Abstract
Recently, the monocyte count to high-density lipoprotein cholesterol ratio (MHR) was found to be associated with the SYNTAX score in patients with both stable coronary artery disease (CAD) and acute coronary syndrome (ACS). The MHR was significantly higher in male patients. However, the sex-specific association of MHR with SYNTAX score in stable CAD was not well explored. Thus, the present study aimed to investigate the association of MHR and presence and severity of CAD evaluated by coronary angiography and the SYNTAX score in males and females.
In total, 873 patients who received selective coronary angiography between March 2017 and July 2018 were included in the present study. Patients were divided into 3 groups according to MHR tertiles. The MHR was calculated by dividing the monocyte count by the high-density lipoprotein cholesterol level. CAD was defined as at least 50% diameter stenosis of a major coronary artery, including the right coronary, left main coronary, left anterior descending, and left circumflex arteries. The SYNTAX score was calculated by 2 experienced interventional cardiologists. SYNTAX score ≥23 was defined as a high SYNTAX score.
Males showed a significantly higher MHR (12.2 [8.9–15.5] vs 9.3 [6.2–12.1], P < .001), accompanied by a higher prevalence of CAD (68.1% vs 53.4%, P < .001). Male sex remained an independent predictor of elevated MHR after correction for confounding factors (adjusted odds ratio [OR] 3.102, P = .001). The association between MHR and SYNTAX score was confirmed only in male stable patients with CAD (r = 0.113, P = .036). Multivariate logistic regression analysis showed that MHR was an independent predictor of SYNTAX score ≥23 only in male patients with CAD. The receiver-operating characteristic curve showed a predictive value of MHR for high SYNTAX score only in males.
A higher MHR in males and a positive correlation of MHR with SYNTAX score were observed only in male stable patients with CAD. Such an easily obtained index may help interventional cardiologists detect high-risk patients before coronary catheterization, but its application may be restricted to males.
Keywords: monocyte count to high-density lipoprotein cholesterol ratio, stable coronary artery disease, SYNTAX score
1. Introduction
Cardiovascular diseases (CVDs) are still a major determinant of global health. In 2013, there were an estimated 8.6 million cases of acute myocardial infarction (AMI) globally.[1] Death rates have fallen; however, the number of CVD deaths has increased over the past 20 years due to the aging and growth of the world's population. The number of CVD deaths increased from 12.3 to 17.3 million, a 41% increase.[2] Accumulating evidence in basic science and population-based studies has proven that inflammation plays a crucial role in the development of atherosclerosis. The higher white blood cell (WBC) level has predictive value for AMI and coronary artery disease (CAD).[3–6] The elevated circulating monocyte (MON) level is an independent risk marker of CAD.[6–9] Serum high-density lipoprotein cholesterol (HDL-C) plays a protective role against developing CAD.[10–14] Recently, a new marker, called the monocyte to HDL-C ratio (MHR), was found to be related to cardiovascular events in chronic kidney disease.[15] It was also found to be an independent predictor of outcome for patients with acute coronary syndrome (ACS).[16–18] The SYNTAX score was developed as an angiographic stratification tool to grade the complexity of coronary lesions and to guide appropriate revascularization strategy selection in patients with CAD with complex multivessel and left main disease.[19] The association of MHR and SYNTAX score in stable CAD was demonstrated in several studies.[20,21] Moreover, it was found that the level of MHR was significantly higher in males.[22] However, the impact of sex on MHR and its relationship with CAD has not been well explored. The present study aimed to investigate the association of MHR and the presence and severity of CAD evaluated by coronary angiography and SYNTAX score in males and females.
2. Methods
In total, 1465 consecutive patients presenting with stable angina pectoris, angina-equivalent symptoms, or suspected asymptomatic cardiac ischemia were referred to Ningbo Medical Center Lihuili Hospital for coronary angiography (CAG) between March 2017 and July 2018 and were enrolled in this retrospective cross-sectional study. All patients received successful CAG procedures. Patients with recent ACS, including ST segment-elevated myocardial infarction (STEMI) and non-ST segment-elevated ACS (NSTE-ACS) (≤6 months before admission to the hospital), any prior percutaneous coronary intervention (PCI), any prior coronary artery bypass grafting (CABG), any decompensated heart failure, any autoimmune disease, any serious acute infection, severe renal insufficiency (serum creatinine [Scr] ≥2.5 mg/dL), severe hepatic disease(alanine aminotransferase [ALT] or aspartate aminotransferase [AST] >3× upper normal value), hyperthyroidism or hypothyroidism, any hematonosis including moderate and severe anemia (hemoglobin [HGB] <90 g/L), and evidence of malignant tumors were excluded. Finally, 873 patients were included in this study.
