Abstract
Background:
Traditional treatment of functional dyspepsia (FD) is unsatisfactory in a subgroup of patients with FD, and the potential role of antidepressant medications also has not been definitely clarified. To provide more evidence for future optimal practice recommendations, we reviewed a 1-year clinical database of antidepressant agents applied in outpatients with FD.
Methods:
Clinical presentations, treatment course, and outcomes were determined by chart review of patients referring to the functional gastrointestinal disorders specialist clinic. One hundred thirty patients with FD were included for further analysis.
Results:
Patients were treated with different antidepressant drugs according to individual symptoms. The most commonly used drugs were flupenthixol melitracen and fluoxetine. Improvement and complete remission occurred in 93.8% and 54.6% of patients, respectively. There was a trend toward superior outcome for citalopram compared to sulpiride and mirtazapine in overall analysis. Meanwhile, regimens containing fluoxetine had significant increased remission rate compared to any other antidepressant regimens in postprandial distress syndrome subgroup analysis. Furthermore, older patients were more likely to achieve remission. However, sex and symptom duration were not associated with symptom remission. Finally, 11.5% of patients experienced adverse events.
Conclusions:
This retrospective cohort study indicated that small dose antidepressant therapy, especially citalopram and fluoxetine, is an effective and well tolerated treatment option for refractory FD.
Keywords: adverse effect, antidepressant, functional dyspepsia, remission
1. Introduction
Functional dyspepsia (FD) is a widely recognized functional gastrointestinal disorder (FGID) characterized by epigastric pain, meal-related fullness or satiety, and absence of an organic cause that readily explains them.[1] The prevalence of uninvestigated dyspepsia or FD, diagnosed by Rome III criteria, has been reported to be 5.3% to 20.4% in the general population.[2] There are 2 FD subtypes according to Rome foundation: epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS).[3]
The diverse clinical manifestations and uncertain pathophysiological mechanisms made it difficult to choose proper medication to manage the condition. Standard treatments include dietary modifications, proton pump inhibitor (PPI), antispasmodics, and prokinetics, which were applied based on predominant symptoms.[4] Majority of the patients with FD seek for medical care continuously because of limited benefits from traditional therapies, which induces considerably impaired quality of life, poor work productivity, and high economic burden on society.[4–7]
Because the current treatment of FD is often unsatisfactory and challenging, many patients with FD refer to alternative therapies. With central perception of peripheral visceral events disturbed in FD, psychiatric illness and manifestation of somatization are prevalent in patients with FGIDs.[8] Furthermore, it has been revealed that PDS is independently associated with psychopathological factors.[9] As a result, the role of antidepressant medications in FD has been investigated in recent researches.[10–13] Therapy aimed at improving mental status and quality of life have been a valuable intervention in FD patients.[14]
The efficacy of antidepressant drugs on FD was inconsistent in different randomized controlled trials (RCTs). A few previous studies have identified positive efficacy of some antidepressant mediations, including reducing dyspepsia symptom score, improving quality of life, and relieving anxiety and depression.[10,15] A recent systematic review and meta-analysis of 13 RCTs in FD did not draw a firm conclusion for the efficacy of antidepressants, because it showed the effect appeared to be limited to antipsychotics and tricyclic antidepressants (TCADs), with fewer trials for other agents.[16] More investigation is required to address this uncertainty.
In our hospital, we have observed successful attempts in using antidepressants to treat patients with refractory FD. We performed a retrospective review to validate this clinical experience and quantify its effect on patients with refractory FD.
2. Methods
This retrospective study evaluated the efficacy and safety of antidepressant medications for management of annoying symptoms of FD. Informed consent from patients was not required as patients’ records were reidentified and anonymized before data analysis. The study was approved by the review board of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University (No. 2017-271-01).
