | DTG + 2 NRTIs | 310 | 3 | (1) R263K |
| (2) R263K | ||||||
| (3) T66I | ||||||
| Ahmed19 | case report | ART experienced; INSTI naive | DTG/OB | 1 | 1 | (1) R263K (D) |
| Fulcher18 | case report | ART naive | DTG/TDF/FTC | 1 | 1 | (1) Q148K, G163E |
| Pena Lopez18b | case report | ART naive | DTG/TDF/FTC | 1 | 1 | (1) E157Q, R263K (CRF14_BG) |
| Seatla18 | case report | ART experienced; INSTI naive | DTG/OB | 1 | 1 | (1) G118R, E138K |
| Cardoso18 | case report | ART experienced; INSTI naive | DTG + 2 NRTIs | 2 | 2 | (1) E157Q, R263K |
| (2) R263K (G) | ||||||
| Lubke18 | case report | ART naive | DTG/TDF/FTC | 1 | 1 | (1) G118R, R263K (F) |
RCT, randomized clinical trial; OB, optimized background; W, weeks; h/o, history of.
Subtypes are indicated in parentheses.
DTG was administered twice daily in a person who was also receiving rifampicin in Pena Lopez18. In Underwood15, the two cases of R263K were reported in the first 48 weeks of the SAILING trial.15 The three additional cases of INSTI DRMs in this trial occurred between weeks 72 and 120. In Wang18,17 the first isolate contained 3 clones with H51Y/G118R and 11 clones with G118R/E138K/R263K. In Lepik17, R263K was detected in two ART-experienced persons and T66I in a previously ART-naive person. Complete sequences were unavailable for all isolates in this table.
Table 3.
Emergent INSTI-associated DRMs in persons with sustained virological suppression receiving dolutegravir (DTG) monotherapy
| AuthorYr | Population | Past ART | DTG ART (q24h) | Subjects | Subjects with DRMs | Emergent INSTI DRMsa |
|---|---|---|---|---|---|---|
| Wijting18 | RCT | 15% INSTI experienced; no h/o VF | DTG × 48W (98 in immediate and delayed switch groups and 4 in a pilot study) | 102 | 5 | (1) R263K |
| (DOMONO) | (2) N155H | |||||
| (3) S230R | ||||||
| (4) E92Q, N155H | ||||||
| (5) 3′ polypurine tract mutations | ||||||
| Blanco18 | RCT | 15% INSTI experienced; no h/o VF on an INSTI regimen | DTG × 24W | 31 | 2 | (1) S147G, Q148R, N155H |
| (DOLAM) | (2) E138K, G140S, N155H | |||||
| Hocqueloux18 | RCT | 17% INSTI experienced | DTG × 24W | 78 | 2 | (1) N155H, S147G |
| (MONCAY) | (2) R263K | |||||
| Katlama16 | cohort | 46% INSTI experienced | DTG × 24W | 28 | 3 | (1) E138K, G140A, Q148R |
| (MONODOLU) | (2) E92Q | |||||
| (3) N155H | ||||||
| Rojas16 | cohort | DTG × 24W | 33 | 1 | (1) G118R | |
| Oldenbuettel17 | cohort | 61% INSTI experienced; no h/o VF on an INSTI regimen | DTG × 24W | 31 | 1 | (1) Q148H, G140S |
| (DOLUMONO) | ||||||
| Brenner16 | case report | h/o first-line ART with EVG-containing regimen | DTG × 8W | 1 | 1 | (1) G118R |
| Malet18 | case report | RAL experienced | DTG | 1 | 1 | (1) N155H |
RCT, randomized clinical trial; VF, virological failure; EVG, elvitegravir; RAL, raltegravir; W, weeks; h/o, history of.
Underlined mutations indicate that the virus emerged in an INSTI-experienced person. In Wijting18, R263K, N155H and S230R were detected in persons in the main DOMONO study, which required a nadir CD4 count >200 cells/mm3, whereas E92Q/N155H and 3′ polypurine tract mutations occurred in four persons in the pilot DOMONO study, which included persons with a nadir CD4 count <200 cells/mm3. The virus with R263K reported by Hocqueloux18 also had a single G to A mutation in the 3′ polypurine tract, whereas two of the six nucleotides in the 3′ polypurine tract were mutated. The G118R mutation in Rojas16 was detected as a minority variant in 7% of viruses by next-generation sequencing. Complete sequences were unavailable for all sequences except Brenner16.
Table 4.
Emergent INSTI-associated DRMs in INSTI-experienced persons receiving dolutegravir (DTG) in combination with an optimized background (OB) regimen
| AuthorYr | Population | DTG ART | Time to VF | Pre-DTG DRMs | Emergent INSTI DRMs |
|---|---|---|---|---|---|
| Eron13 | RCT (VIKING) | DTG 50 mg q24h + OB (Cohort I) | Day 11 | G140S, Y143H, Q148H | L74I/M, E138A |
| Day 11 | L74M, T97A, Y143R, G163R | E138K | |||
| W8 | none | L74I/M, T97A, G140S, Q148H | |||
| W24 | L74M, T97A, E138A, Y143R | N155H | |||
| W24 | L74M, T97A, Y143R | N155H | |||
| DTG 50 mg q12h + OB (Cohort II) | Day 11 | E138A, G140S, Q148H | T97A, E138T | ||
| Day 11 | G140S, Q148H | N155H | |||
| Day 11 | E138K, G140S, Q148H | T97A | |||
| W8 | E138A, G140S, Q148H | E92Q, T97A | |||
| W8 | G140S, Q148H | E138K, N155H | |||
| W16 | G140S, Q148H | T97A, E138K, N155H | |||
| Naeger16 | RCT (VIKING-4) | DTG 50 mg q12h + OB | W12 | E138D, G140S, Q148H | L74M, G149A, N155H |
| W24 | G140S, Q148H | T97A, E138K, G149A | |||
| W24 | E138A/K/T, G140S, Q148H | T97A | |||
| W24 | L74M, Q95K, T97A, Y143C | E138K, S147G | |||
| W4 | G140S, Q148H | T97A | |||
| W32 | G140S, Q148H | T97A | |||
| Castagna18 | cohort | DTG 50 mg q12h + OB | W84 | G140S, Q148H | E138K |
| W132 | T97A, G140S | E138K, Q148H | |||
| W132 | E138A, G140S, Q148H | T97A | |||
| W132 | L74I/M, G140S, Q148H | T66I, T97A | |||
| W172 | G140S, Q148H | L74I, T97A | |||
| W200 | E138A, G140S, Y143H/R/C, Q148H | T97A | |||
| W228 | G140S, Q148H | T97A, E138K | |||
| W276 | E138K, G140S, Q148H | T97A | |||
| W320 | Y143C | T97A, E138K, G140S, Q148H | |||
| Carganico14 | case report | DTG 50 mg q12h + OB | W32 | N155H, E157Q | T97A, S147G |
| Hardy15 | case report | DTG 50 mg q12h + OB | W108 | S147G, V151I, N155H | T97A, E138K |
| Malet15 | case report | DTG 50 mg q12h + OB | W156 | G140S, Q148H | T97A, N155H |
| George18 | case report | DTG 50 mg q12h + OB | W24 | G140S, Q148H | T97A |
| DTG 50 mg q12h + OB | W44 | E138T, G140S, Q148H | T97A | ||
| Seatla18 | case report | DTG 50 mg q12h + OB | W64 | E138K, G140A, Q148R | T97A, S147G |
Several additional baseline and follow-up mutations were noted for Naeger16. However, as sequences were not available for any of the studies, the lists of mutations do not show any mutations that are not known INSTI-associated DRMs or mutations at highly conserved positions. 31% of the persons in Castagna18 had previously been enrolled in one of the VIKING trials. In VIKING Cohorts I and II (Eron13) and in VIKING-4 (Naeger16), subjects had a period of functional dolutegravir monotherapy lead-in of 7 (VIKING-4) to 10 (VIKING Cohorts I and II) days before the ARVs accompanying dolutegravir were optimized. W, weeks.
