Supplemental Digital Content is available in the text
Keywords: COX-2 inhibitor, metformin, National Health Insurance Research Database, rheumatoid arthritis, type 2 diabetes mellitus
Abstract
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory autoimmune disease associated with increased prevalence of type 2 diabetes mellitus (T2DM). Here, we investigated the effect of the combination of cyclooxygenase (COX)-2 inhibitors and metformin on the rate of admission in patients with RA and T2DM and compared it with that of only COX-2 inhibitors.
In total, 1268 subjects with RA and T2DM under COX-2 inhibitor and metformin therapy were selected from the National Health Insurance Research Database of Taiwan, along with 2536 patients as 1:2 sex-, age-, and index year-matched controls without metformin therapy. Cox proportional hazard analysis was used to compare the rate of admission during the 10 years of follow-up.
At the end of the follow-up, 72 enrolled subjects (1.89%) had admission, including 9 from the combination group (0.71%) and 63 from the COX-2 inhibitor group (2.48%). The combination group was associated with a lower rate of admission at the end of follow-up (P < .001). Cox proportional hazard regression analysis revealed the lower rate of admission for subjects under combination therapy (adjusted hazard ratio of 0.275; 95% confidence interval = 0.136-0.557, P < .001).
Patients with RA and T2DM receiving the combination of COX-2 inhibitors and metformin were associated with lower admission rate than those on COX-2 inhibitors alone, and this effect may be attributed to the decrease in the levels of proinflammatory factors.
1. Introduction
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory autoimmune disease characterized with progressive articular damage, thereby causing joint deformities.[1] RA is more prevalent in women than in men and presents as polyarticular disease[2] that may affect the patient's capacity to perform physical activities.[3] Acute onset of polyarthritis is associated with myalgia and fatigue.[4]
Early recognition and treatment with disease-modifying anti-rheumatic drugs (DMARDs) are important to control disease progression.[5] Rapid diagnosis and control may increase remission rates in patients with RA.[6] Moura et al[7] suggested the association between longer exposure to either methotrexate or other DMARDs within the first year after RA diagnosis and longer time to joint replacement. In addition, cyclooxygenase-2 (COX-2) expression has been associated with synovial inflammation in arthritis,[8] and COX-2 inhibitors are known to provide therapeutic benefits.[9]
Several reports have discussed the association between chronic inflammatory disease and insulin resistance[10,11] as well as between multiple immunoregulatory components in RA, insulin resistance, and type 2 diabetes mellitus (T2DM).[12,13] A large percentage of patients with RA is affected with T2DM,[14] and a meta-analysis study revealed the statistically significant increase in the risk of DM prevalence among individuals with RA (odds ratio [OR] = 1.40, 95% confidence interval [CI]: 1.34–1.47).[15]
Several guidelines suggesting the use of metformin as the initial treatment for T2DM[16] have revealed the amelioration in chronic inflammation.[17] Our previous study showed the effect of the combination of metformin and celecoxib against adipose tissue inflammation.[18] In the present study, we clarify the effect of the combination of metformin and COX-2 inhibitor therapy in patients with RA and T2DM and investigate its association with admission or mortality rate using the data from a nationwide health insurance database, the Taiwan National Health Insurance Research Database (NHIRD).
2. Material and methods
2.1. Data sources
We used the data from NHIRD to investigate whether the combination of metformin and COX-2 inhibitor therapy, as compared to COX-2 inhibitor only, in patients with RA and T2DM could lower the admission over a 10-year period from the outpatient Longitudinal Health Insurance Database in Taiwan (2000–2010). The National Health Insurance (NHI) Program was launched in Taiwan in 1995, and as of June 2009, it included contracts with 97% of the medical providers in Taiwan with approximately 23 million beneficiaries or >99% of the entire population in Taiwan.[16] The NHIRD uses International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes to record diagnoses.[19] All diagnoses of T2DM were made by a board-certified medical specialist, and RA was confirmed by a rheumatologic specialist. The Bureau of NHI randomly reviews the records of 1 in 100 ambulatory care visits and 1 in 20 in-patient claims to verify the accuracy of diagnoses.[20] Several studies have demonstrated the accuracy and validity of diagnoses in the NHIRD.[21,22]
2.2. Study design and sampled participants
The present study is a retrospective matched-cohort design. Patients with diagnosed RA and T2DM were selected from January 1, to December 31, 2010 as per ICD-9-CM 714.XX (RA) and ICD-9-CM 250.XX (T2DM). In addition, each enrolled patient was required to have made at least 3 outpatient visits within the study period according to these ICD-9-CM codes under COX-2 inhibitors therapy with or without metformin therapy. The patients diagnosed with RA and/or T2DM before 2000 were excluded. In addition, the patients who received joint replacement surgery before tracking and those aged <18 years were excluded. From a total of 1972 enrolled patients, we excluded 704 patients to obtain 1268 subjects with RA and T2DM on COX-2 inhibitor and metformin therapy (case group). In addition, 2536 patients as 1:2 sex-, age-, and index year-matched controls without metformin therapy (control group) were included in this study (Fig. 1).
