Abstract
Background:
Corticosteroid injection is beneficial in treating carpal tunnel syndrome (CTS) due to its anti-inflammatory effects. However, its side effects limit widespread usage. Recently, several studies have found that polydeoxyribonucleotide offers anti-inflammatory capabilities with fewer side effects, making it an ideal alternative. Nevertheless, there has been no study on its effectiveness in patients with CTS. Therefore, we evaluate the effectiveness of polydeoxyribonucleotide in patients with CTS. Based on the criteria, 30 patients with CTS who received two-consecutive polydeoxyribonucleotide injections (with a week interval) were initially included.
Method:
Patients with CTS were investigated retrospectively. To evaluate the effectiveness of polydeoxyribonucleotide in patients with CTS, numeric rating scale (NRS), cross-sectional area (CSA) of the median nerve, and severity and functional status scores of CTS based on the Boston Carpal Tunnel Syndrome Questionnaire (BCTQ) were assessed.
Results:
There was a significant improvement in the NRS, CSA, and functional and severity scores of BCTQ after two-consecutive polydeoxyribonucleotide injections (P < .05).
Conclusion:
In conclusion, although more research is needed to evaluate the effectiveness of polydeoxyribonucleotide in patients with CTS, the findings here suggest that polydeoxyribonucleotide may be a viable alternative to corticosteroids in patients with CTS.
Keywords: carpal tunnel syndrome, corticosteroid, polydeoxyribonucleotide, ultrasound
1. Introduction
Ultrasound-guided corticosteroid injection is one of the most widely used treatments for patients with carpal tunnel syndrome (CTS).[1] Corticosteroid is beneficial in treating CTS due to its anti-inflammatory effects;[2] however, its side effects – including decreased cognition,[3] mood disorders,[3] glucocorticoid-induced osteoporosis,[4] hypopigmentation,[5] increased risk of infection,[3] suppression of the adrenal system,[3] and disturbances in menstrual patterns[3] – limit widespread usage. Increased blood glucose level, associated with decreased insulin sensitivity, is another major side effect that can be highly problematic for patients with glucose intolerance or diabetes mellitus (DM).[2]
Recently, several studies have found that polydeoxyribonucleotide may be a viable alternative to corticosteroids, as it has anti-inflammatory capabilities but with relatively fewer side effects.[6–8] In addition, we have previously reported the effectiveness of polydeoxyribonucleotide in a patient with CTS.[9] Nevertheless, to the best of our knowledge, there is no study on its effectiveness on patients with CTS to date. Therefore, we attempt to evaluate the effectiveness of polydeoxyribonucleotide on CTS patients
2. Method
2.1. Participants
We investigated the medical records of patients who were clinical diagnosed with idiopathic CTS, confirmed by an electrodiagnostic study, and treated with polydeoxyribonucleotide.[10] Among CTS patients treated with polydeoxyribonucleotide, those with the following conditions were excluded: rheumatoid arthritis, degenerative joint disease in the hand or wrist; flexor tendinitis in the hand or wrist; space occupying lesions of the wrist; coexistent neurologic disease, such as polyneuropathy, proximal median neuropathy, cervical radiculopathy, thyroid disease, and diabetes mellitus; history of fractures, or other trauma to the hand or wrist; and other systematic disease. This study was approved by the Institutional Review Board of Daegu Fatima Hospital. Informed consent was obtained. The study must comply with the Declaration of Helsinki. The research protocol must have been approved by the locally appointed ethics committee and only medical records with research authorization were included.
2.2. Ultrasound-guided intervention
In supine position, ultrasound-guided injection of polydeoxyribonucleotide into the carpal tunnel was performed at the level of the forearm wrist crease, using 5.625 mg/3 mL of polydeoxyribonucleotide (Rejuvenex, PharmaResearch Products, South Korea), with a 27-gauge, 1.5-inch needle.[9] The total injection volume was 3 mL. Lidocaine or saline was not added in conjunction with polydeoxyribonucleotide. Polydeoxyribo-nucleotide injection was given twice with a 1-week interval.