2.1. Subjects
Outpatient electronic medical records were reviewed to identify patients who were referred to the FGID specialist clinic of Sir Run Run Shaw Hospital in 2016. Several criteria were used to determine if the patients with FD were suitable candidates to be included in the review. Adult patients, aged 18 years and older, diagnosed FD (identified by Rome III criteria) were selected for the study if they had initially received antidepressant medication between January 1, 2016 and December 31, 2016.[17] All patients had undergone appropriate gastrointestinal (GI) diagnostic tests (e.g., endoscopy, abdominal ultrasonography, blood, and stool studies) and organic GI diseases that may be a possible explanation for the symptoms had been ruled out before treatment. There was at least 1 follow-up interview after initiation of antidepressant therapy. Subjects with other GI conditions that potentially explain their symptoms or those presented with additional organic GI diseases (e.g., peptic ulcer, reflux esophagitis, inflammatory bowel disease) were excluded from this study. Meanwhile, subjects with active psychiatric disease requiring antidepressants or other ongoing psychiatric interventions were not included either.
After chart review completed, the symptom patterns of included patients were divided into 3 groups as defined by the patients’ most predominant complaint at their initial visit: PDS, EPS, or both.[17]
2.2. Antidepressant therapy
The type and dose of antidepressants were not systematically controlled, and the dosage of medication could be adjusted in the serial of clinical visits for each individual. Patients were treated with different antidepressant agents and dosage according to individual illness features. Patients with abdominal pain were more likely to be given flupenthixol melitracen, while with fullness were more likely to be given sulpiride. Some patients were given regimens combining antidepressants and antipsychotics. Initially, very small dose antidepressants were prescribed, which were gradually increased to certain level of small dose in the follow-up interviews according to the symptom remission percentage and drug tolerance. In cases of slight improvement, strategies such as increasing dosage of monotherapy, or the combination of antidepressants were employed. Accompanying symptom such as poor appetite, solid, or loose stool would also influence the medication selection. Medication adjustment was recorded and categorized by unsatisfactory response, unacceptable side effects or other reasons. PPI, prokinetics, or so were sometimes used as complementary medication for additional symptom relief.
2.3. Assessment of symptoms relief
To measure the response of antidepressant therapy, an established 4-point Likert-type scale (0, no improvement or worse; 1, slight improvement, but requiring more tests or treatment adjustment; 2, moderate improvement, stable regimen but not completely resolved, no further evaluation; 3, clinical remission, near-complete resolution of symptoms, complete satisfaction with treatment) was used in the serial clinical follow-up records.[18] Any other complementary therapy or intervention that may increase symptom relief was recorded.
2.4. Study outcomes
The primary outcome of this current study was to determine symptomatic outcome in a cohort of subjects with FD treated with antidepressant therapies, and to determine if the outcome varied depending on the specific medication administration, symptom manifestation, or other clinical features. Secondary outcomes were to evaluate adverse effects of antidepressant therapy and long-term remission.
2.5. Data collection
Data were extracted from the Sir Run Run Shaw outpatient electronic medical information system and entered into a database by 2 investigators independently. Patient demographics, clinical symptoms, medication history, GI diagnostic tests, antidepressant therapy, and adverse effects were collected. Antidepressant treatment information included the type and dose used, treatment response, and self-reported adherence. Any disagreement was resolved by a third researcher. Thirty months after initial treatment were followed up with telephone interview to observe the disease recurrence rate and medication compliance.
2.6. Statistical analysis
Throughout the manuscript, “improvement” indicated a response rating ≥1 and included the remitted subjects (response rating = 3). Age of individuals and duration of FD symptoms were transformed to the dichotomous variables (young vs old, short vs long) by the median for further analysis. Chi-square test was used to evaluate response rate between different medications. Univariate analysis was used to assess differences between subjects with or without clinical response in demographic and illness features. Logistic regression analysis was performed to determine the independent effects of demographic data and clinical features in predicting response with antidepressant therapy. A 2-tailed value of P < .05 was considered as statistical significance. All statistical analyses were performed using SPSS version 22.