Figure 1.
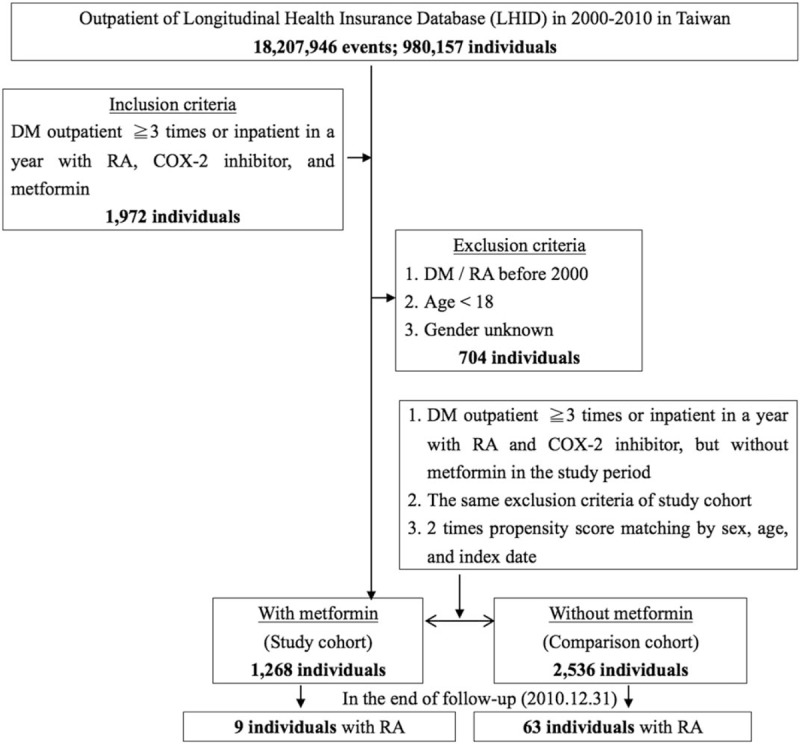
The flowchart of study sample selection from the National Health Insurance Research Database in Taiwan. COX-2 = inhibitor/Metformin: ≧90 days, DM = diabetes mellitus: ICD-9-CM 250, RA = rheumatoid arthritis: ICD-9-CM 714.
The covariates included sex, age, Charlson Comorbidity Index (CCI) removed T2DM, geographical area of residence (north, center, south, and east of Taiwan), urbanization level of residence (level 1 to 4), and monthly income (in New Taiwan Dollars [NTD]; <18,000, 18,000–34,999, ≥35,000). The urbanization level of residence was defined as per the population and various indicators of the level of development. Level 1 was defined as a population >1,250,000 and a specific designation as political, economic, cultural, and metropolitan development. Level 2 was defined as a population between 500,000 and 1249,999 with an important role in the political system, economy, and culture. Urbanization levels 3 and 4 were defined as a population between 149,999 and 499,999 and <149,999, respectively.[23]
2.3. Outcome measures
All study participants were followed from the index date until the onset of receiving joint replacement surgery from the NHI program before the end of 2010.
2.4. Statistical analysis
All analyses were performed using SPSS software version 22 (SPSS Inc., Chicago, IL). We used χ2 and t tests to evaluate the distribution of categorical and continuous variables, respectively. Multivariate cox proportional hazard regression analysis was carried out to determine the risk of receiving joint surgical replacement, and the results were present as hazard ratio (HR) with 95% CI. The difference in the risk of receiving joint surgical replacement between the study and control groups was estimated using the Kaplan–Meier method with the log-rank test. A 2-tailed P value <.05 was considered statistically significant.
2.5. Ethics
This study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The Institutional Review Board of Tri-Service General Hospital approved this study and waived the need for individual written informed consent (TSGH IRB No. 2-105-05-082).
