Abstract
It is not rare to find Immunoglobulin A (IgA) nephropathy (IgAN) combined with other glomerular diseases, which can be called compound IgAN (cIgAN). Till now, clinical-pathological investigation of cIgAN was lacking, especially the severity of “background IgAN lesions.” This research aimed to investigate the incidence, clinical and pathological characteristics of cIgAN, and thus improve the understanding of the clinical significance of this combination.
Patients with cIgAN diagnosed in Peking University People's Hospital from November 2012 to April 2018 were retrospectively analyzed. Patients with IgAN without compound glomerular diseases (sIgAN) were enrolled as a control group.
Among 1407 patients diagnosed with IgAN, 80 (5.69%) were cIgAN patients. Compared with sIgAN, cIgAN patients had a significantly lower prevalence of microscopic hematuria and more urine protein. There were 10 pathological types of glomerular diseases combined with IgAN, led by diabetic nephropathy 37 (46.25%) and membranous nephropathy 14 (17.5%). Histologically, although the mesangial hypercellularity was comparable in 2 groups, cIgAN patients had a lower prevalence of endocapillary proliferation, segmental glomerulosclerosis, and cellular or fibrocellular crescents formation, as well as weaker immunofluorescence intensity for IgA and C3 (all P < .05). Eight out of 27 (29.63%) cIgAN patients with follow-up data (5–48 months) developed irreversible end-stage renal disease requiring dialysis.
The order of incidence of concomitant diseases was similar to that of the pure diseases. The “background IgAN associated lesions” except mesangial hypercellularity were relatively mild in cIgAN group. Those might suggest that in some cases, IgAN seems to be a chance finding, and the combined diseases may play a more important role in the clinicopathological features of the patients than the nephritis caused by IgA deposition. While diagnosing IgAN, other combined glomerular diseases need to be carefully considered by nephrologists and pathologists.
Keywords: combined disease, glomerulonephritis, IgA nephropathy, pathology, renal biopsy
1. Introduction
Immunoglobulin A (IgA) nephropathy (IgAN) is recognized as the most common form of glomerulonephritis all over the world.[1–3] It is characterized by the IgA-dominant or IgA-codominant glomerular staining, mainly involving the mesangium. There is much histological variability under light microscope, ranging from no detectable histologic lesions to diffuse proliferative glomerulonephritis or glomerulosclerosis.[4,5] Under transmission electron microscopy (TEM), virtually all cases of IgAN show mesangial deposits within mesangial regions.
It is not rare to find IgAN combined with other glomerular diseases, which can be called compound IgAN (cIgAN). Some pathological types such as IgAN combined with diabetic nephropathy (DN) or with membranous nephropathy (MN) have been reported respectively.[6–11] But till now, the incidence and constituent ratio of cIgAN was unknown, and clinical–pathological investigation of cIgAN was lacking. Moreover, the complexity of the histological lesions in cIgAN brought difficulties to judge whether IgAN or the combined disease might be the major cause of kidney injury and make treatment decisions.
The purpose of this study is to investigate the incidence, pathological type, and ultrapathological features of cIgAN, with the attempt to find the severity of the “background IgAN associated lesions” and to improve the understanding of cIgAN.
2. Materials and methods
2.1. Study participants
This was a retrospective study. IgAN cases diagnosed from November 2012 to April 2018 in Department of Nephrology and Electron Microscope Laboratory of Peking University People's Hospital were collected. IgAN was defined by the presence of dominant or codominant glomerular staining for IgA in the absence of systemic diseases such as systemic lupus or nephritis associated with hepatitis B. Cases that combined with other glomerular diseases except for merely ischemic lesions were defined as cIgAN and retrospectively analyzed. One hundred nineteen patients with simple IgAN (sIgAN) were randomly selected as disease controls.
This study was approved by the ethics committee of Peking University People's Hospital (2019PHB069-01).
2.2. Methods
2.2.1. Data collection
General information, clinical and laboratory data of cIgAN and sIgAN patients at the time of renal biopsy were collected, including sex, age, serum creatinine, daily urinary protein excretion, serum IgA level, etc. Follow-up data of cIgAN patients were collected from inquiry outpatient case system or through telephone follow-up. The endpoint was defined as developing irreversible end-stage renal disease (ESRD) requiring dialysis or transplantation, and doubling of serum creatinine levels.