The 11 studies of INSTI-naive persons included 4 clinical trials, 1 cohort study and 6 case reports (Table 2). Three of the four clinical trials included 712 ART-experienced patients receiving dolutegravir plus an optimized backbone,15–19 which was required in the two largest trials to include at least one fully active ARV based on a pre-therapy genotypic resistance test.15,17 One of the clinical trials included 120 ART-naive persons receiving dolutegravir plus lamivudine.20 The cohort study included 310 ART-naive and ART-experienced INSTI-naive persons receiving dolutegravir plus two NRTIs.21 The six case reports included three ART-naive persons who received dolutegravir plus tenofovir plus emtricitabine and three ART-experienced persons who received dolutegravir plus an optimized background regimen or two NRTIs.22–27 Overall, 21 persons developed VF and an INSTI resistance mutation. The most common mutations were R263K in 13 persons and G118R in 6 persons. Other non-polymorphic resistance mutations were E138K/T (3 persons), N155H (2 persons), Q148K (1 person), S230R (1 person), T66I (1 person) and H51Y (1 person). The non-polymorphic mutation A49G developed in two persons.
The eight studies of persons receiving dolutegravir monotherapy for treatment simplification included three trials with 211 persons, three cohort studies with 92 persons, and two case reports28–35 (Table 3). Overall, 16 persons developed VF and an INSTI resistance mutation. The most common mutations were N155H (7 persons), Q148H/R (3 persons), R263K (2 persons), G118R (2 persons) and S147G (2 persons). Among the 16 persons with VF and emergent INSTI resistance mutations, 7 had previously received raltegravir or elvitegravir but had not previously experienced VF on a raltegravir- or elvitegravir-containing regimen.
In two studies, in which the 3′ polypurine tract was sequenced, 2 of 17 persons had one or more nucleotide changes in this highly conserved region. One had virus containing two 3′ polypurine tract nucleotide changes (GGGGGG→GGGAGC) compared with baseline and no reported integrase mutations,36 and another containing one polypurine tract nucleotide change also had the integrase mutation R263K.37
The eight studies of persons with a history of VF and INSTI resistance mutations on a previous raltegravir- or elvitegravir-containing regimen included two of the VIKING trials (the Phase IIb VIKING and Phase III VIKING-4 trials), one cohort study and five case reports38–45 (Table 4). In these eight studies, 31 persons were reported to have developed VF on a dolutegravir-containing regimen. The spectrum of mutations selected by dolutegravir in this population differed from the spectrum in INSTI-naive persons. No person developed R263K or G118R. Rather, the most common dolutegravir-selected mutations were T97A (22 persons), E138K/A/T (12 persons), N155H (7 persons), L74M/I (4 persons), Q148H±G140S (3 persons) and S147G (3 persons). The non-polymorphic mutations G149A and F139Y developed in two persons and one person, respectively.
R263K was the common mutation developing during in vitro passage and developing in INSTI-naive persons. G118R, E138K and H51Y also occurred both in vitro and in vivo, with E138K and H51Y occurring only in combination with other mutations. S153Y/F occurred commonly in vitro but has not yet been reported in patients receiving dolutegravir. N155H and Q148 mutations were not selected in vitro but developed in several INSTI-naive persons, particularly during dolutegravir monotherapy. Further studies are required to determine the frequency of dolutegravir-selected mutations outside of integrase in persons with VF on a dolutegravir-containing regimen.
G118R and R263K, which were previously reported in persons receiving raltegravir46 and elvitegravir,47 respectively, occurred in a much higher proportion of persons with VF on dolutegravir compared with the first-generation INSTIs.5,6 Indeed, no completely novel non-polymorphic INSTI-selected mutations were identified in persons receiving dolutegravir except for A49G, G149A and 3′ polypurine tract mutations. However, complete integrase sequences were submitted to GenBank for just 2 of the 63 viruses from dolutegravir-treated persons with VF and INSTI resistance mutations, making it possible that many dolutegravir-selected mutations were not reported.
Effect of INSTI resistance mutations on in vitro dolutegravir susceptibility
A total of 41 studies contained 572 dolutegravir in vitro susceptibility results (Table S1), including 395 results on site-directed mutants and 177 results on clinical isolates (Table S2). The site-directed mutants generally contained raltegravir- and elvitegravir-associated resistance mutations, mutations observed under dolutegravir selection pressure, and additional accessory INSTI-associated mutations. The complete integrase sequence was known for all of the site-directed mutants but was available for only 74 (41.8%) of the 177 clinical virus isolates.
The most commonly used assay was the recombinant virus reporter gene PhenoSense assay (Monogram Biosciences, South San Francisco; n = 279). Most of the remaining assays were recombinant virus reporter gene assays developed by other laboratories, including ViiV, McGill University, the ANRS and the NCI. The site-directed mutants contained 127 distinct mutational patterns, and for 45 patterns susceptibility tests were performed with more than one assay. For these 45 patterns, the range/median ratio, a non-parametric measure of dispersion, was ≤1.0 for 21 patterns, 1.1–2.0 for 20 patterns and >2.0 for 4 patterns. The dispersion appeared to be lowest between the PhenoSense and ViiV assays (Table S3).
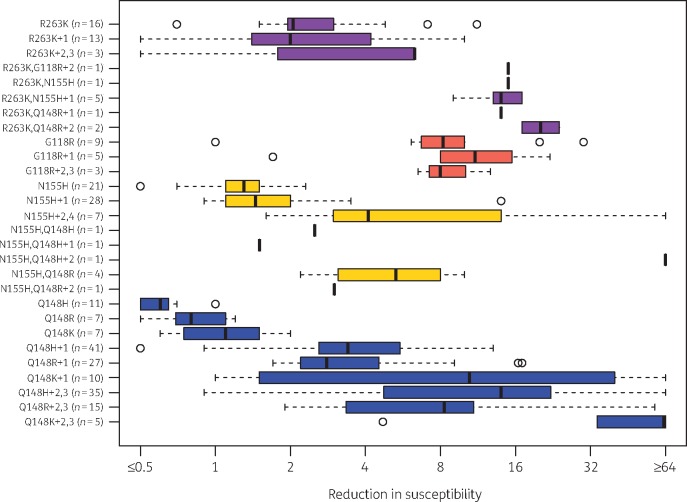
Figure 2 summarizes results on 281 site-directed mutants and clinical isolates containing patterns of INSTI resistance mutations characterized by four signature mutations: R263K, G118R, N155H and Q148H/R/K. The first eight plots in Figure 2 show the fold reductions in susceptibility for 42 isolates containing R263K. The median reduction in dolutegravir susceptibility was ∼2-fold for isolates with R263K alone, ∼5-fold for isolates with R263K plus two additional mutations, and 10- to 15-fold for isolates with R263K plus G118R, N155H or Q148R (usually in combination with one additional INSTI resistance mutation).
Figure 2.