2.3. Outcome measure
To evaluate the effectiveness of PDRN in patients with CTS, the numeric rating scale (NRS), severity, and functional status scores of CTS were assess based on the Boston Carpal Tunnel Syndrome Questionnaire (BCTQ).[11,12] Moreover, we also evaluated the cross-sectional area (CSA) of the median nerve at the wrist crease.[10] All parameters were evaluated at the following periods: initial (T0), 1 week after the 1st PDRN injection (T1), and 1 week after the 2nd PDRN injection (T2) (Fig. 1).
Figure 1.
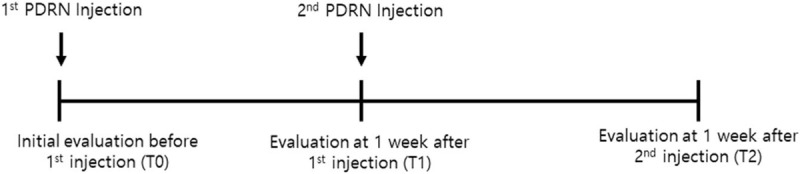
Time table of this study.
2.4. Statistical analysis
All statistical analyses were performed using SPSS for Windows and R package for Windows (version 2.15.2, R Foundation for Statistical Computing, Vienna, Austria). The initial statistical analysis was performed to evaluate the effectiveness of treatments in patients with CTS using a one-way ANOVA analysis with a Tukey post-hoc test to compare the BCTQ scores, NRS measures, and CSA across the three time points. The results are presented as the mean ± standard deviation. P values of <.05 were considered statistically significant.
3. Results
3.1. Demographics
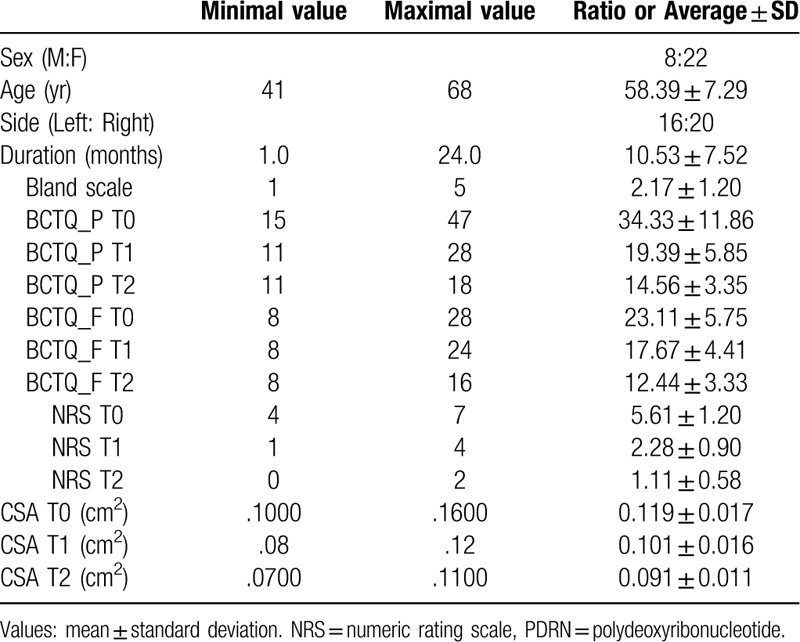
Based on the criteria, 40 patients with CTS, who visited our hospital between June 2017 and November 2018, were initially included. Among them, 10 patients were excluded. Finally, a total of 36 hands of 30 patients were included for analysis (Table 1).
Table 1.
Characteristics of patients with carpal tunnel syndrome.
3.2. Changes in NRS, BCTQ, and CSA
There was a significant improvement in NRS, and the severity score of BCTQ was observed 1 week after the first injection (T1) (P < .05) (Table 2). polydeoxyribonucleotide treatment also showed significant improvement at 1 week after the second injection (T2), as compared with the initial status (T0) (P < .05). Moreover, there were statistically significant improvements at 1 week after the second injection (T2), as compared with the status at 1 week after the first injection (T1) (P < .05) (Table 2).