3. Results
3.1. Subjects
Among the total patients (n = 1524) referred to this specialist clinic for refractory GI symptoms (e.g., abdominal pain, bloating), 9.4% (n = 144) patients met the inclusion criteria for study cohort, 49.9% (n = 761) were excluded because of symptom remission by routine treatment (e.g., PPI, prokinetics, antispasmodics, probiotics, digestive enzyme), 5.6% (n = 86) were excluded for presence of other FGIDs, and 35.1% (n = 533) were excluded for presence of organic diseases (e.g., inflammatory bowel disease, peptic ulcer, reflux esophagitis).
A total of 144 patients meeting inclusion criteria were identified for further review. Fourteen patients were subsequently excluded for failure to follow-up after initiation of antidepressant therapy and 130 cases were available for final analysis (Fig. 1). All of the 130 patients with FD were unsuccessfully managed with routine therapies. The mean patient age was 50.5 years with a range of 18 to 83 years, and 88 patients (67.7%) were female. The average body mass index (BMI) of included patients was 21.2. The incidence of hyperlipidemia, hypertension, and diabetes mellitus in the included patients was 3.4%, 11.4%, and 2.3% respectively.
Figure 1.
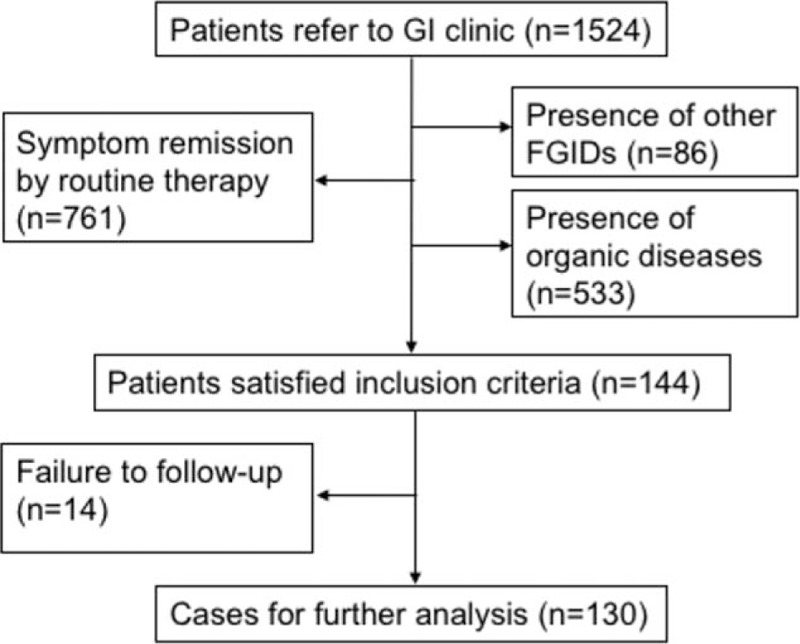
Flow diagram of assessment of patients identified in this retrospective study. FGID = functional gastrointestinal disorder.
Among the included patients, 38 patients (29.2%) had epigastric pain-predominant symptoms, 64 (49.2%) had postprandial distress-predominant symptoms, and the remaining 28 (21.6%) complained of both abdominal pain and fullness. More prevalence of PDS in China was consistent with previous research.[12] The mean duration of GI symptoms before initial evaluation was 4.3 ± 0.3 years. Nine patients had definite life events (e.g., divorce, relative's death) before onset of dyspeptic symptoms.