3. Results
Of the total 3804 enrollees, 1268 were study subjects treated with metformin and 2536 were the 1:2 sex-, age-, and index year-matched controls. Overall, the subjects with RA and T2DM under COX-2 inhibitor and metformin combination treatment tended to show an association with a lower rate of admission than those on COX-2 inhibitor therapy alone (adjusted HR 0.275, 95% CI = 0.136–0.557, P < .001). Figure 2 shows the Kaplan–Meier analysis result for the cumulative risk of admission in case and control groups with statistically significant difference (log rank, P < .001). At the first year of the follow-up, the difference between the 2 groups was significant (log-rank test P < .001). However, no obvious difference in decreasing incidence of RA (Fig S1, log-rank test P = .849) and mortality rate (Fig S2, log-rank test P = .061) was observed between the control and case groups.
Figure 2.
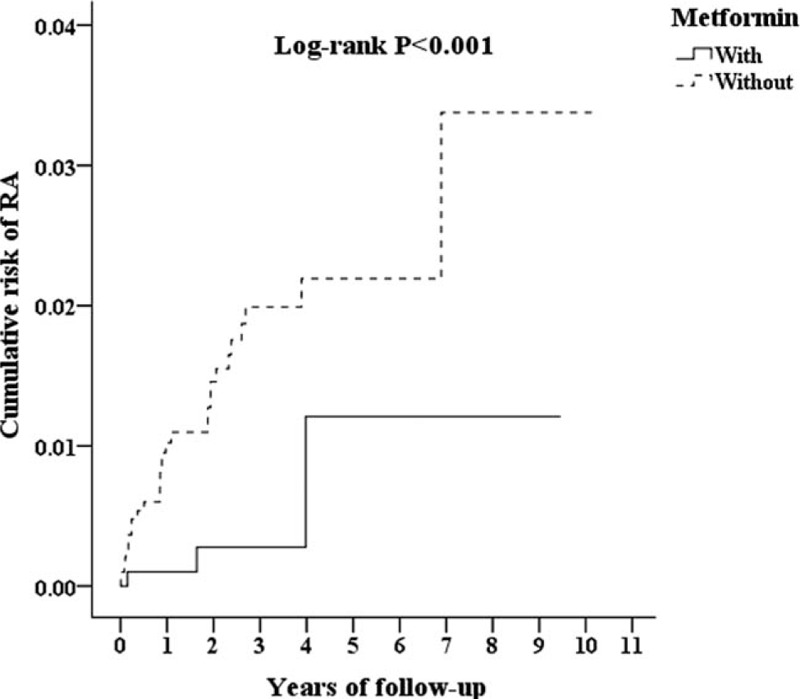
Kaplan–Meier analysis for the cumulative risk of RA among patients with DM and RA, and patients aged 18 years and over on COX-2 inhibitors stratified by metformin with the log-rank test. DM = diabetes mellitus, RA = rheumatoid arthritis
Table 1 shows the sex, age, comorbidities, location, urbanization, level of care, and income of the study subjects and controls. In comparison with the control group, the study subjects exhibited much higher CCI (0.84 vs 0.66, P < .001), a higher tendency to receive therapy in hospital centers (38.09% vs 28.90%, P < .001), and had lived longer in urbanized areas and northern areas of Taiwan (P < .001).
Table 1.
Baseline characteristics of the study population.
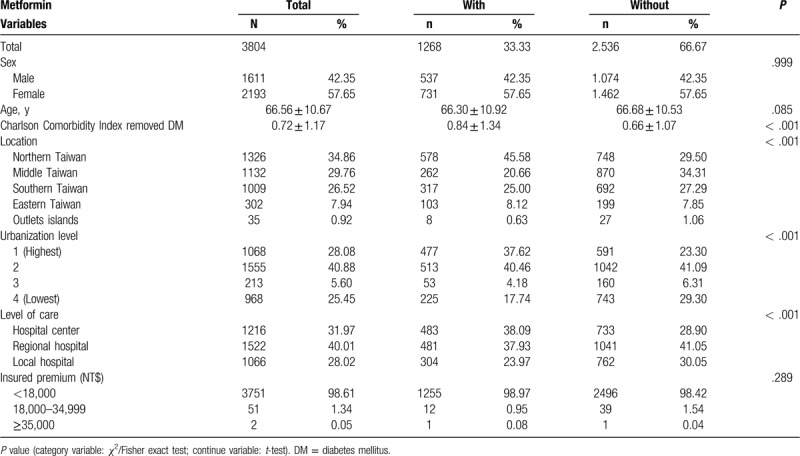
Table 2 shows that at the end of the follow-up period, 72 enrolled subjects (1.89%) had admission, including 9 in the case group (0.71%) and 63 in the control group (2.48%). The case group tended to be associated with a much lower rate of admission at the end of follow-up than the control group (P < .001). In comparison with the control group, the case group showed higher CCI (1.27 vs 1.13, P = .045), a much higher tendency to receive therapy in hospital centers (33.36% vs 29.26%, P < .001), and had lived longer in the northern and eastern areas of Taiwan (P < .001).