The study was approved by the local ethics committee. Informed consent was obtained from all the participants.
All detailed data on the clinical characteristics of the whole population enrolled in this study were collected, including age, sex, history of hypertension (HP), and diabetes mellitus (DM), smoking, drinking, family history of CAD, systolic blood pressure (SBP), and diastolic blood pressure (DBP) at admission, and current use of CVD drugs, including angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs),calcium channel blockers (CCBs), beta-blockers, diuretics, statins, and antiplatelets. Hypertension was defined as repeated blood pressure measurements ≥140/90 mm Hg or current use of antihypertensive drugs. DM was defined as fasting blood glucose (FBG) levels ≥126 mg/dL or glucose level ≥200 mg/dL at any time on repeated measurements, or current use of antidiabetic medications. Smoking was defined as current smoking ≥20 cigarettes per day. A family history of CAD was concluded as the presence of a history of CAD or sudden cardiac death in a 1st-degree relative before the age of 55 years for men and 65 years for women.
Peripheral venous blood samples of the patients were obtained from the antecubital vein after a 12-hour overnight fast. The vacuum blood collection system was purchased from Zhejiang Gongdong Medical Technology Co, Ltd. EDTA anticoagulated tubes (GD050A) were used to collect blood samples for complete blood cell analysis while plain tubes (GD020EK) were used for biochemical analysis. The levels of biochemical parameters, including baseline albumin (ALB), ALT, AST, gamma-glutamyl transferase (GGT), total bilirubin (TBIL), FBG, Scr, uric acid (UA), high-sensitivity C-reactive protein (hsCRP), lipid panel including triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and HDL-C, were measured using an AU5800 Chemistry Analyzer (Beckman Coulter KK, Tokyo, Japan). An automated DxH 800 hematology analyzer (Beckman Coulter Inc, South Kraemer Boulevard Brea, CA) was used to measure complete blood count parameters with differential analysis. All the WBC count, MON count, HGB, and platelet (PLT) count data were recorded. MHR was calculated by dividing the monocyte count by the HDL-C level. Transthoracic echocardiography was performed in all patients, and the left ventricular ejection fraction (LVEF) was evaluated with Simpson's method.
The standard Judkins technique was used for CAG via radial or femoral access. At least 2 orthogonal plane images were taken for the right and left coronary arteries. Philips AlluraXper FD10 cardiovascular X-ray system (Philips Healthcare/Philips Medical Systems BV, Eindhoven, The Netherlands) was used for angiography. CAD was defined as at least 50% diameter stenosis of a major coronary artery including the right coronary artery (RCA), left main artery (LM), left anterior descending artery (LAD), and left circumflex artery (LCx). The SYNTAX score was calculated in all patients by 2 experienced interventional cardiologists who were unaware of the clinical information of the patients from baseline diagnostic CAG. There were no significant discrepancies between the 2 cardiologists. The SYNTAX score was calculated for all coronary lesions with ≥50% diameter stenosis in each vessel ≥1.5 mm, based on the SYNTAX score calculator 2.28 (available online at www.SYNTAXscore.com). The definitions of dominance, total occlusion, trifurcation, bifurcation, aorto-ostial lesion, severe tortuosity, heavy calcification, thrombus, and diffuse disease were based on the online tutorial (www.SYNTAXscore.com). SYNTAX score ≥23 was defined as a high SYNTAX score.[23]
The IBM SPSS statistics version 22.0.0.0 (SPSS Inc, Chicago, IL) was used. The Kolmogorov–Smirnov test was used to evaluate whether the quantitative variables met the criterial of normal distribution. Quantitative variables with a normal distribution are presented as the mean ± standard deviation, and those with nonnormal distribution are presented as the median (interquartile range); categorical variables are presented as the number and percentage values. To compare parametric continuous variables, Student t test or 1-way analysis of variance was used; to compare nonparametric continuous variables, the Mann–Whitney U test or Kruskal–Wallis test was used. The Chi-squared test was used for categorical variables. Correlations between variables were evaluated by the Pearson or Spearman correlation test. Univariate and multivariate logistic regressions were used to identify the independent predictors of MHR ≥14.4 (upper quartile), and high SYNTAX score, confounding factors were defined as age, hypertension, DM, smoking, drinking, family history of CAD, therapy with ACEIs/ARBs, CCBs, beta-blockers, diuretics, statins and antiplatelets, SBP, DBP, ALB, ALT, AST, GGT, TBIL, FBG, Scr, UA, TG, TC, LDL-C, hsCRP, WBC, HGB, PLT, and LVEF. A receiver-operating characteristic (ROC) curve was also used to demonstrate the sensitivity and specificity of MHR and the optimal cutoff value for predicting a high SYNTAX score in stable patients with CAD. A 2-tailed value of P < .05 was considered statistically significant.