3.2. Treatment response
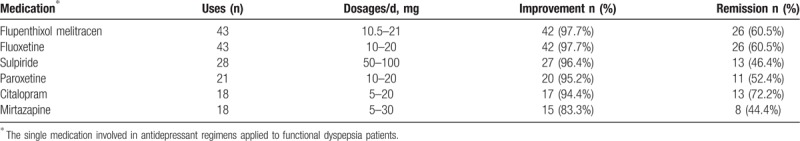
Thirty-three subjects (25.4%) of all had been treated with only 1 antidepressant, 91 (70%) were treated with 2, and 6 (4.6%) had changed medication consecutively. Antidepressant agents, in descending order of referring frequency were flupenthixol melitracen, fluoxetine, sulpiride, paroxetine, citalopram, mirtazapine, sertraline, duloxetine, amitriptyline, and venlafaxine. The selection of specific medications for different patients was on the basis of the individual psychiatric features. The most commonly applied regimens were flupenthixol melitracen plus fluoxetine and sulpiride plus fluoxetine. The dosages employed were smaller than regular use in department of mental health.[19]
Following initiation of antidepressants therapy, symptom improvement was obtained in 122 patients (93.8%) at certain time in follow-up (within 1 year): symptoms remission (score = 3) occurred in 71 patients (54.6%), and the average time to obtain remission was 3.4 months; moderate improvement (score = 2) was obtained in 33 patients (26.2%). Once remission was achieved, only 3 patients relapsed after tapering the dose. Clinical response of antidepressant treatment applied in 10 or more patients is listed in Table 1. There was a trend toward superior outcome for citalopram compared to sulpiride (Chi-square 2.97, P = .085) and mirtazapine (Chi-square 2.86, P = .091), whereas no difference of remission rate was found between other medications. In addition, remission rate was not related with patient BMI or metabolic diseases. Complete symptom remission occurred in 46.9% of subjects with PDS versus 57.9% for subjects with EPS and 67.9% with both PDS and EPS. In PDS subgroup analysis, regimens contained fluoxetine had significant increased remission rate compared to any other antidepressant regimens (Chi-square 7.519, P = .006), whereas there was no difference of remission rate between medications based on EPS subgroup or symptom patterns. Overall, the patients reported a high degree of satisfaction with antidepressant treatments.
Table 1.
Clinical response rates during treatment with antidepressant medications.
When subjects with symptom remission were compared with the remainder, young patients tended to lead a poorer outcome (P = .065). Furthermore, when given sulpiride or fluoxetine, patients with younger age were associated with a poorer outcome (P = .001 and P = .005, respectively).
However, sex and symptom duration were not associated with response to antidepressants. Logistic regression analysis did not identify any independent predictors of favorable outcomes.
3.3. Adverse events
There were 15 (11.5%) of 130 patients experienced adverse events. No serious adverse events were reported and the most frequently encountered side effects were as follows: drowsiness occurred in 5 patients, dry mouth in 4 patients, dizziness in 4 patients, and weakness in 7 patients. Some of these patients experienced more than 1 kind of side effects and these side effects occurred in various different antidepressant or combination therapy. Only 3 persons required a change in medications because of intolerable drowsiness or dry mouth.
3.4. Long-term follow-up
In the 30-month telephone follow-up of 92 accessible patients, 19 (20.6%) patients with poor compliance had stopped the medication before finishing the full course of treatment because of tolerable symptom or not fully satisfactory effect, the rest 73 (79.4%) patients finishing the full course of treatment with the average course of treatment of 10.4 months. Thirty-four (37%) patients had symptom remission without relapse, whereas 39 (42.4%) patients experienced the symptom relapse (1–30) months after they finished the full course of treatment, with mean relapse duration of 9 months, requiring another course of therapy. Logistic regression analysis did not identify any independent predictors for relapse among medication duration, symptom relief scale, age, sex, BMI, disease duration, or metabolic diseases.
4. Discussion
In present study, we reviewed a 1-year clinical experience to explore whether antidepressant therapies were valuable for treatment of patients with FD. This study provided real-world insights for future randomized trials in the application of antidepressants for FD. The findings from this retrospective review showed the benefit of antidepressants in treating refractory FD patients who were unresolved by traditional therapies. Four major findings were identified for future clinical treatment: antidepressants achieved a high response rate in patients with FD; citalopram had a trend toward to be superior to sulpiride and mirtazapine in overall analysis, whereas regimens that contained fluoxetine had significant increased remission rate compared to any other antidepressant regimens in PDS subgroup analysis; small dosages of antidepressants were employed and few adverse effects occurred during improvement; and older patients seemed to have a better outcome.