Box plots of in vitro dolutegravir susceptibility results for 176 site-directed mutants and 105 clinical isolates containing R263K, G118R, N155H and/or Q148H/R/K. Purple plots indicate the fold reduction in susceptibility for viruses containing R263K with or without G118R, N155H and Q148H/R. Orange plots indicate the fold reduction in susceptibility for viruses containing G118R. Yellow plots indicate the fold reduction in susceptibility for viruses containing N155H with or without Q148H/R. Blue plots indicate the fold reduction in susceptibility for viruses containing Q148H/R/K. In addition to the four signature mutations, mutation patterns were characterized by the number of additional INSTI resistance mutations. The number of isolates with each pattern is shown in parentheses.
The next three plots in Figure 2 summarize the levels of reduced susceptibility for 17 isolates containing G118R without R263K. G118R alone or in combination with T66A, L74I and T97A reduced dolutegravir susceptibility between 5- and 15-fold. Two of the site-directed mutants contained a CRF02_AG integrase backbone. One G118R-containing clinical isolate lacking other reported INSTI resistance mutations had 30-fold reduced susceptibility; however, the complete list of mutations in this isolate was not available.48 Although both R263K and G118R were associated with reduced enzymatic activity and replication capacity, 49–51 the relative rarity of G118R may be due to its usual requirement for mutations at two nucleotides rather than one nucleotide, regardless of subtype.34
The next eight plots summarize the levels of reduced susceptibility for 64 isolates containing N155H (without R263K or G118R). N155H alone or in combination with one additional INSTI resistance mutation usually yielded <2-fold reduced dolutegravir susceptibility. However, in combination with Q148H/R or two or more additional resistance mutations, reduced susceptibility ranged from 2-fold to >15-fold. The highest levels of reduced susceptibility were found in a site-directed mutant containing the extremely rare mutation pair L74F+V75I52 and in clinical isolates containing N155H plus Q148H, G140S and T97A43 and N155H plus T97A, E138K and S147G.42
The final nine plots in Figure 2 summarize the levels of reduced susceptibility for 158 isolates containing Q148H/R/K without R263K, G118R or N155H. Q148H/R/K alone did not cause measurably reduced dolutegravir susceptibility. However, the median reduction in susceptibility was about 3-fold for the commonly occurring combination Q148H+G140S, 5- to 10-fold for Q148R+G140A/S and 10- to 20-fold for Q148K+E138K. In combination with an additional resistance mutation, including the polymorphic accessory resistance mutations, L74M and T97A, the reduction in susceptibility reached higher levels. The contribution of accessory mutations to reduced dolutegravir susceptibility was most striking in clinical isolates, possibly because these were likely to have additional background mutations that facilitate reduced susceptibility.39,44,53
Considering the above data, it is notable that of 251 isolates in the Stanford HIV Drug Resistance Database with Q148H/R/K, only 14 (5.6%) did not also include a G140 or E138 mutation: Q148H occurred alone in 3 (1.9%) of its 160 occurrences, Q148R in 11 (15.7%) of its 70 occurrences, and Q148K in 0 of its 11 occurrences.
Several isolates lacking each of the four signature mutations in integrase were also reported to have ≥2-fold reduced dolutegravir susceptibility, including isolates with F121Y,54 S230R,55 E92Q+G140A56 and T66K+L74M, V151L and S153Y.57 Additionally, a site-directed mutant containing the five nucleotide changes selected in vitro in the 3′ polypurine tract region displayed 23-fold reduced dolutegravir susceptibility.11
Studies in which dolutegravir plus an optimized background regimen was used for the treatment of INSTI-experienced persons showed that in viruses with Q148 mutations, a 3- to 4-fold reduction in dolutegravir susceptibility was associated with a measurably reduced response to therapy and that a 10-fold reduction in susceptibility was associated with a markedly reduced response to therapy.58,59
There is extensive cross-resistance between dolutegravir and bictegravir.53,60,61 The levels of reduced susceptibility are generally lower for bictegravir than dolutegravir for most patterns of INSTI resistance mutations. However, the clinical significance of these results is uncertain because bictegravir has not been used for salvage therapy in persons harbouring viruses with INSTI resistance mutations.
Our summary of published phenotypic data combined with data on the clinical significance of phenotypic thresholds provides partial insight into the concept of the genetic barrier to dolutegravir resistance. With few exceptions, two or more integrase mutations appear to be required to reduce dolutegravir susceptibility more than 3- to 4-fold and three or more mutations appear required to reduce dolutegravir susceptibility more than 10-fold.
However, the concept of the genetic barrier to resistance is complicated by several factors. First, it is not known whether the phenotypic thresholds for clinically significant reduced susceptibility cited above also apply to the R263K or G118R mutational pathways. Second, several INSTI resistance mutations, particularly G118R and R263K, reduce HIV-1 replication capacity49–51 suggesting that the barrier to resistance is not simply a function of the number of mutations required for reducing susceptibility. Third, R263K, the most common mutation detected at the time of VF in INSTI-naive persons receiving dolutegravir, usually reduces susceptibility only about 2-fold, raising the question as to whether continued dolutegravir therapy with increased adherence and/or at the twice daily 50 mg dosage would lead to virus resuppression. Finally, the published phenotypic data on clinical isolates do not account for possible unreported compensatory integrase mutations and for the potential effects of mutations outside of integrase.
Virological efficacy in populations at increased risk of drug resistance
There were 22 clinical trials and 19 cohort studies meeting one of the following inclusion criteria: (i) ART-naive persons receiving monotherapy or dual therapy (Table S4); (ii) ART-experienced virologically suppressed persons receiving monotherapy (Table S5) or dual therapy (Table S6); (iii) ART-experienced, INSTI-naive persons with active virus replication (Table 5); and (iv) INSTI-experienced persons with active virus replication (Table 6). Baseline genotypic resistance testing was performed in each of the clinical trials in persons with active virus replication, to determine subject eligibility and/or to select the ARVs to be used in combination with dolutegravir (Tables 5, 6 and S4). We excluded 11 cohort studies containing highly heterogeneous populations with varying proportions of ART-naive and ART-experienced persons, varying proportions of ART-experienced persons with virological suppression, or varying numbers of ARVs used in combination with dolutegravir.62–72
Table 5.
Dolutegravir (DTG)-containing regimens in ART-experienced INSTI-naive persons
| AuthorYr | Population | Past ART | DTG ART (q24h) | Subjects | Weeks | Percentage VF (95% CI)a | Percentage resistance (95% CI)b |
|---|---|---|---|---|---|---|---|
| Aboud19 | RCT (DAWNING) | VF on a 1st-line NNRTI regimen | DTG + 2 NRTIs (1 NRTI predicted to be fully active) | 312 | 24 | 17.6 (13.7–22.4) | 0 (0–1.5) |
| 48 | 36.2 (30.9–41.8) | 0.6 (0.1–2.3) | |||||
| Cahn13 | RCT (SAILING) | h/o resistance to ≥2 ARV classes | DTG + OB (1 to 2 ARVs predicted to be fully active) | 354 | 48 | 29.1 (24.4–34.1) | 0.6 (0.1–2) |
| Vavro18 | trial (P1093) | heavily treated adolescents | DTG + OB | 61 | 48 | 31.1 (19.9–44.3) | 4.9 (1–13.7) |
| Lepik17 | cohort | infrequent h/o NRTI resistance (<10%) | DTG + 2 NRTIs | 252 | 48 | 16.7 (12.3–21.9) | 0.8 (0.1–2.8) |
| ALL, 48 weeksc | 979 | 48 | 28.0 (18.6–37.5), I2 = 91 | 0.7 (0.2–1.2), I2 = 0 |
OB, optimized background; W, weeks; h/o, history of.