All renal biopsies were processed according to standard techniques for light microscopy, immunofluorescence, and electron microscopy. The definitions of pathological lesions referred to and were modified from the Oxford Classification.[12] Glomerular lesions which may be “IgAN characteristic” under light microscopy were recorded and analyzed, including mesangial hypercellularity (0, non, <4 mesangial cells/mesangial area; 1, mild, 4–5 mesangial cells/mesangial area; 2, moderate, 6–7 mesangial cells/mesangial area; and 3, severe, >7mesangial cells/mesangial area; the mesangial hypercellularity score is the mean score for all glomeruli), endocapillary proliferation (0, non, no proliferation; 1, focal and segmental, <50% of glomeruli proliferated; and 2, global, >50% of glomeruli globally proliferated), cellular and fibrocellular crescents, segmental glomerulosclerosis, and global glomerulosclerosis. Tubular atrophy/interstitial fibrosis was assessed by the percentage of cortical area involved by the tubular atrophy or interstitial fibrosis, whichever is greater (0, 0%–25%; 1, 26%–50%; 2, >25%). Biopsies from sIgAN patients were graded according to the Oxford Classification of IgAN.[13,14] The intensity of the immunofluorescence staining signals for immunoglobulin (Ig)G, IgA, IgM, C3, C1q, and fibrinogen was scored as 0 to 4. Electron microscopy was performed with an FEI Tecnai (Hillsboro) electron microscope. Each glomerulus was carefully observed, including mesangial cells, mesangial matrix, endothelium cells, podocytes, glomerular basement membrane, deposits, and any special substances.
2.2.2. Statistical analysis
Statistical analysis was performed using SPSS version 16.0 (chicoga, IL). Continuous data were described as mean ± standard deviation or median and range, and categorical data were described as frequencies and percentages. Differences of quantitative parameters between groups were assessed using the Student t test or Mann–Whitney U test. Differences of qualitative results were compared using the Chi-squared test. P < .05 was considered significant.
3. Results
3.1. Incidence and constituent ratio of cIgAN
A total of 1407 IgAN patients were diagnosed from November 2012 to April 2018, of which 80 patients meet the diagnostic criteria of cIgAN, accounting for 5.69% of all IgAN.
There were 10 pathological types of glomerular disease in cIgAN patients: DN (2 with type 1 diabetes, and others with type 2 diabetes), MN, minimal change disease, Anti-neutrophil cytoplasmic antibodies (ANCA)-related glomerulonephritis, thin basement membrane nephropathy, obesity-related glomerulopathy, lipoprotein glomerulopathy, acute postinfectious glomerulonephritis, and Alport syndrome. The constituent ratio of each pathological type of cIgAN was shown in Table 1.
Table 1.
The constituent ratio of each pathological type of compound IgA nephropathy.

3.2. Clinical characteristics of cIgAN patients
Among the cIgAN patients, 52 (65%) were males, with a male to female ratio of 1.86:1. The age of the patient was 45.36 ± 13.58 (18–74) years old. Five (6.25%) patients had gross hematuria during the course of the disease, and 62 (77.5%) patients showed microscopic hematuria. Serum albumin was 29.77 ± 7.76 g/L. Serum IgA levels were examined in 57 patients, which was 2.97 ± 1.20 g/L, and elevated in 9 (15.79%) patients. Serum creatinine was 33 to 1119 μmol/L, with a median of 107.3 μmol/L. Estimated glomerular filtration rate (eGFR) was 70.20 ± 37.33 mL/minutes/1.73 m2. Urine protein quantification was 5.28 ± 4.28 g/24 hours. Thirty-four patients (42.5%) showed nephrotic syndrome. More clinical characteristics of patients with cIgAN and sIgAN were displayed in Table 2.
Table 2.
Demographic and clinical characteristics of compound IgA nephropathy and simple IgA nephropathy without compound glomerular diseases.