Table 2.
Characteristics of study population at the study endpoint.
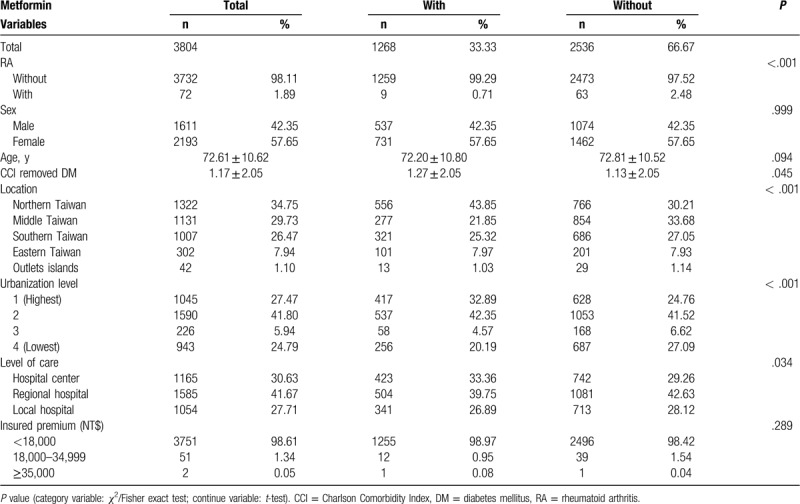
Table 3 shows the results of Cox regression analysis of the factors associated with the rate of admission. The study subjects under metformin therapy showed a tendency of association with a lower rate of admission (adjusted HR 0.275, 95% CI = 0.136–0.557, P < .001) and showed good adherence (>80%) of medication possession ratio (MPR) with a lower rate of admission (adjusted HR 0.567, 95% CI = 0.012–0.627, P = .015). Furthermore, the study subjects with older age and those who lived in non-Northern areas of Taiwan were associated with a lower rate of admission. Otherwise, the study subjects who received therapy in hospital centers were associated with a higher rate of admission.
Table 3.
Factors of RA evaluated using Cox regression analysis.
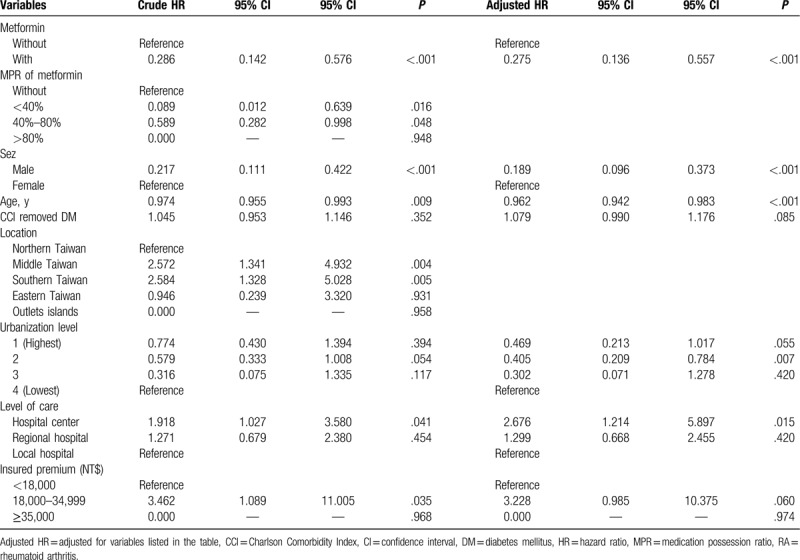
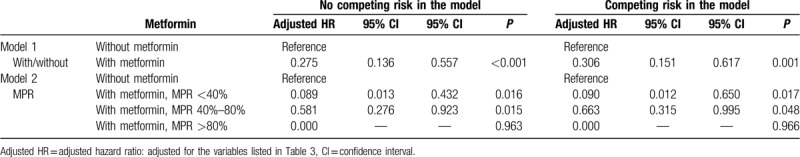
In the subgroups stratified by sex, urbanization, level of care, and monthly income, the female study subjects and those living in higher urbanization levels of residence, being treated in the regional hospitals, and with monthly insurance premiums of NT$ <18000 were associated with a lower risk of admission in the case group than in the control group (adjusted HR of 0.820 [P < .001], 0.252 [P = .028], 0.096 [P = .022], and 0.260 [P < .001], respectively, Table 4). Among patients on metformin treatment and stratified by MPR, the sensitivity test for factors of RA by using Cox regression showed those with MPR <40% and 40% to 80% were associated with lower admission rates (adjusted HR 0.089 [P = .016] and 0.581 [P = .015], respectively, Table 5) and Fine & Gray's competing risk model[24] showed those with MPR <40% and 40% to 80% were associated with lower admission rates (adjusted HR 0.090 [P = .017] and 0.663 [P = .048], respectively, Table 5).