VF, confirmed virus load ≥50 copies/mL or treatment discontinuation for any reason. For the cohort studies, the proportion of persons with VF after the median time of follow-up was provided.
Percentage of those receiving DTG ART developing an INSTI resistance mutation.
Pooled proportions and 95% CI of VF and VF with INSTI resistance estimated using a random-effects meta-regression. Follow-up meeting presentations provided additional drug resistance data for Aboud19 (DAWNING) and Cahn13 (SAILING). Vavro18 also included 5 day functional monotherapy in 10 patients and weight-adjusted dosing. The follow-up meeting presentation for the SAILING included additional cases of VF plus drug resistance after week 48.16
Table 6.
Dolutegravir (DTG) plus optimized background (OB) in persons with virological failure and INSTI resistance on a raltegravir (RAL)- or elvitegravir (EVG)-containing regimen
| AuthorYr | Population | Past ART | DTG ART | Subjects | Weeks | Percentage VF (95% CI)a |
|---|---|---|---|---|---|---|
| Eron13 | RCT (VIKING – Cohort I) | heavily treated; h/o RAL VF and resistance | DTG (50 mg q24h) + OB | 27 | 24 | 59.3 (38.8–77.6) |
| RCT (VIKING – Cohort II) | heavily treated; h/o RAL VF and resistance | DTG (50 mg q12h) + OB | 24 | 24 | 25 (9.8–46.7) | |
| Castagna14 | RCT (VIKING 3) | heavily treated; h/o RAL VF and resistance | DTG (50 mg q12h) + OB | 183 | 24 | 31.1 (24.5–38.4) |
| Akil15; Naeger16 | RCT (VIKING 4; with vs without OB × 7 days) | heavily treated; h/o INSTI VF and resistance | DTG (50 mg q12h) + OB | 30 | 24 | 53.3 (34.3–71.7) |
| 48 | 60 (40.6–77.3) | |||||
| Castagna18b | Cohort | heavily treated; h/o INSTI VF and resistance | DTG (50 mg q12h) + OB | 190 | 24 | 27.9 (21.6–34.8) |
| 48 | 38.9 (32–46.3) | |||||
| ALL, 24 weeksc | 454 | 24 | 36.9 (26.9–47.0), I2 = 75 | |||
| ALL, 48 weeksc | 220 | 48 | 47.9 (27.5–68.3), I2 = 79 |
h/o, history of.
VF, confirmed virus load ≥50 copies/mL or treatment discontinuation for any reason. For the cohort studies, the proportion of persons with VF after the median time of follow-up was provided.
31% of the persons in Castagna18 had previously been enrolled in one of the VIKING trials.
Pooled proportions and 95% CI of VF and VF with INST resistance estimated using a random-effects meta-regression.
Note: In VIKING Cohorts I and II and in VIKING-4, subjects had a period of functional dolutegravir monotherapy lead-in of 7 (VIKING-4) to 10 (VIKING Cohorts I and II) days before the ARVs accompanying dolutegravir were optimized.
ART-naive persons
There were two small studies of ART-naive persons treated with dolutegravir monotherapy. One was a cohort study of 20 ART-naive persons with baseline plasma HIV-1 RNA levels <100000 copies/mL, of whom 18 maintained virological suppression when receiving dolutegravir monotherapy for a median of 13 months.73 There was also one dose-finding 10 day dolutegravir monotherapy trial, which recruited 28 participants.74 Among those receiving 50 mg daily, the mean reduction in virus load was ∼2.5 log copies/mL. No INSTI resistance mutations or reduction in INSTI susceptibility was observed.
Three clinical trials described 856 ART-naive persons treated with dolutegravir/lamivudine dual therapy20,75,76 (Table S4). Each excluded persons with baseline plasma HIV-1 RNA levels >10000075 or >500000 copies/mL20 or genotypic evidence of lamivudine resistance. The largest trial was a randomized controlled trial (GEMINI) that reported a non-inferior 91% virological response rate at week 48 for 716 persons receiving dolutegravir/lamivudine compared with a similar number of persons receiving dolutegravir plus tenofovir/emtricitabine.75 The remaining two trials were pilot trials that enrolled 120 persons for 48 weeks (A5353)20,77 or 20 persons for 48 weeks.76 The VF rate was 10% in these two trials. One person in A5353 with VF developed the dolutegravir resistance mutation R263K.20 Although dolutegravir/lamivudine is unlikely to be used in regions without baseline genotypic resistance testing, the success of this regimen indicates that dolutegravir does not require two fully active NRTIs to be highly effective.
ART-experienced persons with virological suppression
There were four clinical trials and four cohort studies in which virologically suppressed persons were treated with dolutegravir monotherapy (Table S5). The four clinical trials included 279 persons, of whom 26 (9.3%) developed VF and 9 (3.2%) developed INSTI resistance mutations over periods ranging from 24 to 48 weeks.29,37,78,79 The four cohort studies included 113 persons, of whom 6 (5.3%) developed VF and 5 (4.4%) developed INSTI resistance mutations over periods ranging from 24 to 48 weeks.31–33,80
The dolutegravir monotherapy arm was discontinued prematurely in three of the four randomized controlled trials because of an increase in VF and INSTI resistance mutations compared with the control arm either at week 2429 or between weeks 24 and 48.37,78 The pooled proportion of persons with VF and INSTI resistance mutations was 2.4% (95% CI 0.4%–4.3%; I2 26%) at study termination. However, the raw proportion was 3.6% (i.e. 14 cases in 392 treated persons). An analysis of the DOMONO trial reported that a longer interval between the time of HIV-1 diagnosis and ART initiation, a lower CD4 nadir and a higher PBMC HIV-1 DNA level while virologically suppressed were associated with an increased risk of VF during dolutegravir monotherapy.28
As the risk of VF plus INSTI resistance was unacceptably high in these studies,1,81 further monotherapy studies will be unlikely except possibly for certain populations that appear to be at low risk of VF, including those who initiated ART shortly after HIV-1 infection or who had low PBMC HIV-1 DNA levels, factors associated with a smaller, less heterogeneous latent virus population.28,79
Table S6 lists the 13 studies of virologically suppressed persons on a stable ART regimen switched to dolutegravir dual therapy with lamivudine, rilpivirine, unboosted atazanavir or ritonavir-boosted darunavir.29,82–93 Dolutegravir/rilpivirine was used in one randomized clinical trial and in four cohort studies totalling 1067 persons. The randomized controlled SWORD trial demonstrated that dolutegravir/rilpivirine maintained 95% virological suppression at week 48 in 513 persons and was non-inferior to the control arm of continued unchanged ART. Dolutegravir/lamivudine was used in two randomized clinical trials, one single-arm pilot trial and three cohort studies totalling 504 persons. Dolutegravir/atazanavir and dolutegravir with boosted darunavir were each used in one small cohort study.
In all but three studies, the proportion of persons with VF was ≤10%, and no person developed INSTI resistance mutations. The pooled proportion of VF at week 48 was 8.6% (95% CI 4.8%–12.3%). The extent of heterogeneity was high, with an I2 of 82%, likely reflecting the inclusion of cohort studies and clinical trials, the heterogeneous ART histories of the study subjects, and the different dual drug combinations. Although persons in the larger studies were at low risk of VF (i.e. having no history of VF or of resistance to INSTIs, rilpivirine or lamivudine), several of the smaller studies included persons with multiple past VFs, including some harbouring viruses with reduced lamivudine susceptibility.