Table 2.
Comparison of the clinical parameters of patients with CTS after 2 consecutive PDRN injection.

In the severity score of BCTQ, there was a significant improvement was observed 1 week after the first injection (T1) (P < .05) (Table 2). polydeoxyribonucleotide treatment also showed significant improvement at 1 week after the second injection (period 2), as compared with the initial status (T0) (P < .05). Moreover, there were statistically significant improvements at 1 week after the second injection (T2), as compared with the status at 1 week after the first injection (T1) (P < .05) (Table 2).
In the CSA of the median nerve, there was a significant improvement 1 week after the first injection (T1) (P < .05) (Table 2). In addition, polydeoxyribonucleotide treatment showed a significant improvement at 1 week after the second injection (T2), as compared with the initial status (T0) (P < .05). However, there was no statistically significant improvement 1 week after the second injection (T2), as compared with the status at 1 week after the first injection (T1) (P ≥ .05) (Table 2, Fig. 2).
Figure 2.
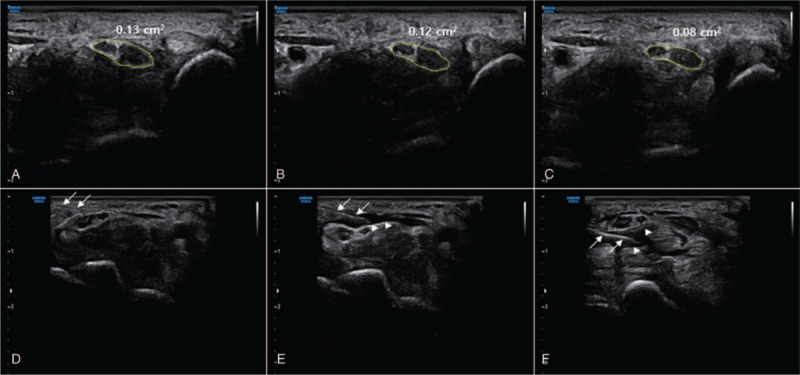
Serials ultrasound image of the median nerve treated with 2 consecutive polydeoxyribonucleotide injections. (A) Median nerve at initial state (T0). (B) Median nerve at 1 week after 1st polydeoxyribonucleotide injection (T1). (C) Median nerve at 1 week after 2nd polydeoxyribonucleotide injection (T2). Cross-sectional area (CSA) showed decrease after 2 consecutive polydeoxyribonucleotide injections. (D) During procedure, a needle near median nerve was observed (arrow). (E)(F) A needle (arrow) and a flow of polydeoxyribonucleotide (arrowhead) around the median nerve were observed.
4. Discussion
Our study showed the effectiveness of polydeoxyribonucleotide in patients with CTS. In the results of our study, polydeoxiribonucleotide showed its effectiveness in pain scale (NRS), functional scale (BCTQ), and ultrasonographic finding (CSA of the median nerve). Polydeoxyribonucleotide is obtained from salmon sperm, and it is a mixture of deoxyribonucleotide polymers with chain lengths ranging from 50 bp to 2000 bp.[13,14] The structure of polydeoxyribonucleotide consists of a low molecular weight fraction of deoxyribonucleic acid (DNA), composed of a linear polymer of deoxyribonucleotides with phosphodiester bonds, in which the monomer units are represented by purine and pyrimidine nucleotides.[13]
Polydeoxyribonucleotide has been reported to stimulate the A2A receptors under pathologic conditions of low tissue perfusion.[15] Adenosine, a purine nucleoside that is released from a variety of cells in response to several types of injury of stress, has been suggested to regulate excessive inflammation via an interaction with one or more of its four known receptors (A1, A2A, A2B, and A3).[16] Although the stimulation of adenosine receptor has differing effects on the release of pro-inflammatory cytokines, the stimulation of adenosine A2A receptor specifically has been demonstrated to inhibit TNF-α production in human peripheral blood mononuclear cells (PBMCs).[13] Moreover, polydeoxyribonucleotide has been shown to lower the circulating levels and cartilage expression of the inflammatory cytokines interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) in a mouse model of rheumatoid arthritis.[17]