3. Results
A total of 873 patients (508 males, mean age 62.8 ± 9.6 years) were included in this study, and clinical, laboratory examination, and angiographic data for females and males are listed in Table 1. Males showed significantly higher MHRs (12.2 [8.9–15.5] vs 9.3 [6.2–12.1], P < .001), accompanied by higher prevalence of CAD (68.1% vs 53.4%, P < .001), and triple vessel disease (15.7% vs 9.0%, P = .004). Males were younger; had higher DBP; higher levels of ALT, AST, GGT, TBIL, Scr, UA, WBC, MON, and HGB; and lower levels of LVEF and HDL. Males were more likely to be smoking, drinking, and on antiplatelet therapy.
Table 1.
Clinical and laboratory characteristics of the whole study population.
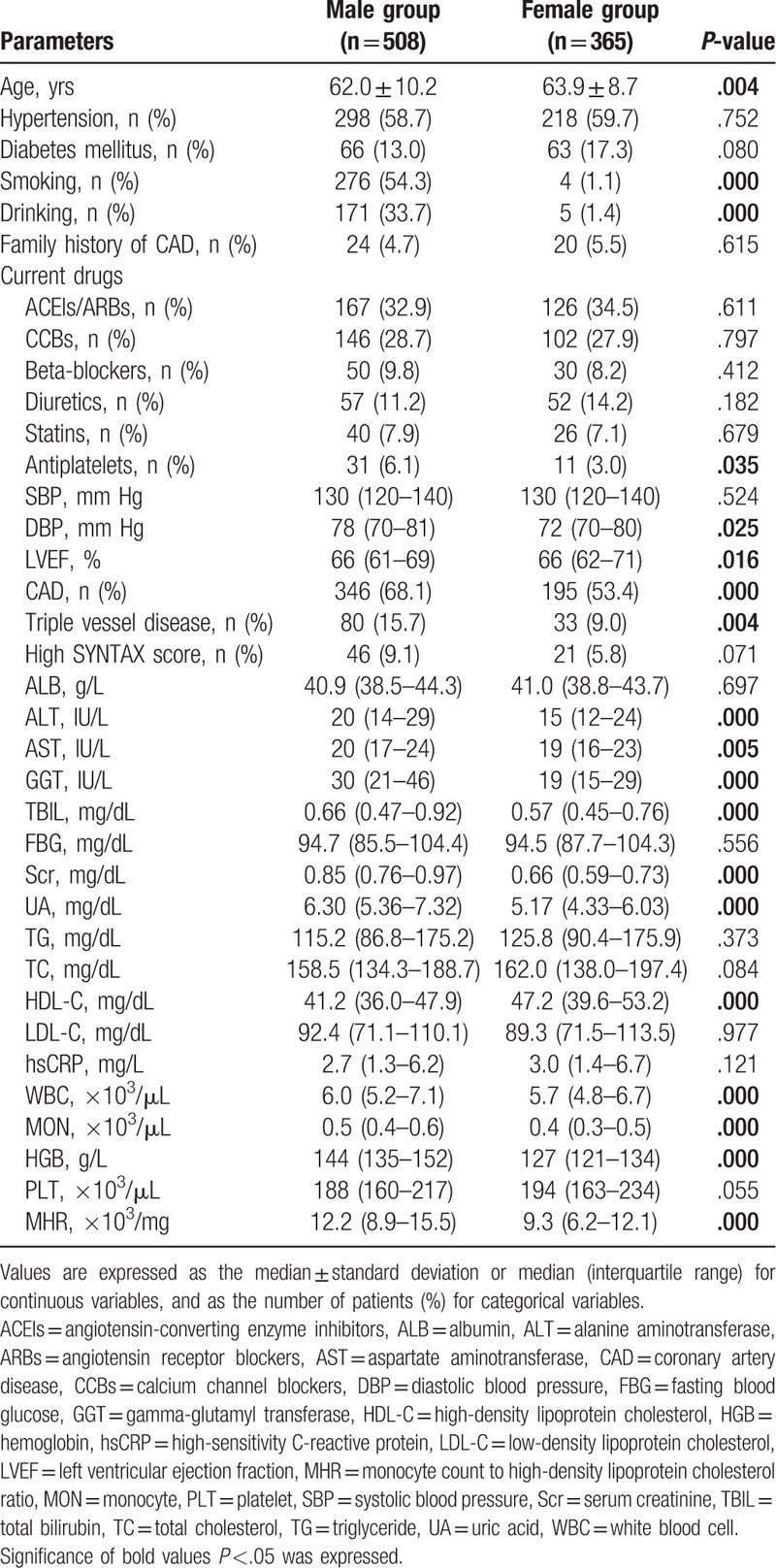
There was a positive correlation between MHR and male sex (r = 0.305, P < .001) in the Spearman correlation analysis. As shown in Table 2, multivariate logistic regression analysis confirmed the association of male sex and MHR ≥14.4 (upper quartile) after correction for confounding factors (adjusted odds ratio [OR] 3.102 95% confidence interval [CI] 1.616–5.954, P = .001).