Compared with sIgAN, cIgAN patients were significantly older (45.36 ± 13.58 versus 38.66 ± 13.27, P = .001), male susceptibility (65% versus 49.58%, P = .032), with lower serum albumin (29.77 ± 7.76 versus 36.77 ± 7.13, P < .001), lower prevalence of microscopic hematuria (77.5% versus 94.92%, P < .001), more urine protein (5.28 ± 4.28 versus 2.36 ± 2.73, P < .001), and higher prevalence of nephrotic syndrome (42.5% versus 7.63%, P < .001). No significant difference was found in the renal disease course, the prevalence of hypertension, serum IgA level, serum creatinine, eGFR, and the prevalence of gross hematuria (Table 2).
3.3. Immunofluorescence and histological characteristics of cIgAN patients
Immunofluorescence assay showed that besides IgA (Fig. 1A and E), IgG was mesangial positive in 6 patients (7.5%), glomerular capillary wall positive in 27 patients (33.75%) (Fig. 1 B and F), and C3 was positive in 75 patients (93.75%). The staining intensity score for mesangial IgA, IgG, and C3 were 2.28 ± 0.57, 0.12 ± 0.49, and 2.01 ± 0.82, respectively. Compared with sIgAN patients, the intensity for C1q was significantly stronger, while the intensity for IgA and C3 were significantly weaker in the cIgAN group (P < .05) (Table 3).
Figure 1.
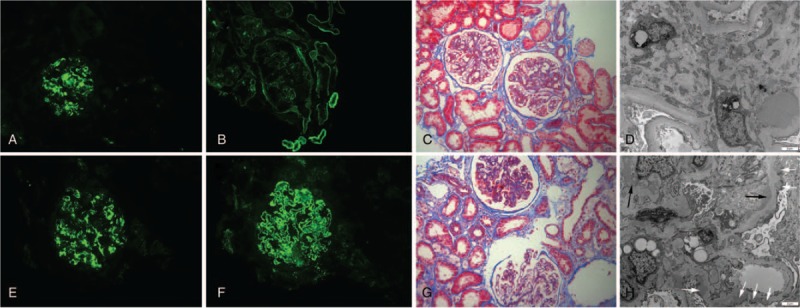
Representative immunofluorescence, light microscopy, and electron microscopy on biopsy specimen from cIgAN patients combined with diabetic nephropathy (A–D) and membranous nephropathy (E–H). (A and E) Immunofluorescence reveals mesangial staining for IgA (200×). (B) Immunofluorescence reveals linear GBM and tubular basement membrane staining for IgG (200×). (C) Masson trichrome stain shows mild (the left glomerular) to moderate (the right glomerular) mesangial hypercellularity (200×). (D) Electron microscopy shows diffuse thickening of glomerular basement membrane and increased mesangial matrix. The electron dense deposits can be seen in the mesangial region, with diffuse podocyte foot-process effacement (4200×). (F) Immunofluorescence reveals fine granular GBM staining for IgG (200×). (G) Masson trichrome stain shows thickened GBMs and mild mesangial hypercellularity (200×). (H) Electron microscopy shows GBM thickening, with electron dense deposits in the subepithelial region (white arrow) and mesangial region (black arrow), and with diffuse podocyte foot-process effacement (4200×). cIgAN = compound IgA nephropathy, GBM = glomerular basement membrane.
Table 3.
Pathological characteristics of compound IgA nephropathy and sIgAN under light microscopy and immunofluorescence microscopy.

Under light microscopy, all the cIgAN patients had mild to moderate mesangial hypercellularity: mild in 56 (70%) (Fig. 1G) and moderately in 24 (30%) (Fig. 1C). Endocapillary proliferation was seen in 27 (33.75%) patients: segmental in 24 (30%) and global in 3(3.75%). Cellular or fibrocellular crescents were found in 18 (22.5%) patients. Fifteen (18.75%) patients had segmental sclerosis and 50 (62.5%) had global sclerosis (Table 3).
Patients in sIgAN group were scored according to Oxford classification (MEST-C). Twenty-nine (24.37%) patients were scored as M1, 51 (42.86%) patients were scored as E1, 52 (43.70%) patients were scored as S1, 61 (51.26%) patients were scored as T1, 54(45.38%) patients were scored as T2, 51 (42.86%) patients were scored as C1, and 15 (12.61%) patients were scored as C2.