These anti-inflammatory effects of polydeoxyribonucleotide via immune cells indicate that it may be a potential alternative treatment to corticosteroids. Moreover, polydeoxyribonucleotide has been demonstrated to exert profibrinolytic and antithrombotic activities through a stimulation of vascular prostacyclin and vascular endothelial growth factor (VEGF) production during low tissue perfusion in both ischemic conditions and wound healing via the stimulation of A2A receptors.[13] Moreover, polydeoxyribonucleotide may also exhibit cytoprotective effects by decreasing calcium entry into the cells, possibly through adenosine receptors.[18,19] Considering that concurrent primary ischemia and fibrosis may occur in the connective tissue components of the median nerve of CTS,[10,20,21] these anti-fibrinolytic and anti-ischemic effects of polydeoxyribonucleotide may also indicate that it can be an alternative treatment to corticosteroids. Considering previous studies,[22] the absolute effectiveness of polydeoxyribonucleotide may be slightly lower than that of dexamethasone given the same dose. Nevertheless, polydeoxy-ribonucleotide appears to be effective in treating CTS, suggesting it can be a promising alternative to corticosteroids in patients with CTS.
There are a few limitations in this study. First, this study enrolled a small number of patients and is a retrospective study. However, to the best of our knowledge, it is the first study to evaluate the effectiveness of polydeoxyribonucleotide in patients with CTS. Therefore, we think that this study, as the first preliminary study evaluating the effectiveness of polydeoxyribonucleotide in patients with CTS, is valuable. Further prospective study with a larger number of patients with CTS may be necessary to evaluate the safety and efficacy of polydeoxyribonucleotide in patients with CTS. Second, we investigated only the short-term effect of polydeoxyribonucleotide in patients with CTS. Hence, a long-term study may be necessary to evaluate its long-term effectiveness in patients with CTS.
In conclusion, although more effort is needed to evaluate the effectiveness of PDRN in patients with CTS, polydeoxyribonucleotide appears to be a viable alternative to corticosteroids in patients with CTS.
Author contributions
Data curation: Joonyoung Huh, Hyun-jung Cho.
Methodology: Kwang Seok Shim.
Writing – original draft: Donghwi Park.
Writing – review & editing: Byung Joo Lee, Donghwi Park.
Footnotes
Abbreviations: BCTQ = Boston Carpal Tunnel Syndrome Questionnaire, CSA = cross-sectional area, CTS = carpal tunnel syndrome, LPS = lipopolysaccharide, NO = nitric oxide, PDRN = polydeoxyribonucleotide.
How to cite this article: Huh J, Shim KS, Cho Hj, Lee BJ, Park D. Polydeoxyribonucleotide injection in the treatment of patients with carpal tunnel syndrome. Medicine. 2019;98:41(e17522).
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF- 2017R1D1A1B03033127). The authors have no conflicts of interest to disclose.