Table 2.
Univariate and multivariate analysis of the relationship between male sex and MHR ≥14.4 (upper quartile).
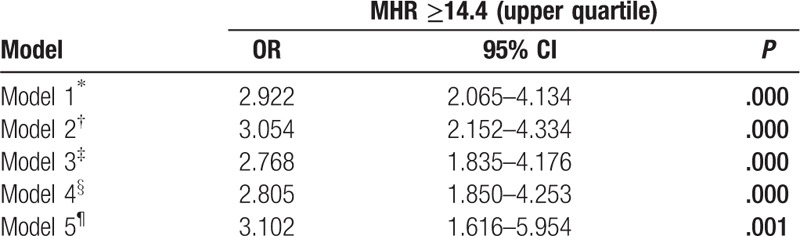
Clinical, angiographic characteristics, and laboratory tests according to the MHR tertiles in males (Tertile 1 group, MHR < 9.8, n = 159; Tertile 2 group, MHR = 9.8–14.2, n = 178; Tertile 3 group, MHR ≥ 14.2, n = 171) are presented in Table 3. Patients with elevated MHR had higher levels of Scr, UA, TG, WBC, and MON, but lower levels of ALB, TBIL, TC, and HDL-C. Patients with elevated MHR displayed a higher prevalence of hypertension, ACEIs/ARBs therapy, diuretic therapy, and LCx and RCA involvement. There was a trend toward a higher prevalence of CAD (P = .078) (Fig. 1A) in the upper MHR tertile groups. Pearson correlation analysis showed that MHR was correlated with hsCRP (r = 0.198, P < .001) and WBC (r = 0.477, P < .001) in males. Moreover, the Pearson correlation analysis showed a positive relationship between MHR and SYNTAX score in male stable patients with CAD (r = 0.113, P = .036) (Fig. 2A). As shown in Table 4, multivariate logistic regression analysis confirmed the association of MHR with high SYNTAX score in male stable patients with CAD after correction for confounding factors, (adjusted OR, 1.146 [95% CI, 1.049–1.251], P = .002). ROC curve analysis showed a positive MHR value in predicting a high SYNTAX score in male stable patients with CAD. The sensitivity was 84.8%, and the specificity was 34.0% with a cutoff value of 10.0 (area under the curve [AUC] = 0.616 [95% CI 0.530–0.701], P = .011) (Fig. 3A).
Table 3.
Clinical, angiographic, and laboratory characteristics according to MHR tertiles in males.
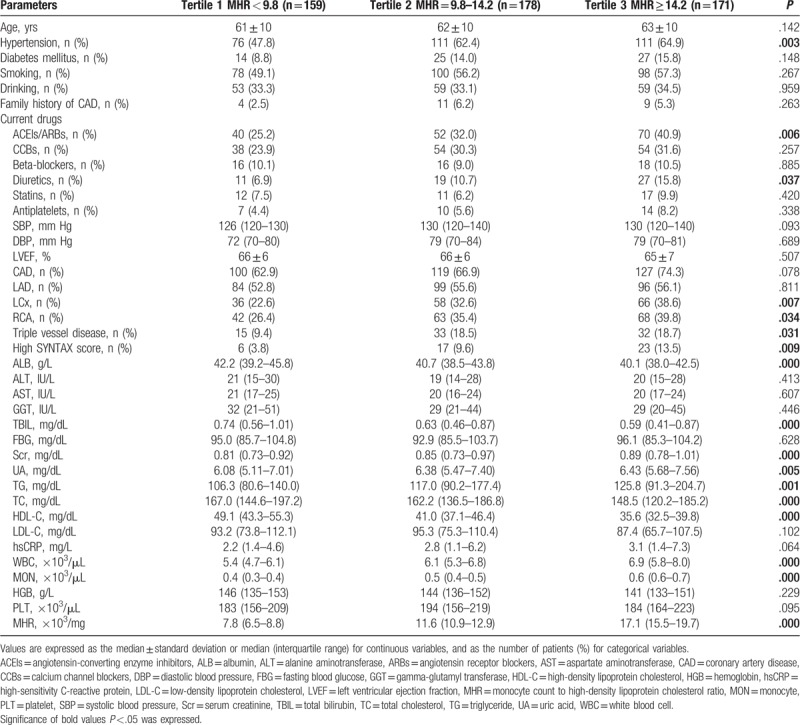
Figure 1.