Compared with sIgAN, cIgAN patients had lower prevalence of endocapillary proliferation (33.75% versus 42.68%, P = .027), segmental glomerulosclerosis (18.75% versus 43.70%, P < .001), and cellular or fibrocellular crescents formation (22.5% versus 55.46%, P < .001). The prevalence of global glomerulosclerosis was comparable but the average number of global sclerosis was lower in cIgAN group. Moreover, the average number of crescents, segmental sclerosis, or the global sclerosis was significantly smaller in the cIgAN group (P < .05). The severity of tubulointerstitial scarring was comparable in 2 groups (Table 3).
3.4. TEM characteristics of cIgAN patients
Under electron microscopy, electron dense deposits were seen in the mesangial region in all but 1 cIgAN patients, with mesangial hypercellularity and matrix increasing in varying degrees (Fig. 1 D and H). We failed to find definite electron dense deposits in 1 cIgAN patient combined with DN, whose immunofluorescence revealed the presence (2+) of IgA and C3 within the mesangial area. Electron dense deposits were also seen within subendothelial regions in 12 (15%) patients, within subepithelial regions in 19 (23.75%) patients, and within glomerular basement membrane in 10 (12.5%) patients.
In comparison with sIgAN, the TEM findings of cIgAN patients were more complicated and various, according to the combined diseases (e.g., diffuse thickening of glomerular basement membrane and mesangial matrix increasing were seen in patients combined with DN and glomerular basement membrane thickening and subepithelial electron dense deposition were seen in patients combined with MN) (Fig. 1 D and H). Some common TEM findings of patients with cIgAN and sIgAN were displayed in Table 4.
Table 4.
Glomerular pathological lesions of compound IgA nephropathy and simple IgA nephropathy without compound glomerular diseases under transmission electron microscopy.

3.5. Treatment and outcome of cIgAN patients
Forty-four (55%) patients were treated with angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, and 30 patients (37.5%) received immunosuppressive therapy with steroids and/or cytotoxic agents.
Follow-up data were available for 27 patients with cIgAN, including 16 patients combined with DN. They were followed up for 5 to 48 months (median 18 months). Eight patients (29.63%), all combined with DN (50% of followed-up patients combined with DN), developing ESRD. Two patients (7.41%), both combined with DN (12.5% of followed-up patients combined with DN), achieved doubling of serum creatinine levels during follow-up.
4. Discussion
IgAN is defined by the presence of IgA-dominant or codominant immune deposits within glomeruli. The diagnosis of IgAN is generally made according to the immunofluorescence and light microscopic findings, together with the clinical data. In routine diagnostic work, it is not very rare to find IgAN coexisting with other glomerular diseases. In this study, we firstly reported that the incidence of this coexistence was 5.69% in all IgAN patients. The combined diseases covered various pathological types of glomerular diseases.
With the increase in the incidence of diabetes, DN has become one of the important causes of chronic kidney disease in China. It has been reported that IgAN was a common finding in diabetic patients.[6,10,11] The results of this study also showed that IgAN combined with DN was the most common type of cIgAN. In 1 case which combined with DN, we failed to detect any deposit by TEM. It was previously reported that the absence of deposits by TEM was from 0.5% to 25% of cases.[15] The absence of deposits could not rule out the diagnosis of IgAN due to the limited range of observation by TEM.
MN was the second common type of cIgAN patients of the present study. There have been a few reports of the concurrence of IgAN and MN.[7–9] Some speculated that the combination was caused by the occurrence of superimposed MN on a background of preexisting mild IgAN. Others took it as a unique entity rather than due to chance alone. In the present study, we found that the incidence of cIgAN types seems in accordance with the order of incidence of combined disease in China, supporting the “chance hypothesis.” However, the etiology of this combination is still to be explored.