This observation appeared to highlight the role of visceral afferent mechanisms in patients with FD. These demographic features were similar to those from prior reports of patients with FD.[12,20] Patients with FD have been found to display visceral hypersensitivity and abnormal central processing of pain.[21,22] In hypersensitive patients with FD, anxiety is significantly and negatively correlated with discomfort threshold, pain threshold, and compliance.[23] It has been identified that the receptors of many psychotropic medications including selective serotonin reuptake inhibitor (SSRI), 5-hydroxytryptamine (5-HT)-1A receptor agonists, and TCADs are located throughout the brain and GI tract, and their ability on modulating depression remission, motor function, and visceral pain perception contributes to the efficacy of neuromodulators in patients with FD.[24,25] It seems that antidepressants may reduce the severity of comorbid psychological symptoms, including anxiety and depression which may exacerbate symptoms.[16] SSRI is a class of antidepressants, and has been approved to reduce somatization and improve effective response to chronic visceral pain, which are independent of its antidepressive actions.[26] Antidepressants are also associated with sleep and gastric accommodation restoration.[27–29] As a result, these drugs have been proposed as potential therapies for FD.
A meta-analysis has been conducted to assess the effects of psychotropic drugs compared with placebo on FD symptoms and adverse events.[16] Because of the different drugs of 5-HT-1A receptor agonists used or few trials included, the results were conflicting. The dosage of psychotropic drugs used in those studies was much larger than those we employed in our gastroenterology clinic for patients with FD. That's the reason much higher adverse effects occurrence rate (21.9%) was reported, which led to high drop-out rate in these trials. Moreover, whether psychotropic treatments were more effective than established drugs, such as PPI, could not be concluded from that meta-analysis.
Our study has identified that psychotropic treatments is credible in management of FD symptoms. Because the included patients of this study were those who have been unsuccessfully managed with PPIs and prokinetics, this study indicated that antidepressants may be more effective than traditional therapies. We also noted that improvement and remission of FD symptoms occurred with small dosages of antidepressant medications, so that larger dosages usually used for psychiatric disorders were not necessary.[4] Furthermore, employment of low dosages obviously minimized the side effects that are likely to interfere with compliance and efficacy measures. Efforts must be taken by the physician to have a good explanation at the initiation of therapy because most patients have negative connotations of antidepressants. Moreover, this study also suggested younger patients were less likely to get remission than older patients. Differences in their pressure and stress exposure, lifestyle, and compliance may partially explain this observation; however, further investigation is warranted. In this perspective, in addition to the optimized antidepressants, the study calls for the comprehensive care and intervention for the younger patients.
Furthermore, clinical response during the trial seemed to be associated with antidepressant regimens and symptom patterns. Flupenthixol melitracen and fluoxetine were mostly used for their relatively higher remission rate and widely employed in outpatient. Previous clinical studies focused on only 1 or 2 antidepressants for patients with FD, and have found that certain drugs (mirtazapine, amitriptyline) can significantly improve symptom (early satiety, pain).[10,30,31] In our study, citalopram was found to be superior to sulpiride and mirtazapine in overall analysis and regimens containing fluoxetine was superior to any other antidepressant regimens in PDS subgroup analysis. Furthermore, long-term follow-up indicated that the small dose antidepressant could help more than one third of the patient with FD who do not benefit from routine treatment remain remitted for >30 months. Thus, this observation provided evidence for the future application of these medications.
However, there were some limitations to this study. Firstly, this retrospective chart review meant that not all patients had systematic questionnaires to address psychiatric disorders. Secondly, different antidepressant regimens, dosing variety, and patient follow-up intervals were inherent to the retrospective study considerably weakened the conclusion. Finally, complementary taking of PPI, prokinetics, or other medication could be a confounding factor, even when they were firstly proved insufficient in symptom relief from the prior routine therapy.