References
- [1].Wang JC, Liao KK, Lin KP, et al. Efficacy of combined ultrasound-guided steroid injection and splinting in patients with carpal tunnel syndrome: a randomized controlled trial. Arch Phys Med Rehabilit 2017;98:947–56. doi:10.1016/j.apmr.2017.01.018. [DOI] [PubMed] [Google Scholar]
- [2].Pasieka AM, Rafacho A. Impact of glucocorticoid excess on glucose tolerance: clinical and preclinical evidence. Metabolites 2016;6.doi:10.3390/metabo6030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: A comprehensive review: Ocular, cardiovascular, muscular, and psychiatric side effects and issues unique to pediatric patients,\. J Am Acad Dermatol 2017;76:201–7. [DOI] [PubMed] [Google Scholar]
- [4].Kim YU, Karm MH, Cheong Y, et al. Effect of epidural steroid injection on bone mineral density in postmenopausal women according to antiosteoporotic medication use. Pain Phys 2016;19:389–96. [PubMed] [Google Scholar]
- [5].Salvatierra AR, Alweis R. Permanent hypopigmentation after triamcinolone injection for tennis elbow. J Community Hosp Intern Med Perspect 2016;6:31814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ryu K, Ko D, Lim G, et al. Ultrasound-guided prolotherapy with polydeoxyribonucleotide for painful rotator cuff tendinopathy. Pain Res Manag 2018;8286190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Do HK, Lee JH, Lim JY. Polydeoxyribonucleotide injection in the patients with partial-thickness tear of supraspinatus tendon: a prospective and pilot study using ultrasound. Phys Sportsmed 2018;46:213–20. [DOI] [PubMed] [Google Scholar]
- [8].Yoon YC, Lee DH, Lee MY, et al. Polydeoxyribonucleotide injection in the treatment of chronic supraspinatus tendinopathy: a case-controlled, retrospective, comparative study with 6-month follow-up. Arch Phys Med Rehabil 2017;98:874–80. [DOI] [PubMed] [Google Scholar]
- [9].Park JS, Park D. Effect of polydeoxyribonucleotide injection in a patient with carpal tunnel syndrome. Am J Phys Med Rehabil 2018;97:e93–5. [DOI] [PubMed] [Google Scholar]
- [10].Park D. Ultrasonography of the transverse movement and deformation of the median nerve and its relationships with electrophysiological severity in the early stages of carpal tunnel syndrome. PMR 2017;9:1085–94. [DOI] [PubMed] [Google Scholar]
- [11].Heybeli N, Kutluhan S, Demirci S, et al. Assessment of outcome of carpal tunnel syndrome: a comparison of electrophysiological findings and a self-administered Boston questionnaire. J Hand Surg 2002;27:259–64. [DOI] [PubMed] [Google Scholar]
- [12].Mondelli M, Reale F, Sicurelli F, et al. Relationship between the self-administered Boston questionnaire and electrophysiological findings in follow-up of surgically-treated carpal tunnel syndrome. J Hand Surg 2000;25:128–34. [DOI] [PubMed] [Google Scholar]
- [13].Altavilla D, Bitto A, Polito F, et al. Polydeoxyribonucleotide (PDRN): a safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc Hematolog Agents Med Chem 2009;7:313–21. [DOI] [PubMed] [Google Scholar]
- [14].Bianchini P, Tellini N, Morani AM, et al. Pharmacological data on polydeoxyribonucleotide of human placenta. Int J Tissue React 1981;3:151–4. [PubMed] [Google Scholar]
- [15].Squadrito F, Bitto A, Irrera N, et al. Pharmacological activity and clinical use of PDRN. Front Pharmacol 2017;8:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nature reviews. Drug Discov 2006;5:247–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bitto A, Polito F, Irrera N, et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A((2)A) receptor. Arthr Rheumat 2011;63:3364–71. [DOI] [PubMed] [Google Scholar]
- [18].Módis K, Gero D, Nagy N, et al. Cytoprotective effects of adenosine and inosine in an in vitro model of acute tubular necrosis. Br J Pharmacol 2009;158:1565–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cronstein BN, Rose FR, Pugliese C. Adenosine, a cytoprotective autocoid: effects of adenosine on neutrophil plasma membrane viscosity and chemoattractant receptor display. Biochim Biophys Acta 1989;987:176–80. [DOI] [PubMed] [Google Scholar]
- [20].Ettema AM, Amadio PC, Zhao C, et al. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am 2004;86-A:1458–66. [DOI] [PubMed] [Google Scholar]
- [21].Kim JK, Koh YD, Kim JS, et al. Oxidative stress in subsynovial connective tissue of idiopathic carpal tunnel syndrome. J Orthop Res 2010;28:1463–8. [DOI] [PubMed] [Google Scholar]
- [22].Park D, Yu KJ, Cho JY, et al. The effectiveness of 2 consecutive intra-articular polydeoxyribonucleotide injections compared with intra-articular triamcinolone for hemiplegic shoulder pain: a STROBE-complaint retrospective study. Medicine 2017;96:e8741. [DOI] [PMC free article] [PubMed] [Google Scholar]