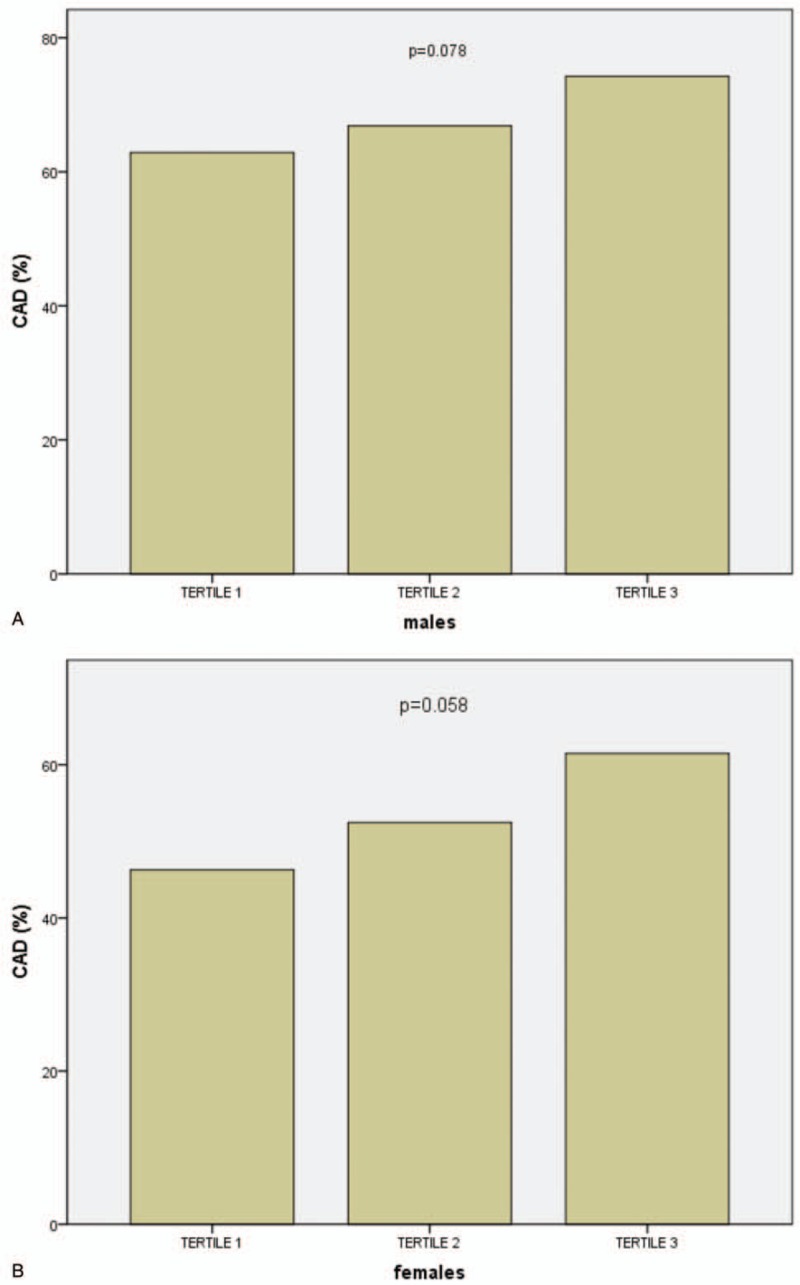
Bar graph showing the trend toward a higher coronary artery disease (CAD) incidence in the upper monocyte count to high-density lipoprotein cholesterol ratio tertile groups in both males (A) and females (B).
Figure 2.