In summary, this retrospective cohort study indicates that small-dose antidepressant therapy is an effective and well tolerated treatment option to improve symptom in refractory FD, resulting in great patient satisfaction and minimal adverse effects. Notable effects of citalopram in patients with FD and fluoxetine in PDS subgroup were observed. More efforts should be made to expand access to antidepressants and validate its efficacy and safety, considering its potential for managing the diverse and refractory symptoms of FD.
Author contributions
Conceptualization: Ning Dai.
Data curation: Liang Luo, Jinhua Shen, Mengsha Cen.
Methodology: Liang Luo, Lijun Du.
Resources: Lijun Du.
Validation: Jinhua Shen.
Writing – original draft: Lijun Du.
Writing – review and editing: Liang Luo, Ning Dai.
Footnotes
Abbreviations: 5-HT = 5-hydroxytryptamine, BMI = body mass index, EPS = epigastric pain syndrome, FD = functional dyspepsia, FGID = functional gastrointestinal disorder, GI = gastrointestinal, PDS = postprandial distress syndrome, PPI = proton pump inhibitor, RCT = randomized controlled trial, SSRI = selective serotonin reuptake inhibitor, TCAD = tricyclic antidepressant.
How to cite this article: Luo L, Du L, Shen J, Cen M, Dai N. Benefit of small dose antidepressants for functional dyspepsia. Medicine. 2019;98:41(e17501).
Grant information/financial support: This work was supported by the Zhejiang Provincial Medical and Healthy Science and Technology Projects (Grant No. 2018254219) and Zhejiang Provincial Traditional Chinese Medical and Healthy Science and Technology Projects (Grant No. 2017ZQ019).
The authors have no conflicts of interest to disclose.
References
- [1].Tack J, Talley NJ. Functional dyspepsia—symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol 2013;10:134–41. [DOI] [PubMed] [Google Scholar]
- [2].Oshima T, Miwa H. Epidemiology of functional gastrointestinal disorders in Japan and in the world. J Neurogastroenterol Motil 2015;21:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Drossman DA, Dumitrascu DL. Rome III: new standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 2006;15:237–41. [PubMed] [Google Scholar]
- [4].Saad RJ, Chey WD. Review article: current and emerging therapies for functional dyspepsia. Aliment Pharmacol Ther 2006;24:475–92. [DOI] [PubMed] [Google Scholar]
- [5].Mason JM, Moayyedi P, Young PJ, et al. Population-based and opportunistic screening and eradication of Helicobacter pylori. An analysis using trial baseline data. Leeds H. pylori Study Group. Int J Technol Assess Health Care 1999;15:649–60. [PubMed] [Google Scholar]
- [6].Aro P, Talley NJ, Agreus L, et al. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther 2011;33:1215–24. [DOI] [PubMed] [Google Scholar]
- [7].Talley NJ, Verlinden M, Jones M. Quality of life in functional dyspepsia: responsiveness of the Nepean Dyspepsia Index and development of a new 10-item short form. Aliment Pharmacol Ther 2001;15:207–16. [DOI] [PubMed] [Google Scholar]
- [8].Wu JC. Psychological co-morbidity in functional gastrointestinal disorders: epidemiology, mechanisms and management. J Neurogastroenterol Motil 2012;18:13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hsu YC, Liou JM, Liao SC, et al. Psychopathology and personality trait in subgroups of functional dyspepsia based on Rome III criteria. Am J Gastroenterol 2009;104:2534–42. [DOI] [PubMed] [Google Scholar]
- [10].Talley NJ, Locke GR, Saito YA, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multicenter, randomized controlled study. Gastroenterology 2015;149:340.e2–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Filipovic BF, Randjelovic T, Ille T, et al. Anxiety, personality traits and quality of life in functional dyspepsia-suffering patients. Eur J Intern Med 2013;24:83–6. [DOI] [PubMed] [Google Scholar]