Table 4.
Factors of RA stratified by variables listed in the table using Cox regression analysis.
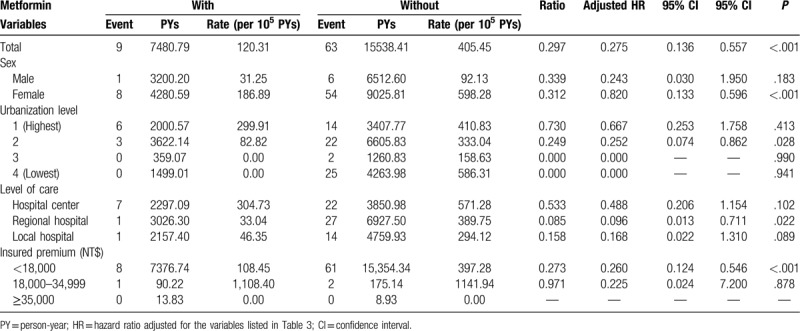
Table 5.
Sensitivity test for factors of RA by using Cox regression and Fine and Gray's competing risk model.
4. Discussion
Patients with RA exhibit impairments in health-related quality of life in comparison with age- and sex-matched populations without arthritis.[25] These patients have a higher burden of cardiovascular diseases, hypertension, diabetes, obesity, and hyperlipidemia as compared with healthy subjects.[26] Although the wide utilization of DMARDs and biologic agents has improved the quality of life of patients with RA, the rates of total hip arthroplasty, total knee arthroplasty, and other comorbidity remain high.[27] Both traditional nonsteroidal anti-inflammatory diseases and COX-2 inhibitors are commonly prescribed to relieve patients from pain and inflammation, and COX-2 inhibitors are associated with a lower incidence of symptomatic ulcers.[9,28] A previous study has shown a statistically significant increase in the risk of T2DM prevalence among individuals with RA.[15,29] One Italian cross-sectional cohort study showed increased prevalence of T2DM and impair fasting glucose of RA patients when compared with age- and sex-matched control individuals. Both RA-specific features, such as disease duration, corticosteroids exposure, and radiographic damage, and cardiovascular risk factors were significantly associated with glucose metabolism abnormalities.[30] The current guidelines from the American Diabetes Association recommend early initiation of metformin as a first-line drug for monotherapy in patients with T2DM.[16] However, no studies have investigated the association between the combination of COX-2 inhibitors and metformin therapy in patients with RA and T2DM and admission or mortality rates.
We found that the patients with RA and T2DM under COX-2 inhibitor and metformin therapy were associated with lower admission rates than those on COX-2 inhibitors only. Even after adjustment for comorbidities and other covariates, the overall adjusted HR was 0.275 (P < .001). Kaplan–Meier analysis result revealed that the study subjects had a significantly lower 10-year risk of admission than the controls. In addition, it took only 1 year to achieve a significantly adjusted HR. However, in comparison with the control group, the case group had no obvious difference in decreasing incidence or mortality rate. Our study is the first report to indicate that patients with RA and T2DM under the combination of COX-2 inhibitors and metformin were associated with lower admission risks in a nationwide, population-based study.