The other types of combination with thin basement membrane nephropathy, ANCA-related glomerulonephritis, obesity-related glomerulopathy, minimal change disease, Alport syndrome, and lipoprotein glomerulopathy of IgAN have been reported as cases.[16–23]
As well known, the histological changes of IgAN may be various and complex.[4,24,25] The combination with another glomerular disease would make it even more complicated. No histological lesion is truly specific for IgAN. The lesions, such as glomerular sclerosis, mesangial expansion, and crescents, can exist in different diseases. In the present study, when analyzing the histological lesions of cIgAN, we focused on the glomerular pathological lesions which were commonly found and proved to be important for prognosis of IgAN. Those lesions, including mesangial hypercellularity, endothelial proliferation, segmental sclerosis, and crescents,[14] can be taken as possibly “background IgAN associated lesions” (“background lesions” for short) in this study. We found that mesangial hypercellularity was comparable in the 2 groups. But the other “background lesions” including endocapillary proliferation, segmental glomerulosclerosis, and crescents formation had significantly lower incidence in cIgAN patients, although acute postinfectious glomerulonephritis (with endocapillary proliferation) and ANCA-related glomerulonephritis (with crescents formation) were combined in some cases. Similarly, in clinical analysis, we found that although some of the clinical manifestations such as proteinuria seemed more severe in cIgAN group, the incidence of microscopic hematuria was significantly lower in cIgAN group than in sIgAN group. Moreover, the immunofluorescence intensity for IgA and C3 were significantly weaker in the cIgAN group compared with the sIgAN group. According to all the above results, we might carefully speculate that at least in some patients in cIgAN group, the IgA molecules found in mesangial area were just opportunistic depositions instead of having any pathogenic effect, while the other glomerular disease played the major role in the course of the kidney disease, and the more severe clinical manifestations might due to the combined disease rather than so-called “IgAN.” But in present study, we had not enough evidence to differentiate “IgA deposition” from “IgA nephropathy.” More studies are needed to prove or disprove this hypothesis.
Techniques used in renal pathology, as we know, include light microscopy, immunofluorescence, and TEM. Light microscopy, including several staining methods, is the most complicated and most important part for distinction and evaluation of most lesions of the tissues. Immunofluorescence can identify specific molecules, especially immune substances within tissues. TEM is helpful to identify tiny lesions which cannot be clearly shown by other techniques. The role of TEM is more important in some nonimmune kidney diseases, such as diseases mainly due to podocyte lesion, basement membrane lesion, or organized deposits.
All of those above techniques are necessary for diagnosis of cIgAN and pathologists should pay more attention to every part of renal pathology, as the clinicians should identify any special manifestation to prevent misdiagnosis and mistreatment. When the diagnosis of IgAN is proposed under immunofluorescence microscope, it is necessary to carefully rule out the other glomerular diseases hidden behind IgAN, especially when clinical manifestations, other immunofluorescence findings or light microscopy findings cannot be explained by IgAN alone. Every part of the glomeruli and the structure and location of the electronic deposits should be carefully observed under TEM. For example, in most cases, the diagnosis of DN combined with IgAN can be made according to a history of diabetes, positive immune staining for IgA, and typical diabetic histological features such as mesangial matrix expansion or nodules formation. But in 6 patients in our study, the histological lesions were mild under light microscopy, and the diagnosis of early DN was added after TEM observation, according to the finding of diffuse thickening of the glomerular basement membrane. So TEM is necessary in this situation.
As expected, we found that TEM often brought more detailed information associated with the combined disease than light microscopy. We can divide the combined diseases of cIgAN into several categories according to the different roles which TEM plays:
-
1.
Category 1: There may be clinical clues such as microscopic hematuria, deafness, family history of chronic kidney disease, or nephrotic syndrome, but immunofluorescence and light microscopy are not characteristic, so decisive diagnosis must be made under TEM. Thin basement membrane nephropathy, Alport syndrome, minimal change disease, and some early DN are in this category.
-
2.
Category 2: There may be clinical clues, but definite diagnosis must be based on all the pathological methods including immunofluorescence, light microscopy, and TEM, or the TEM characteristics of the disease are helpful to make a definite diagnosis. DN, MN, and acute postinfectious glomerulonephritis are in this category.
-
3.
Category 3: There is clinical evidence but no characteristic lesions under TEM. The role of TEM is to exclude other possible diseases and make the diagnosis more confident. ANCA-related glomerulonephritis and obesity-related glomerulopathy are in this category.