- [12].Mak AD, Wu JC, Chan Y, et al. Dyspepsia is strongly associated with major depression and generalised anxiety disorder—a community study. Aliment Pharmacol Ther 2012;36:800–10. [DOI] [PubMed] [Google Scholar]
- [13].Van Kerkhoven LA, Laheij RJ, Aparicio N, et al. Effect of the antidepressant venlafaxine in functional dyspepsia: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 2008;6:746–52. quiz 718. [DOI] [PubMed] [Google Scholar]
- [14].Lee HJ, Lee SY, Kim JH, et al. Depressive mood and quality of life in functional gastrointestinal disorders: differences between functional dyspepsia, irritable bowel syndrome and overlap syndrome. Gen Hosp Psychiatry 2010;32:499–502. [DOI] [PubMed] [Google Scholar]
- [15].Tan VP, Cheung TK, Wong WM, et al. Treatment of functional dyspepsia with sertraline: a double-blind randomized placebo-controlled pilot study. World J Gastroenterol 2012;18:6127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ford AC, Luthra P, Tack J, et al. Efficacy of psychotropic drugs in functional dyspepsia: systematic review and meta-analysis. Gut 2015;66:411–20. [DOI] [PubMed] [Google Scholar]
- [17].Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006;130:1466–79. [DOI] [PubMed] [Google Scholar]
- [18].Patel A, Sayuk GS, Kushnir VM, et al. Sensory neuromodulators in functional nausea and vomiting: predictors of response. Postgrad Med J 2013;89:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Culpepper L, Muskin PR, Stahl SM. Major depressive disorder: understanding the significance of residual symptoms and balancing efficacy with tolerability. Am J Med 2015;1289 suppl:S1–5. [DOI] [PubMed] [Google Scholar]
- [20].Olafsdottir LB, Gudjonsson H, Jonsdottir HH, et al. Natural history of functional dyspepsia: a 10-year population-based study. Digestion 2010;81:53–61. [DOI] [PubMed] [Google Scholar]
- [21].Li X, Cao Y, Wong RK, et al. Visceral and somatic sensory function in functional dyspepsia. Neurogastroenterol Motil 2013;25:246–53. e165. [DOI] [PubMed] [Google Scholar]
- [22].Vandenberghe J, Dupont P, Van Oudenhove L, et al. Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology 2007;132:1684–93. [DOI] [PubMed] [Google Scholar]
- [23].Van Oudenhove L, Vandenberghe J, Geeraerts B, et al. Relationship between anxiety and gastric sensorimotor function in functional dyspepsia. Psychosom Med 2007;69:455–63. [DOI] [PubMed] [Google Scholar]
- [24].Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry 2010;81:1372–3. [DOI] [PubMed] [Google Scholar]
- [25].Bouras EP, Talley NJ, Camilleri M, et al. Effects of amitriptyline on gastric sensorimotor function and postprandial symptoms in healthy individuals: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol 2008;103:2043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saito H, Wakai J, Sekiguchi M, et al. The effect of selective serotonin reuptake inhibitor (SSRI) on pain-related behavior in a rat model of neuropathic pain. Eur Spine J 2014;23:2401–9. [DOI] [PubMed] [Google Scholar]
- [27].Aarts N, Zuurbier LA, Noordam R, et al. Use of selective serotonin reuptake inhibitors and sleep quality: a population-based study. J Clin Sleep Med 2016;15:989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tack J, Broekaert D, Coulie B, et al. Influence of the selective serotonin re-uptake inhibitor, paroxetine, on gastric sensorimotor function in humans. Aliment Pharmacol Ther 2003;17:603–8. [DOI] [PubMed] [Google Scholar]
- [29].Talley NJ, Locke GR, 3rd, Herrick LM, et al. Functional Dyspepsia Treatment Trial (FDTT): a double-blind, randomized, placebo-controlled trial of antidepressants in functional dyspepsia, evaluating symptoms, psychopathology, pathophysiology and pharmacogenetics. Contemp Clin Trials 2012;33:523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011;34:638–48. [DOI] [PubMed] [Google Scholar]
- [31].Tack J, Ly HG, Carbone F, et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin Gastroenterol Hepatol 2016;14:385.e4–92.e4. [DOI] [PubMed] [Google Scholar]