Inflammatory disorders are associated with increased risks of cardiometabolic events, which may vary with anti-inflammatory therapy and duration.[29] A recent study showed that RA was highly associated with multiple risks of cardiometabolic diseases (RR 1.70, 95% CI = 1.59 to 1.83).[31] In addition, RA is associated with the same risk of myocardial infarction (MI) as T2DM, and the risk of MI in patients with RA generally corresponds to that in non-RA subjects older than 10 years.[32] Despite the increased risk, the relatively modest absolute numbers of cardiovascular events in patients with RA present a challenge to investigators.[33] One study showed a high proportion (7.1%) of patients in this single-center cohort developed new-onset T2DM in RA patients in the short-term thus suggesting a possible synergistic interaction between uncontrolled disease activity and glucose metabolism derangement in determining a worse cardiovascular phenotype of RA patients with comorbid T2DM.[34] Hence, there is a need to rely on biomarkers of disease activity or burden to probe disease mechanisms or develop treatment regimens.[35]
Recent article emphasize the evidence effect of metformin on immunological mechanisms related to the development and maintenance of autoimmunity and its potential relevance in treatment of autoimmune diseases.[36] Metformin demonstrated anti-inflammatory properties on macrophages via AMP-dependent and -independent mechanisms and active RA synovial tissue shows abundance of M1 macrophages[18] where treatment with metformin resulted in reduced serum levels of interleukin-6 and tumor necrosis factor (TNF)-α and in the AMPK-mediated modulation of macrophage polarization with a shift toward an anti-inflammatory M2 phenotype.[37] One study evaluated the effect of metformin on collagen antibody-induced arthritis, a well-established animal model of RA resulted in a significant improvement of arthritis score, with reduced bone destruction, inflammatory cytokines production associated with the AMPK/mTOR-mediated inhibition of STAT3 signaling.[38]
Our previous study revealed the additional effect of metformin and celecoxib against lipid dysregulation and adipose tissue inflammation in high-fat fed rats with insulin resistance and fatty liver.[39] The combination therapy with celecoxib and metformin resulted in the reduction in macrophage infiltration rate and decreased the levels of TNF-α, monocyte chemoattractant protein-1, and leptin in the adipose tissue of high-fat fed rats. Furthermore, the combination of COX-2 inhibitor and metformin could be associated lower rates of joint replacement in patients with osteoarthritis and T2DM.[40] The pro-inflammatory factors in patients receiving combination therapy have been already investigated in preclinical and clinical studies and synthetized in systematic reviews,[41–43] a growing body of evidence is investigating the possible benefits of anti-inflammatory therapies beyond the joints in RA.
We, therefore, hypothesize that the patients with RA and T2DM on the combination therapy with COX-2 inhibitors and metformin may show a decrease in these inflammatory factors, and could be associated with reduced admission and mortality rates than those on COX-2 inhibitors only. In addition, the combination therapy may have additional effects against the inflammatory factors in RA, resulting in a decrease in admission rates.
However, the reasons why females, subgroups living in higher urbanized areas and receiving therapy in hospital centers, and those with lower monthly insurance premiums showed association with lower rates for joint replacement surgery are unknown, and may warrant further studies.
The present study has a few limitations. First, our study lacks the analysis of the disease duration, disease severity, and patient's perception. The patients with RA or T2DM could be identified using the insurance claim data; however, the data on severity, disease duration, and effect on diabetes (as HbA1c level) were not available. Clinical and demographic factors associated with change and maintenance of disease severity in a large registry of patients with RA indicate that a substantial proportion of patients remain in moderate disease, emphasizing the need for treat-to-target strategies for RA patients.[44] Second, medical treatment may be effective by decreasing inflammatory factors; however, the details regarding RA assessment were not available in the NHIRD. Furthermore, the Eun et al's study[45] showed a common disease in a case–control study of a 1:4 case–control ratio is one way to achieve higher statistical power. Our study used the 1:2 ratio for the 2 groups because the groups of study design criteria condition cannot fit the 1:4 ratio then we adjusted the ratio of 1:2. Finally, a longer follow-up period may be necessary to clarify the admission or mortality risk for patients.
5. Conclusion
Patients with T2DM are at a higher risk of developing RA than those without T2DM. The combination of COX-2 inhibitors and metformin therapy in patients with RA and T2DM was associated with lower admission rates than single metformin therapy. Although the mechanisms responsible for this association are still unclear, inflammatory factors may contribute to the decrease in admission rates. Further studies are warranted to evaluate the mechanism underlying the decreased risk of RA with T2DM.
Author contributions
Conceptualization: Chieh-Hua Lu, Chien-Hsing Lee, Sheng-Chiang Su, Jhih-Syuan Liu, Fu-Huang Lin, Chang-Huei Tsao, Po-Shiuan Hsieh, Yi-Jen Hung, Chang-Hsun Hsieh, Wu-Chien Chien.