Our analyses of dolutegravir monotherapy and dual therapy in virologically suppressed persons included 20 studies that overlapped with 19 distinct studies in a recent systematic review of VF during dolutegravir-based monotherapy and dual therapy in virologically suppressed persons.94 We excluded three studies from this previous review, including two that had <10 persons95,96 and one that included persons with both virological suppression and active virus replication.64 We included four studies that were published following the completion date of this previous review.37,84,97
ART-experienced, INSTI-naive persons without virological suppression
There were two RCTs, one single-arm Phase I/II trial and one cohort study of dolutegravir-containing regimens in non-virologically suppressed, ART-experienced, INSTI-naive persons15,19,21,48 (Table 5). Pre-therapy genotypic resistance testing was performed on all persons in three clinical trials and on an unspecified proportion of persons in the cohort study.
In the DAWNING trial, dolutegravir + two NRTIs was superior to ritonavir-boosted lopinavir + two NRTIs in persons with VF on a first-line NNRTI-containing regimen, who were predicted to have one fully active NRTI—usually either zidovudine or tenofovir. The overall rate of VF at week 48 was 36.2%, with two persons (0.6% of subjects) developing one or more INSTI resistance mutations.
In the SAILING trial, dolutegravir plus an optimized background was superior to raltegravir plus an optimized background in persons with a history of resistance to two or more ARV classes, who nonetheless had a baseline genotypic resistance test, indicating that at least one ARV in addition to dolutegravir or raltegravir was fully active. In the dolutegravir arm, the proportion of persons with VF in this treatment-experienced population was 29.1%. Two persons (0.6% of subjects) developed one or more INSTI resistance mutations during the 48 week trial15 while another three (0.8% of subjects) developed such mutations after week 48.16
In the heavily treated adolescent population enrolled in the Phase I/II P1093 dose-finding trial, 39% of 23 subjects developed VF by week 48 and one developed emergent INSTI resistance.18 A subsequent analysis of two additional treatment cohorts containing 38 additional adolescents later recruited to the study identified two additional cases of emergent INSTI resistance, one at week 52 and another at week 192.19 In the single cohort study, the proportion of persons with VF and emergent INSTI resistance mutations were 16.7% and 0.8%, respectively, after a median of 60 weeks.21
The pooled proportion of VF at week 48 was 28.0% (95% CI 18.6%–37.5%), with a high degree of heterogeneity (I2 91%), reflecting the lower VF rate in the cohort study compared with the three clinical trials. The pooled proportion of VF plus INSTI resistance mutations at week 48 was 0.7% (95% CI 0.2%–1.2%; I2 0%).
Despite its high success rate in ART-experienced, INSTI-naive persons undergoing genotypic resistance testing, it is not known whether a regimen such as dolutegravir/tenofovir/lamivudine will be effective in persons with VF on a first-line NNRTI-containing regimen who harbour NRTI resistance mutations such as K65R and M184V. Both of these NRTI resistance mutations reduce HIV-1 replication fitness98 and NRTIs often appear to retain residual activity against NRTI-resistant viruses.99 Nonetheless, the proportion of persons with VF and emergent INSTI resistance during the first 48 weeks of therapy in low- and middle-income countries would likely be higher than the 0.7% observed in the studies of ART-experienced, INSTI-naive persons in which baseline genotypic resistance testing was performed in an attempt to avoid functional dolutegravir monotherapy.
INSTI-experienced persons without virological suppression
One multi-part clinical trial (VIKING Cohorts I and II, VIKING-3 and VIKING-4) and one cohort study investigated the response to dolutegravir plus an optimized background in persons with a history of INSTI resistance as a result of previous VF on a raltegravir or elvitegravir-containing regimen38–40,59,100 (Table 6). Overall, the VIKING studies included 264 persons and the cohort study included 190 persons. Of the 190 persons in the cohort study, 31% had previously been enrolled in one of the VIKING studies. Dolutegravir at 50 mg twice daily was received in all persons except for a small number of persons in the dose-finding VIKING Cohort I trial. In VIKING Cohorts I and II and in VIKING-4, subjects had a period of functional dolutegravir monotherapy lead-in of 7 days (VIKING-4) to 10 days (VIKING Cohorts I and II) before the ARVs accompanying dolutegravir were optimized.
In nearly all persons in the VIKING trials and in the cohort study, the number of optimized background ARVs predicted to be fully active was low and the overall VF rate was high, ranging from 25% to 59% at week 24 and from 39% to 60% at week 48. The VIKING study defined genotypic dolutegravir resistance as Q148H/R/K in combination with one or more of the following accessory INSTI resistance mutations: L74M/I, T97A, E138A/K/T or G140S/A/C. In an analysis of the 183 person VIKING-3 study, the risk of VF at week 24 was 21% in the absence of a Q148 mutation, 42% in the presence of Q148H/R/K plus one accessory mutation, and 76% in the presence of Q148H/R/K and two accessory mutations.59
Using in vitro susceptibility data obtained by the PhenoSense assay, the risk of VF was 24% for persons with viruses having <4-fold reduced dolutegravir susceptibility, 46% for persons with viruses having 4- to 10-fold reduced susceptibility, and 73% for persons with viruses having >10-fold reduced susceptibility.59 A similar analysis performed by the FDA found that a ≥3-fold (rather than 4-fold) reduction in dolutegravir susceptibility was associated with a reduced virological response.58
Conclusions
The spectrum of dolutegravir-selected mutations and their effects on in vitro susceptibility is emerging but not yet complete because integrase sequences have been published for a small proportion of cases of dolutegravir-associated VF, these cases have been reported primarily in subtype B viruses, and the effects of dolutegravir-selected mutations outside of integrase require further study.
The risk of VF and INSTI resistance on a dolutegravir-containing regimen depends on the ARVs used in combination with dolutegravir, a person’s prior ART experience, and whether a person is stably virologically suppressed. In INSTI-naive persons, several dolutegravir-containing two-drug combinations are likely to be highly effective for first-line therapy, treatment simplification, and even salvage therapy provided dolutegravir’s companion drug is fully active. However, actual and functional dolutegravir monotherapy is associated with non-trivial risks of VF and emergent INSTI resistance.
The risk of functional monotherapy has implications for the use of dolutegravir plus two NRTIs in NRTI-experienced persons in low- and middle-income countries where genotypic resistance testing is not routinely available to guide therapy in persons with VF on a first-line NRTI/NNRTI-containing regimen or where viral load testing is not routinely available to confirm virological suppression in persons transitioning from a first-line NRTI/NNRTI-containing regimen. Studies designed to quantify this risk and to develop strategies to minimize it are urgently needed.
Funding
S. Y. R., P. L. T., J. P. A. I. and R. W. S. were supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (award number R24AI136618). All other authors: the study was carried out as part of routine work.
Transparency declarations
R. W. S. has received research funding from Janssen Pharmaceuticals and Vela Diagnostics and has consulted for Abbott Diagnostics. P. M. G. has received research funding from Viiv Pharmaceuticals and Gilead Sciences. All other authors: none to declare.
Supplementary Material
References
- 1. Vitoria M, Hill A, Ford N. et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? AIDS 2018; 32: 1551–61. [DOI] [PubMed] [Google Scholar]
- 2. Dorward J, Lessells R, Drain PK. et al. Dolutegravir for first-line antiretroviral therapy in low-income and middle-income countries: uncertainties and opportunities for implementation and research. Lancet HIV 2018; 5: e400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meintjes G, Moorhouse MA, Carmona S. et al. Adult antiretroviral therapy guidelines 2017. South Afr J HIV Med 2017; 18: 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. July 2018. HIV Treatment - Interim Guidance https://www.who.int/hiv/pub/guidelines/ARV2018update/en/.