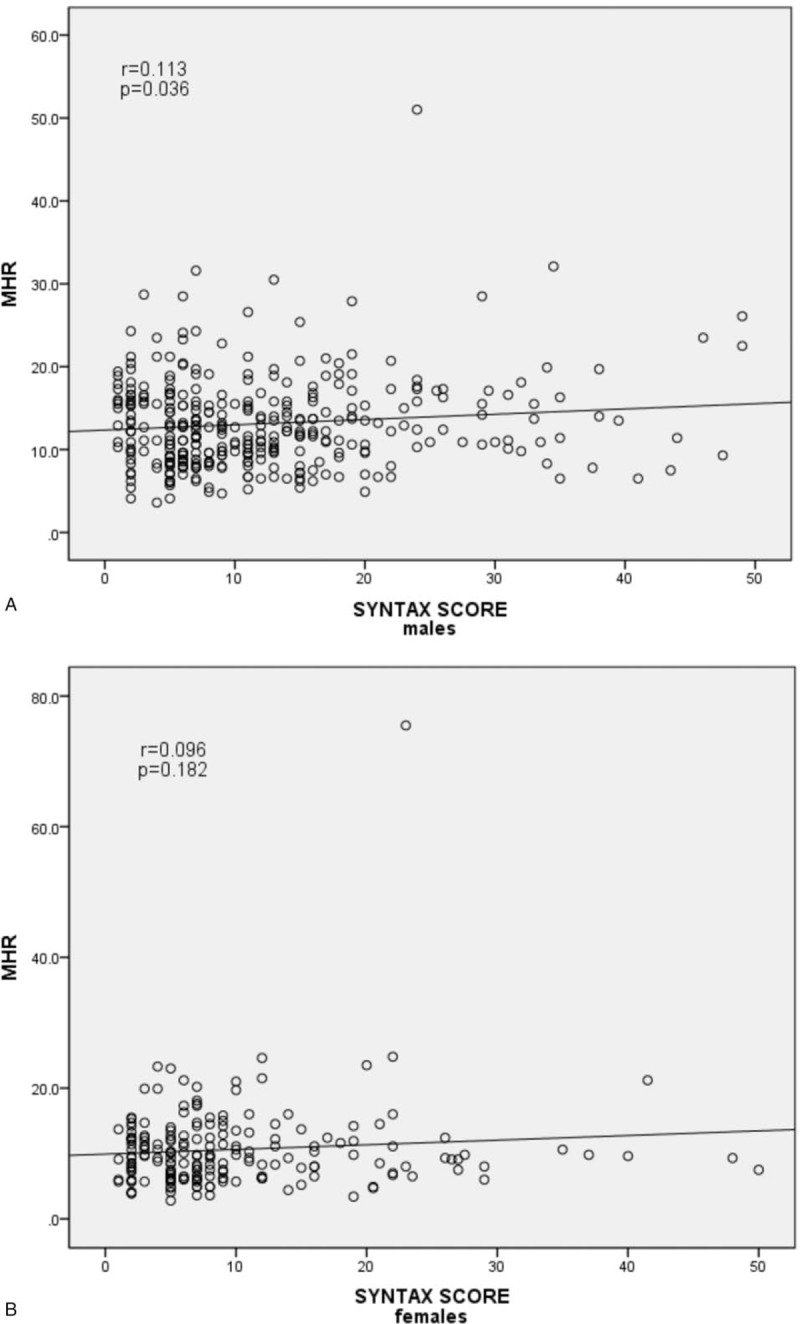
Scatter/dot graph showing a positive relationship between monocyte count to high-density lipoprotein cholesterol ratio and SYNTAX score in male stable patients with coronary artery disease (CAD) (A) but not in female stable patients with CAD (B).
Table 4.
Univariate and multivariate logistic regression analysis for predictors of SYNTAX score ≥23 in male patients with CAD.
Figure 3.