Data curation: Chi-Hsiang Chung.
Formal analysis: Chi-Hsiang Chung.
Writing – original draft: Chieh-Hua Lu.
Writing – review & editing: Chang-Hsun Hsieh, Wu-Chien Chien.
Supplementary Material
Footnotes
Abbreviations: CCI = Charlson Comorbidity Index, CI = confidence interval, COX-2 = cyclooxygenase-2, DMARD = disease-modifying anti-rheumatic drug, HR = hazard ratio, LHID = Longitudinal Health Insurance Database, MCP-1 = monocyte chemoattractant protein, MI = myocardial infarction, MPR = medication possession ratio, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, RA = rheumatoid arthritis, T2DM = type 2 diabetes mellitus, TNF-α = tumor necrosis factor alpha.
How to cite this article: Lu CH, Chung CH, Lee CH, Su SC, Liu JS, Lin FH, Tsao CH, Hsieh PS, Hung YJ, Hsieh CH, Chien WC. Combination of COX-2 inhibitor and metformin attenuates rate of admission in patients with rheumatoid arthritis and diabetes in Taiwan. Medicine. 2019;98:41(e17371).
This study was funded by Tri-Service General Hospital Research Foundation (TSGH-C104-122, TSGH-C105-119, TSGH-C106-002, TSGH-C106-098, TSGH-C107-104, TSGH-C108-003).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017;389:2328–37. [DOI] [PubMed] [Google Scholar]
- [2].Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001;358:903–11. [DOI] [PubMed] [Google Scholar]
- [3].Cooney JK, Law RJ, Matschke V, et al. Benefits of exercise in rheumatoid arthritis. J Aging Res 2011;2011:681640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cojocaru M, Cojocaru IM, Silosi I, et al. Extra-articular Manifestations in Rheumatoid Arthritis. Maedica 2010;5:286–91. [PMC free article] [PubMed] [Google Scholar]
- [5].Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet 2017;389:2338–48. [DOI] [PubMed] [Google Scholar]
- [6].van Nies JA, Krabben A, Schoones JW, et al. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis 2014;73:861–70. [DOI] [PubMed] [Google Scholar]
- [7].Moura CS, Abrahamowicz M, Beauchamp ME, et al. Early medication use in new-onset rheumatoid arthritis may delay joint replacement: results of a large population-based study. Arthritis Res Ther 2015;17:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lipsky PE, Isakson PC. Outcome of specific COX-2 inhibition in rheumatoid arthritis. The Journal of rheumatology Supplement 1997;49:9–14. [PubMed] [Google Scholar]
- [9].Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000;284:1247–55. [DOI] [PubMed] [Google Scholar]
- [10].Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 2003;3:745–56. [DOI] [PubMed] [Google Scholar]
- [11].Firestein GS. The T cell cometh: interplay between adaptive immunity and cytokine networks in rheumatoid arthritis. J Clin Invest 2004;114:471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bokarewa M, Nagaev I, Dahlberg L, et al. Resistin, an adipokine with potent proinflammatory properties. J Immunol 2005;174:5789–95. [DOI] [PubMed] [Google Scholar]
- [13].Aggarwal BB. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum Dis 2000;59Suppl 1:i6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Giacomelli R, Ruscitti P, Alvaro S, et al. IL-1beta at the crossroad between rheumatoid arthritis and type 2 diabetes: may we kill two birds with one stone? Expert Rev Clin Immunol 2016;12:849–55. [DOI] [PubMed] [Google Scholar]
- [15].Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: a systematic review and meta-analysis. Clin Exp Rheumatol 2015;33:115–21. [PubMed] [Google Scholar]
- [16].Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med 2017;166:572–8. [DOI] [PubMed] [Google Scholar]
- [17].Diaz A, Romero M, Vazquez T, et al. Metformin improves in vivo and in vitro B cell function in individuals with obesity and type-2 diabetes. Vaccine 2017;35:2694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Laria A, Lurati A, Marrazza M, et al. The macrophages in rheumatic diseases. J Inflamm Res 2016;9:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Association CH: ICD-9-CM English-Chinese Dictionary. Taipei, Taiwan: Chinese Hospital Association Press; 2000. [Google Scholar]
- [20].Guilherme A, Virbasius JV, Puri V, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chang CH, Toh S, Lin JW, et al. Cancer risk associated with insulin glargine among adult type 2 diabetes patients–a nationwide cohort study. PLoS One 2011;6:e21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rau HH, Hsu CY, Lin YA, et al. Development of a web-based liver cancer prediction model for type II diabetes patients by using an artificial neural network. Comput Methods Programs Biomed 2016;125:58–65. [DOI] [PubMed] [Google Scholar]