- 5. White KL, Raffi F, Miller MD.. Resistance analyses of integrase strand transfer inhibitors within phase 3 clinical trials of treatment-naive patients. Viruses 2014; 6: 2858–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee S-Y, Sankaran K, Varghese V. et al. HIV-1 protease, reverse transcriptase, and integrase variation. J Virol 2016; 90: 6058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wensing AM, Calvez V, Günthard HF. et al. Update of the drug resistance mutations in HIV-1. Top Antivir Med 2017; 2017: 24: 132–3. [PMC free article] [PubMed] [Google Scholar]
- 8. Blanco J-L, Varghese V, Rhee S-Y. et al. HIV-1 integrase inhibitor resistance and its clinical implications. J Infect Dis 2011; 203: 1204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heredia A, Hassounah S, Medina-Moreno S. et al. Monotherapy with either dolutegravir or raltegravir fails to durably suppress HIV viraemia in humanized mice. J Antimicrob Chemother 2017; 72: 2570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kovarova M, Benhabbour SR, Massud I. et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat Commun 2018; 9: 4156.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malet I, Subra F, Charpentier C. et al. Mutations located outside the integrase gene can confer resistance to HIV-1 integrase strand transfer inhibitors. mBio 2017; 8: e00922–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hachiya A, Kirby KA, Ode H. et al. Disruption of HIV-1 LTR sequence by a nucleocapsid mutation leads to DTG resistance. In: Abstracts of the CROI, Washington, US, 2019. Abstract 74.
- 13. Van Duyne R, Kuo LS, Pham P. et al. Mutations in the HIV-1 envelope glycoprotein can broadly rescue blocks at multiple steps in the virus replication cycle. Proc Natl Acad Sci USA 2019; 116: 9040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wei X, Lipscomb JT, Santos Tino A. et al. LTR translocation mutations under high-level cabotegravir maintain HIV replication. In: Abstracts of the CROI, Seattle, US, 2019. Abstract 545LB.
- 15. Cahn P, Pozniak AL, Mingrone H. et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382: 700–8. [DOI] [PubMed] [Google Scholar]
- 16. Underwood M, DeAnda F, Dorey D. et al. Resistance post week 48 in ART-experienced, integrase inhibitor-naive subjects with dolutegravir (DTG) vs. raltegravir (RAL) in SAILING (ING111762). In: Abstracts of the 13th European Meeting on HIV & Hepatitis Treatment Strategies & Antiviral Drug Resistance, Barcelona, Spain, 2015. Abstract 6.
- 17. Wang R, Horton J, King K. et al. Resistance through week 48 in the DAWNING study comparing dolutegravir (DTG) plus 2 nucleoside RT inhibitors (NRTIs) compared with lopinavir/ritonavir (LPV/r) plus 2 NRTIs in second-line treatment. In: Abstracts of the 22nd International AIDS Conference, Amsterdam, the Netherlands, 2018. Abstract THPEB071.
- 18. Viani RM, Alvero C, Fenton T. et al. Safety, pharmacokinetics and efficacy of dolutegravir in treatment-experienced HIV-1 infected adolescents: forty-eight-week results from IMPAACT P1093. Pediatr Infect Dis J 2015; 34: 1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vavro C, Ruel T, Wiznia A. et al. Emergence of resistance in HIV-1 integrase (IN) following dolutegravir (DTG) treatment in 6 to 18 year old participants enrolled in the P1093 study. In: Abstracts of the 22nd International AIDS Conference, Amsterdam, the Netherlands, 2018. Abstract THPEB114.
- 20. Taiwo BO, Zheng L, Stefanescu A. et al. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of HIV-1-infected participants with HIV-1 RNA <500000 copies/mL. Clin Infect Dis 2018; 66: 1689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lepik KJ, Harrigan PR, Yip B. et al. Emergent drug resistance with integrase strand transfer inhibitor-based regimens. AIDS 2017; 31: 1425–34. [DOI] [PubMed] [Google Scholar]
- 22. Ahmed N, Flavell S, Ferns B. et al. Development of the R263K mutation to dolutegravir in an HIV-1 subtype D virus harboring 3 class-drug resistance. Open Forum Infect Dis 2019; 6: ofy329.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fulcher JA, Du Y, Zhang T-H. et al. Emergence of integrase resistance mutations during initial therapy containing dolutegravir. Clin Infect Dis 2018; 67: 791–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pena Lopez MJ, Chueca N, Hernandez-Febles M. et al. Virological failure through the R263K pathway to a first line dolutegravir containing regimen. In: Abstracts of the 22nd International AIDS Conference, Amsterdam, the Netherlands, 2018. Abstract THPEB041.
- 25. Seatla KK, Avalos A, Moyo S. et al. Four-class drug-resistant HIV-1 subtype C in a treatment experienced individual on dolutegravir-based antiretroviral therapy in Botswana. AIDS 2018; 32: 1899–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cardoso M, Baptista T, Diogo I. et al. Two cases of dolutegravir failure with R263K mutation. AIDS 2018; 32: 2639–40. [DOI] [PubMed] [Google Scholar]
- 27. Lubke N, Jensen B, Huttig F. et al. Failure of dolutegravir-containing first-line regimen. In: Abstracts of the European Meeting on HIV & Hepatitis Treatment Strategies & Antiviral Drug Resistance, Rome, Italy, 2018. Abstract 30.
- 28. Wijting I, Rutsaert S, Rokx C. et al. Predictors of virological failure in HIV-1-infected patients switching to dolutegravir maintenance monotherapy. HIV Med 2018; 20: 63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blanco JL, Rojas J, Paredes R. et al. Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother 2018; 73: 1965–71. [DOI] [PubMed] [Google Scholar]
- 30. Hocqueloux L, Allavena C, Prazuck T. et al. Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for HIV-1-infected virologically suppressed patients: results from the randomized non-inferiority MONCAY trial. In: Abstracts of the 22nd International AIDS Conference, Amsterdam, the Netherlands, 2018. Abstract TUAB0103.