From the limited follow-up data, we can find that the prognosis of cIgAN combined with DN was poor, with half of them progressing into ESRD within 4 years. The prognosis seems worse than other Chinese IgAN cohorts previously reported, which showed that 7% to 13.7% patients progressed to ESRD during a longer follow-up.[5,26] The prognosis also seemed worse than the Chinese cohort of DN, which showed an overall 5-year renal survival rate of 61.0%.[27] Further research was needed to answer whether the poor prognosis was associated with IgA deposition.
Besides the lack of follow-up data, there are other limitations in present study. First, the sample size of cIgAN was limited, and we did not find all types of glomerular diseases that may combine with IgAN. Second, the “background lesions” were possibly and relatively characteristic for IgAN, and more specific molecular markers such as underglycosylation of IgA1, anti-abnormal glycosylation antibodies, and other protein or mRNA markers[28] should be analyzed in further researches. Third, some comparison between cIgAN group and sIgAN groups, such as age and c1q score, has little significance, for cIgAN includes various compound diseases. Fourth, we only included patients combined with nonischemic glomerular diseases. IgAN combined with tubulointerstitial injury and ischemic kidney injury is more common and can be easily diagnosed without TEM, and IgAN with malignant hypertensive renal injury has been studied before.[29]
In conclusion, IgAN can be combined with various types of glomerular diseases, especially DN and MN. “Background IgAN lesions” were relatively mild in the cIgAN group, which suggested that at least in some situation, the combined diseases might play a more important role in the clinicopathological features and prognosis of the patients than “IgA nephropathy.” Comprehensive analysis of clinical, histological features, and TEM characteristics are necessary for the diagnosis of cIgAN. In further studies, biomarkers at molecular level and genetic level should be studied to reveal the different mechanisms of “IgA deposition” and “IgA nephropathy.”
Author contributions
Conceptualization: Lei Jiang, Shuying Zheng, Li Zuo, Hongxia Shi.
Data curation: Lei Jiang, Bao Dong, Yu Yan, Shuying Zheng, Yanan Hu.
Formal analysis: Lei Jiang.
Investigation: Lei Jiang.
Methodology: Lei Jiang.
Resources: Bao Dong, Yu Yan.
Writing – original draft: Lei Jiang.
Writing – review & editing: Bao Dong, Yu Yan, Li Zuo, Hongxia Shi.
Footnotes
Abbreviations: cIgAN = compound IgA nephropathy, DN = diabetic nephropathy, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, GBM = glomerular basement membrane, IgAN = IgA nephropathy, MN = membranous nephropathy, sIgAN = simple IgA nephropathy without compound glomerular diseases, TEM = transmission electron microscopy.
How to cite this article: Jiang L, Dong B, Yan Y, Zheng S, Hu Y, Zuo L, Shi H. Clinicopathological analysis of IgA nephropathy combined with other glomerular diseases. Medicine. 2019;98:41(e17388).
This work was supported by Peking University People's Hospital Research and Development Funds (RDY2017–16).
The authors have no conflicts of interest to disclose.