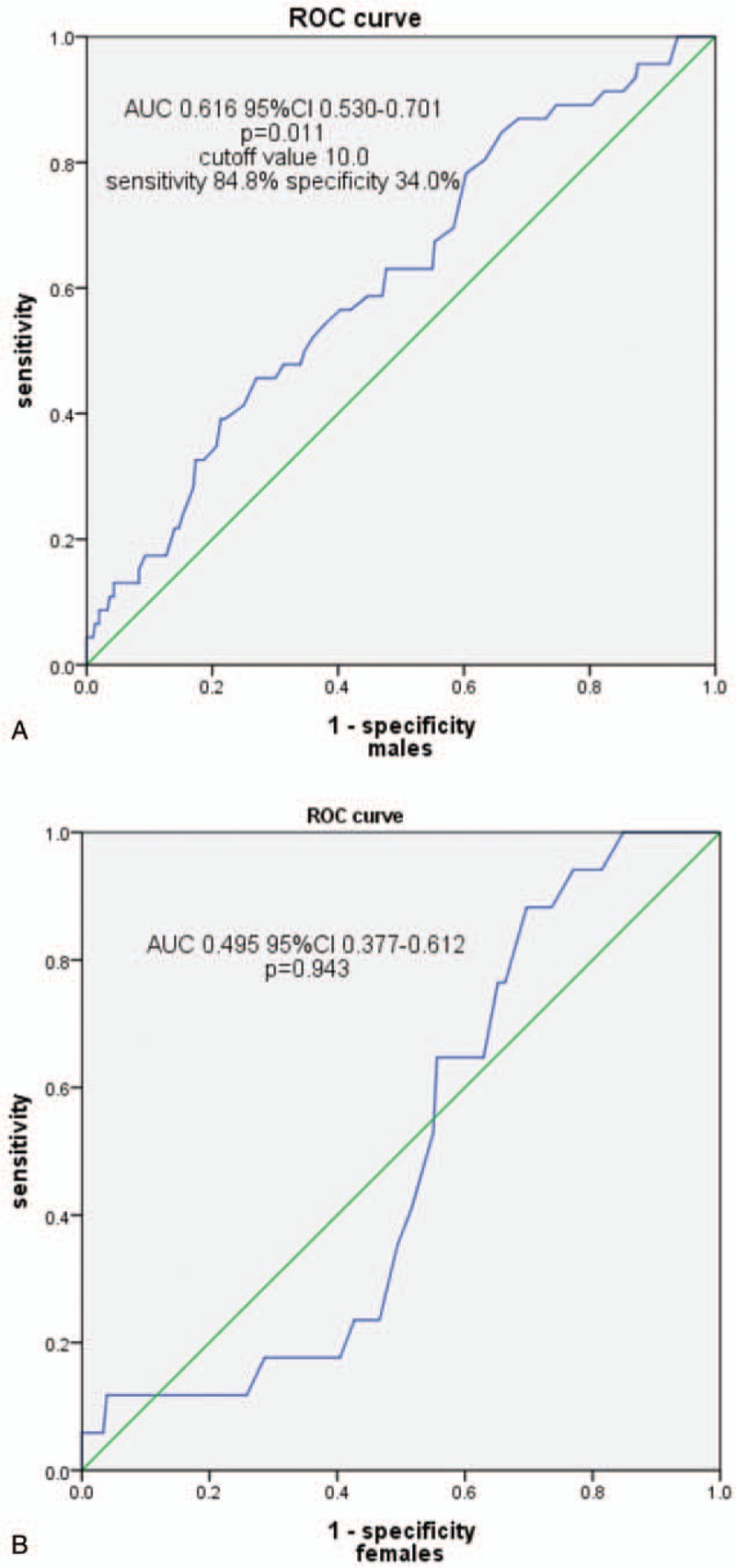
Receiver-operating characteristic (ROC) curve showing the monocyte count to high-density lipoprotein cholesterol ratio cutoff value of 10.0 predicting a high SYNTAX score with a sensitivity of 84.8% and specificity of 34.0% in male stable patients with coronary artery disease (CAD) (A) but no predictive value in female stable patients with CAD (B). AUC = area under the curve, CI = confidence interval.