- 31. Katlama C, Soulie C, Caby F. et al. Dolutegravir as monotherapy in HIV-1-infected individuals with suppressed HIV viraemia. J Antimicrob Chemother 2016; 71: 2646–50. [DOI] [PubMed] [Google Scholar]
- 32. Rojas J, Blanco JL, Marcos MA. et al. Dolutegravir monotherapy in HIV-infected patients with sustained viral suppression. J Antimicrob Chemother 2016; 71: 1975–81. [DOI] [PubMed] [Google Scholar]
- 33. Oldenbuettel C, Wolf E, Ritter A. et al. Dolutegravir monotherapy as treatment de-escalation in HIV-infected adults with virological control: doluMono cohort results. Antivir Ther 2017; 22: 169–72. [DOI] [PubMed] [Google Scholar]
- 34. Brenner BG, Thomas R, Blanco JL. et al. Development of a G118R mutation in HIV-1 integrase following a switch to dolutegravir monotherapy leading to cross-resistance to integrase inhibitors. J Antimicrob Chemother 2016; 71: 1948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malet I, Ambrosio FA, Subra F. et al. Pathway involving the N155H mutation in HIV-1 integrase leads to dolutegravir resistance. J Antimicrob Chemother 2018; 73: 1158–66. [DOI] [PubMed] [Google Scholar]
- 36. Wijting IEA, Lungu C, Rijnders BJA. et al. HIV-1 resistance dynamics in patients failing dolutegravir maintenance monotherapy. J Infect Dis 2018; 218: 688–97. [DOI] [PubMed] [Google Scholar]
- 37. Hocqueloux L, Raffi F, Prazuck T. et al. Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for virologically suppressed people living with chronic HIV infection: the randomized non-inferiority MONCAY trial. Clin Infect Dis 2019; doi:10.1093/cid/ciy1132. [DOI] [PubMed] [Google Scholar]
- 38. Eron JJ, Clotet B, Durant J. et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING study. J Infect Dis 2013; 207: 740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naeger LK, Harrington P, Komatsu T. et al. Effect of dolutegravir functional monotherapy on HIV-1 virological response in integrase strand transfer inhibitor resistant patients. Antivir Ther 2016; 21: 481–8. [DOI] [PubMed] [Google Scholar]
- 40. Castagna A, Ferrara M, Galli L. et al. Long-term efficacy of dolutegravir in treatment-experienced subjects failing therapy with HIV-1 integrase strand inhibitor-resistant virus. J Antimicrob Chemother 2018; 73: 177–82. [DOI] [PubMed] [Google Scholar]
- 41. Carganico A, Dupke S, Ehret R. et al. New dolutegravir resistance pattern identified in a patient failing antiretroviral therapy. J Int AIDS Soc 2014; 17: 19749.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hardy I, Brenner B, Quashie P. et al. Evolution of a novel pathway leading to dolutegravir resistance in a patient harbouring N155H and multiclass drug resistance. J Antimicrob Chemother 2015; 70: 405–11. [DOI] [PubMed] [Google Scholar]
- 43. Malet I, Thierry E, Wirden M. et al. Combination of two pathways involved in raltegravir resistance confers dolutegravir resistance. J Antimicrob Chemother 2015; 70: 2870–80. [DOI] [PubMed] [Google Scholar]
- 44. George JM, Kuriakose SS, Dee N. et al. Rapid development of high-level resistance to dolutegravir with emergence of T97A mutation in 2 treatment-experienced individuals with baseline partial sensitivity to dolutegravir. Open Forum Infect Dis 2018; 5: ofy221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seatla KK, Avalos A, Mphoyakgosi T. et al. Preliminary virologic outcomes and prevalence of integrase strand transfer inhibitor resistance mutations among highly treatment experienced patients receiving dolutegravir in Botswana. In: Abstracts of the 22nd International AIDS Conference, Amsterdam, the Netherlands, 2018. Abstract LBPEB019.
- 46. Malet I, Fourati S, Charpentier C. et al. The HIV-1 integrase G118R mutation confers raltegravir resistance to the CRF02_AG. J Antimicrob Chemother 2011; 66: 2827–30. [DOI] [PubMed] [Google Scholar]
- 47. Margot NA, Hluhanich RM, Jones GS. et al. In vitro resistance selections using elvitegravir, raltegravir, and two metabolites of elvitegravir M1 and M4. Antiviral Res 2012; 93: 288–96. [DOI] [PubMed] [Google Scholar]
- 48. Aboud M, Kaplan R, Lombaard J. et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019; 19: 253–64. [DOI] [PubMed] [Google Scholar]
- 49. Quashie PK, Mesplede T, Han Y-S. et al. Characterization of the R263K mutation in HIV-1 integrase that confers low-level resistance to the second-generation integrase strand transfer inhibitor dolutegravir. J Virol 2012; 86: 2696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quashie PK, Mesplede T, Han Y-S. et al. Biochemical analysis of the role of G118R-linked dolutegravir drug resistance substitutions in HIV-1 integrase. Antimicrob Agents Chemother 2013; 57: 6223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Quashie PK, Oliviera M, Veres T. et al. Differential effects of the G118R, H51Y, and E138K resistance substitutions in different subtypes of HIV integrase. J Virol 2015; 89: 3163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hachiya A, Kirby KA, Ido Y. et al. Impact of HIV-1 integrase L74F and V75I mutations in a clinical isolate on resistance to second-generation integrase strand transfer inhibitors. Antimicrob Agents Chemother 2017; 61: e00315–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang WW, Cheung PK, Oliviera N. et al. Accumulation of multiple mutations in vivo confers cross-resistance to new and existing integrase inhibitors. J Infect Dis 2018; 218: 1773–6. [DOI] [PubMed] [Google Scholar]
- 54. Malet I, Gimferrer Arriaga L, Artese A. et al. New raltegravir resistance pathways induce broad cross-resistance to all currently used integrase inhibitors. J Antimicrob Chemother 2014; 69: 2118–22. [DOI] [PubMed] [Google Scholar]
- 55. Pham HT, Labrie L, Wijting IEA. et al. The S230R integrase substitution associated with viral rebound during DTG monotherapy confers low level INSTI drug resistance. J Infect Dis 2018; 218: 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Andreatta KN, Chang S, Martin R. et al. Integrase inhibitor resistance selections initiated with drug resistant HIV-1. In: Abstracts of the CROI, Boston, USA, 2018. Abstract 546.
- 57. Yoshinaga T, Kobayashi M, Seki T. et al. Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection. Antimicrob Agents Chemother 2015; 59: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.FDA. FDA: TIVICAY (dolutegravir) Tablets for Oral Use 2013.
- 59. Castagna A, Maggiolo F, Penco G. et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis 2014; 210: 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tsiang M, Jones GS, Goldsmith J. et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother 2016; 60: 7086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Smith SJ, Zhao XZ, Burke TR. et al. Efficacies of cabotegravir and bictegravir against drug-resistant HIV-1 integrase mutants. Retrovirology 2018; 15: 37.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Capetti AF, Sterrantino G, Cossu MV. et al. Salvage therapy or simplification of salvage regimens with dolutegravir plus ritonavir-boosted darunavir dual therapy in highly cART-experienced subjects: an Italian cohort. Antivir Ther 2017; 22: 257–62. [DOI] [PubMed] [Google Scholar]
- 63. Palacios R, Mayorga M, González-Domenech CM. et al. Safety and efficacy of dolutegravir plus rilpivirine in treatment-experienced HIV-infected patients: the DORIVIR study. J Int Assoc Provid AIDS Care 2018; 17: 2325958218760847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Revuelta-Herrero JL, Chamorro-de-Vega E, Rodriguez GCG. et al. Effectiveness, safety, and costs of a treatment switch to dolutegravir plus rilpivirine dual therapy in treatment-experienced HIV patients. Ann Pharmacother 2018; 52: 11–8. [DOI] [PubMed] [Google Scholar]
- 65. Jabłonowska E, Siwak E, Bociąga-Jasik M. et al. Real-life study of dual therapy based on dolutegravir and ritonavir-boosted darunavir in HIV-1-infected treatment-experienced patients. PLoS One 2019; 14: e0210476.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Charpentier C, Peytavin G, Le MP. et al. High virological suppression regardless of the genotypic susceptibility score after switching to a dolutegravir-based regimen: week 48 results in an observational cohort. J Antimicrob Chemother 2018; 73: 1665–71. [DOI] [PubMed] [Google Scholar]
- 67. Todd S, Rafferty P, Walker E. et al. Early clinical experience of dolutegravir in an HIV cohort in a larger teaching hospital. Int J STD AIDS 2017; 28: 1074–81. [DOI] [PubMed] [Google Scholar]
- 68. Rusconi S, Adorni F, Tau P. et al. Dolutegravir (DTG)-containing regimens after receiving raltegravir (RAL) or elvitegravir (EVG): durability and virological response in a large Italian HIV drug resistance network (ARCA). J Clin Virol 2018; 105: 112–7. [DOI] [PubMed] [Google Scholar]
- 69. Armenia D, Gori C, Forbici F. et al. Evaluation of virological response and resistance profile in virologically suppressed HIV-1 infected patients switching to integrase inhibitor based treatment in clinical settings. In: Abstracts of the European Meeting on HIV & Hepatitis Treatment Strategies & Antiviral Drug Resistance, Rome, Italy, 2018. Abstract 42.