References
- [1].Lee SM, Rao VM, Franklin WA, et al. IgA nephropathy: morphologic predictors of progressive renal disease. Hum Pathol 1982;13:314–22. [DOI] [PubMed] [Google Scholar]
- [2].D’Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 2004;24:179–96. [DOI] [PubMed] [Google Scholar]
- [3].D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 1987;64:709–27. [PubMed] [Google Scholar]
- [4].Roberts IS. Pathology of IgA nephropathy. Nat Rev Nephrol 2014;10:445–54. [DOI] [PubMed] [Google Scholar]
- [5].Jiang L, Liu G, Lv J, et al. Concise semiquantitative histological scoring system for immunoglobulin A nephropathy. Nephrology (Carlton) 2009;14:597–605. [DOI] [PubMed] [Google Scholar]
- [6].Liu D, Huang T, Chen N, et al. The modern spectrum of biopsy-proven renal disease in Chinese diabetic patients-a retrospective descriptive study. PeerJ 2018;6:e4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen P, Shi SF, Qu Z, et al. Characteristics of patients with coexisting IgA nephropathy and membranous nephropathy. Ren Fail 2018;40:213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen X, Chen Y, Shi K, et al. Comparison of prognostic, clinical, and renal histopathological characteristics of overlapping idiopathic membranous nephropathy and IgA nephropathy versus idiopathic membranous nephropathy. Sci Rep 2017;7:11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hu R, Xing G, Wu H, Zhang Z. Clinicopathological features of idiopathic membranous nephropathy combined with IgA nephropathy: a retrospective analysis of 9 cases. Diagn Pathol 2016;11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sharma SG, Bomback AS, Radhakrishnan J, et al. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol 2013;8:1718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhuo L, Zou G, Li W, et al. Prevalence of diabetic nephropathy complicating non-diabetic renal disease among Chinese patients with type 2 diabetes mellitus. Eur J Med Res 2013;18:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roberts IS, Cook HT, Troyanov S, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int 2009;76:546–56. [DOI] [PubMed] [Google Scholar]
- [13].Coppo R, Troyanov S, Bellur S, et al. VALIGA study of the ERA-EDTA Immunonephrology Working Group. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 2014;86:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Trimarchi H, Barratt J, Cattran DC, et al. IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017;91:1014–21. [DOI] [PubMed] [Google Scholar]
- [15].Jennette JC, D’Agati VD, Olson JL, et al. Heptinstall's Pathology of the Kidney. 7th edPhiladelphia, PA: Lippincott Williams & Wilkins (LWW); 2014. [Google Scholar]
- [16].Jieyuan C, Hongwen Z. Childhood IgA nephropathy combined with Alport syndrome: a report of 2 cases and literature review. J Clin Pediatr 2017;35:9–12. [Google Scholar]
- [17].Li XW, Liang SS, Le WB, et al. Long-term outcome of IgA nephropathy with minimal change disease: a comparison between patients with and without minimal change disease. J Nephrol 2016;29:567–73. [DOI] [PubMed] [Google Scholar]
- [18].Qazi RA, Bastani B. Co-existence of thin basement membrane nephropathy with other glomerular pathologies; a single center experience. J Nephropathol 2015;4:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang YZ, Shi SF, Chen YQ, et al. Clinical features of IgA nephropathy with serum ANCA positivity: a retrospective case-control study. Clin Kidney J 2015;8:482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Herlitz LC, Bomback AS, Stokes MB, et al. IgA nephropathy with minimal change disease. Clin J Am Soc Nephrol 2014;9:1033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bantis C, Stangou M, Schlaugat C, et al. Is presence of ANCA in crescentic IgA nephropathy a coincidence or novel clinical entity? A case series. Am J Kidney Dis 2010;55:259–68. [DOI] [PubMed] [Google Scholar]
- [22].Tanaka M, Yamada S, Iwasaki Y, et al. Impact of obesity on IgA nephropathy: comparative ultrastructural study between obese and non-obese patients. Nephron Clin Pract 2009;112:c71–8. [DOI] [PubMed] [Google Scholar]
- [23].Amenomori M, Haneda M, Morikawa J, et al. A case of lipoprotein glomerulopathy successfully treated with probucol. Nephron 1994;67:109–13. [DOI] [PubMed] [Google Scholar]
- [24].Kusano T, Takano H, Kang D, et al. Endothelial cell injury in acute and chronic glomerular lesions in patients with IgA nephropathy. Hum Pathol 2016;49:135–44. [DOI] [PubMed] [Google Scholar]
- [25].Pan M, Zhang J, You X, et al. Renal outcomes in primary IgA nephropathy patients with segmental glomerular necrosis: a case-control study. Hum Pathol 2018;75:47–54. [DOI] [PubMed] [Google Scholar]
- [26].Shi SF, Wang SX, Jiang L, et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the Oxford classification. Clin J Am Soc Nephrol 2011;6:2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].An Y, Xu F, Le W, et al. Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol Dial Transplant 2015;30:257–66. [DOI] [PubMed] [Google Scholar]
- [28].Knoppova B, Reily C, Maillard N, et al. The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol 2016;7:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jiang L, Zhang JJ, Lv JC, et al. Malignant hypertension in IgA nephropathy was not associated with background pathological phenotypes of glomerular lesions. Nephrol Dial Transplant 2008;23:3921–7. [DOI] [PubMed] [Google Scholar]