- [23].Chang CY, Chen WL, Liou YF, et al. Increased risk of major depression in the three years following a femoral neck fracture—a national population-based follow-up study. PLoS One 2014;9:e89867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Andersen PK, Geskus RB, de Witte T, et al. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Managed Care 2007;13suppl 9:S237–51. [PubMed] [Google Scholar]
- [26].Hitchon CA, Boire G, Haraoui B, et al. Self-reported comorbidity is common in early inflammatory arthritis and associated with poorer function and worse arthritis disease outcomes: results from the Canadian Early Arthritis Cohort. Rheumatology 2016;55:1751–62. [DOI] [PubMed] [Google Scholar]
- [27].Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manage Care 2008;14:234–54. [PubMed] [Google Scholar]
- [28].Scott DL, Wolfe F, Huizinga TW. Rheum Arthritis. Lancet 2010;376:1094–108. [DOI] [PubMed] [Google Scholar]
- [29].Dregan A, Charlton J, Chowienczyk P, et al. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 2014;130:837–44. [DOI] [PubMed] [Google Scholar]
- [30].Ruscitti P, Ursini F, Cipriani P, et al. Prevalence of type 2 diabetes and impaired fasting glucose in patients affected by rheumatoid arthritis: results from a cross-sectional study. Medicine 2017;96:e7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dregan A, Chowienczyk P, Molokhia M. Cardiovascular and type 2 diabetes morbidity and all-cause mortality among diverse chronic inflammatory disorders. Heart 2017;103:1867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lindhardsen J, Ahlehoff O, Gislason GH, et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 2011;70:929–34. [DOI] [PubMed] [Google Scholar]
- [33].Peters MJ, Nurmohamed MT. Cardiovascular risk management in rheumatoid arthritis: are we still waiting for the first step? Arthritis Res Ther 2013;15:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ruscitti P, Ursini F, Cipriani P, et al. Poor clinical response in rheumatoid arthritis is the main risk factor for diabetes development in the short-term: a 1-year, single-centre, longitudinal study. PLoS One 2017;12:e0181203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 2015;36:482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ursini F, Russo E, Pellino G, et al. Metformin and autoimmunity: a “new deal” of an old drug. Front Immunol 2018;9:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jing Y, Wu F, Li D, et al. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mo Cell Endocrinol 2018;461:256–64. [DOI] [PubMed] [Google Scholar]
- [38].Kang KY, Kim YK, Yi H, et al. Metformin downregulates Th17 cells differentiation and attenuates murine autoimmune arthritis. Int Immunopharmacol 2013;16:85–92. [DOI] [PubMed] [Google Scholar]
- [39].Lu CH, Hung YJ, Hsieh PS. Additional effect of metformin and celecoxib against lipid dysregulation and adipose tissue inflammation in high-fat fed rats with insulin resistance and fatty liver. Eur J Pharmacol 2016;789:60–7. [DOI] [PubMed] [Google Scholar]
- [40].Lu CH, Chung CH, Lee CH, et al. Combination COX-2 inhibitor and metformin attenuate rate of joint replacement in osteoarthritis with diabetes: A nationwide, retrospective, matched-cohort study in Taiwan. PLoS One 2018;13:e0191242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ruscitti P, Ursini F, Cipriani P, et al. IL-1 inhibition improves insulin resistance and adipokines in rheumatoid arthritis patients with comorbid type 2 diabetes: an observational study. Medicine 2019;98:e14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ursini F, Russo E, Ruscitti P, et al. The effect of non-TNF-targeted biologics and small molecules on insulin resistance in inflammatory arthritis. Autoimmun Rev 2018;17:399–404. [DOI] [PubMed] [Google Scholar]
- [43].Ruscitti P, Cipriani P, Di Benedetto P, et al. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1beta via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3(NLRP3)-inflammasome activation: a possible implication for therapeutic decision in these patients. Clin Exp Immunol 2015;182:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Reed GW, Collier DH, Koenig AS, et al. Clinical and demographic factors associated with change and maintenance of disease severity in a large registry of patients with rheumatoid arthritis. Arthritis Res Ther 2017;19:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hong EP, Park JW. Sample size and statistical power calculation in genetic association studies. Genomics Inform 2012;10:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