- 70. Sorstedt E, Carlander C, Flamholc L. et al. Effect of dolutegravir in combination with nucleoside reverse transcriptase inhibitors (NRTIs) on people living with HIV who have pre-existing NRTI mutations. Int J Antimicrob Agents 2018; 51: 733–8. [DOI] [PubMed] [Google Scholar]
- 71. Lee S-A, Kim S-W, Chang H-H. et al. Effectiveness, safety, and tolerability of a switch to dual therapy with dolutegravir plus cobicistat-boosted darunavir in treatment-experienced patients with human immunodeficiency virus. Infect Chemother 2018; 50: 252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mascolini M, Sustained viral suppression with dolutegravir and boosted darunavir dual therapy among highly treatment-experienced individuals. Sustained viral suppression after switch to darunavir/dolutegravir. In: Abstracts of the IDWeek, San Francisco, US, 2018. Abstract 1766.
- 73. Lanzafame M, Nicolè S, Gibellini D. et al. Dolutegravir monotherapy in HIV-infected naive patients with an HIV-RNA load <100000 copies/mL: a medium-term follow-up. J Antimicrob Chemother 2017; 72: 2136–8. [DOI] [PubMed] [Google Scholar]
- 74. Min S, Song I, Borland J. et al. Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother 2010; 54: 254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cahn P, Madero JS, Arribas JR. et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2018; 393: 143–55. [DOI] [PubMed] [Google Scholar]
- 76. Cahn P, Rolon MJ, Figueroa MI. et al. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc 2017; 20: 21678.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nyaku AN, Zheng L, Gulick RM. et al. Dolutegravir plus lamivudine for initial treatment of HIV-1-infected participants with HIV-1 RNA <500000 copies/mL: week 48 outcomes from ACTG 5353. J Antimicrob Chemother 2019; 74: 1376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wijting I, Rokx C, Boucher C. et al. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV 2017; 4: e547–54. [DOI] [PubMed] [Google Scholar]
- 79. Braun D, Turk T, Hampel B. et al. Simplification to dolutegravir monotherapy is non-inferior compared to continuation of combination antiretroviral therapy in patients who initiated combination antiretroviral therapy during primary HIV infection: a randomized, controlled, non-inferiority trial. In: Abstracts of the 22nd International AIDS Conference, Amsterdam, the Netherlands, 2018. Abstract TUAB0102.
- 80. Gubavu C, Prazuck T, Niang M. et al. Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother 2016; 71: 1046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.US Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 82. Llibre JM, Hung C-C, Brinson C. et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391: 839–49. [DOI] [PubMed] [Google Scholar]
- 83. Gantner P, Cuzin L, Allavena C. et al. Efficacy and safety of dolutegravir and rilpivirine dual therapy as a simplification strategy: a cohort study. HIV Med 2017; 18: 704–8. [DOI] [PubMed] [Google Scholar]
- 84. Capetti AF, Sterrantino G, Cossu MV. et al. Switch to dolutegravir plus rilpivirine dual therapy in cART-experienced subjects: an observational cohort. PLoS One 2016; 11: e0164753.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Diaz A, Casado JL, Dronda F. et al. Dolutegravir plus rilpivirine in suppressed heavily pretreated HIV-infected patients. In: Abstracts of the 20th International AIDS Conference, Durban, South Africa, 2016. Abstract TUPDB0106.
- 86. Bonijoly T, Cabie A, Cotte L. et al. Week-48 efficacy and safety of dolutegravir + rilpivirine dual therapy as a switch strategy in a real-life cohort study. In: Abstracts of the European AIDS Conference, Milan, Italy, 2017. Abstract PE9/15.
- 87. Taiwo BO, Marconi VC, Berzins B. et al. Dolutegravir plus lamivudine maintain HIV-1 suppression through week 48 in a pilot randomized trial. Clin Infect Dis 2018; 66: 1794–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Joly V, Burdet C, Landman R. et al. Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: results of the ANRS 167 trial (LAMIDOL). J Antimicrob Chemother 2018; 74: 739–45. [DOI] [PubMed] [Google Scholar]
- 89. Maggiolo F, Gulminetti R, Pagnucco L. et al. Lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis 2017; 17: 215.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Borghetti A, Baldin G, Lombardi F. et al. Efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multicentre cohort of patients with suppressed HIV-1 replication. HIV Med 2018; 19: 452–4. [DOI] [PubMed] [Google Scholar]
- 91. Reynes J, Meftah N, Tuaillon E. et al. Dual regimen with dolutegravir and lamivudine maintains virologic suppression even in heavily treatment experienced HIV-infected patients: 96 weeks results from maintenance DOLULAM study. In: Abstracts of the IAS Conference on HIV Science, Paris, France, 2017. Abstract 2755.
- 92. Castagna A, Gulminetti R, Rusconi S. et al. Durability and safety of a dual antiretroviral regimen with dolutegravir and unboosted atazanavir in HIV-1 infected patients with virological suppression. In: Abstracts of the European AIDS Conference, Milan, Italy, 2017. Abstract PE9/63.
- 93. Navarro J, Santos JR, Silva A. et al. Efficacy of once-daily dolutegravir plus boosted-darunavir as a switch strategy in HIV-infected heavily-treated patients. In: Abstracts of the European AIDS Conference, Milan, Italy, 2017. Abstract PE9/93.
- 94. Wandeler G, Buzzi M, Anderegg N. et al. Virologic failure and HIV drug resistance on simplified, dolutegravir-based maintenance therapy: systematic review and meta-analysis. F1000Res 2018; 7: 1359.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rokx C, Schurink CAM, Boucher CAB. et al. Dolutegravir as maintenance monotherapy: first experiences in HIV-1 patients. J Antimicrob Chemother 2016; 71: 1632–6. [DOI] [PubMed] [Google Scholar]
- 96. Sculier D, Doco-Lecompte T, Yerly S. et al. Stable HIV-1 reservoirs on dolutegravir maintenance monotherapy: the MONODO study. HIV Med 2018; 19: 572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Braun DL, Turk T, Tschumi F. et al. Non-inferiority of simplified dolutegravir monotherapy compared to continued combination antiretroviral therapy that was initiated during primary HIV infection: a randomized, controlled, multi-site, open-label, non-inferiority trial. Clin Infect Dis 2019; doi: 10.1093/cid/ciy1131. [DOI] [PubMed] [Google Scholar]
- 98. Oliveira M, Ibanescu RI, Pham HT. et al. The M184I/V and K65R nucleoside resistance mutations in HIV-1 prevent the emergence of resistance mutations against dolutegravir. AIDS Lond Engl 2016; 30: 2267–73. [DOI] [PubMed] [Google Scholar]
- 99. Paton NI, Kityo C, Thompson J. et al. Nucleoside reverse-transcriptase inhibitor cross-resistance and outcomes from second-line antiretroviral therapy in the public health approach: an observational analysis within the randomised, open-label, EARNEST trial. Lancet HIV 2017; 4: e341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Akil B, Blick G, Hagins DP. et al. Dolutegravir versus placebo in subjects harbouring HIV-1 with integrase inhibitor resistance associated substitutions: 48-week results from VIKING-4, a randomized study. Antivir Ther 2015; 20: 343–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.