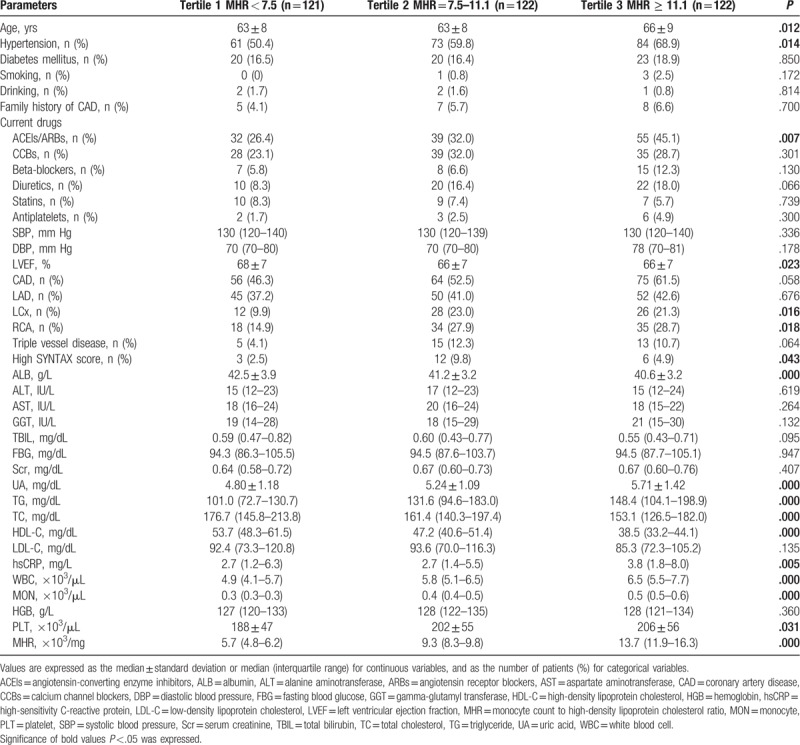
Clinical, angiographic characteristics, and laboratory tests according to the MHR tertiles in females (Tertile 1 group, MHR <7.5, n = 121; Tertile 2 group, MHR = 7.5–11.1, n = 122; Tertile 3 group, MHR ≥11.1, n = 122) are presented in Table 5. Patients with elevated MHR were older; had higher levels of UA, TG, hsCRP, WBC, MON, and PLT; but had lower levels of LVEF, ALB, TC, and HDL-C. Patients with elevated MHR displayed a higher prevalence of hypertension, ACEIs/ARBs therapy, and LCx and RCA involvement. There was a trend toward a higher prevalence of CAD (P = .058) (Fig. 1B) in the upper MHR tertile groups. The Pearson correlation analysis showed that MHR was correlated with hsCRP (r = 0.105, P = .049) and WBC (r = 0.412, P < .001) in females. However, the Pearson correlation analysis showed no relationship between MHR and SYNTAX score in female stable patients with CAD (P = .181) (Fig. 2B). Multivariate logistic regression showed no relationship between MHR and high SYNTAX score in female stable patients with CAD after correction for confounding factors, adjusted OR 1.104 (95% CI 0.979–1.246), P = .107. ROC curve analysis showed a negative MHR value in predicting a high SYNTAX score in female stable patients with CAD (P = .728) (Fig. 3B).
Table 5.
Clinical, angiographic, and laboratory characteristics according to MHR tertiles in females.
4. Discussion
The main findings obtained in the present study were as follows: male sex was independently associated with a higher level of MHR, and the MHR was independently associated with SYNTAX score only in male stable patients with CAD.
Atherosclerosis was characterized by the accumulation of lipids and fibrous elements in the large arteries. The link between lipids and atherosclerosis was considered the most important until the 1970s, based on numerous experimental and clinical studies investigating relationships between hyperlipidemia and atherosclerosis.[24] When evaluating CAD risk, it was shown that a low level of HDL-C must be taken into consideration as well as TC and LDL-C from the data in the Framingham Heart Study.[25] Several studies revealed that HDL-C was associated with the severity of CAD.[26–31] On the contrary, inflammation was revealed to be related to atherosclerosis initiation and progression by increasing evidence.[32,33] Monocytes and monocyte-derived cells, such as macrophages and dendritic and foam cells, are involved in all stages of atherosclerosis, including the uptake of oxidized lipids, lesion development, and ultimate plaque disruption.[34] The accumulation of foam cells, smooth muscle cells, and extracellular matrix eventually results in the formation of atherosclerotic plaques.[24] Under normal circumstances, the capacity of promonocyte proliferation is only partially utilized. When the demand for monocytes increases, the cycle time of promonocyte shortens, allowing more monocyte production, in turn, with an increased number of immature monocytes being released into the blood.[35] A higher circulating monocyte level was found in patients with CAD in several studies of population in Japan and Turkey.[36,37] A lower HDL-C level was found in subjects with mild renal dysfunction and was associated with elevated monocytes and atherosclerosis.[38]
Taking into consideration the counteraction of the 2 factors, it was hypothesized that the MHR, which combines the monocyte count and HDL-C level, is a new predictive index in cardiovascular disease. It was reported that an increased MHR is associated with a worse cardiovascular outcome and is an independent predictor of major adverse cardiovascular events in chronic kidney disease patients.[15] Subsequently, the MHR has been intensively studied in cardiovascular diseases over the past 4 years. The predictive role of the MHR has been confirmed in bare metal stent restenosis,[39,40] slow coronary flow,[41] stent thrombosis after primary PCI (pPCI) in STEMI patients,[42] coronary lesion severity and future cardiovascular events in patients with ACS,[16] and short-term and long-term mortality in STEMI patients who undergo successful pPCI.[17] The SYNTAX score was regarded as a robust tool to grade the complexity and severity of coronary lesions and to guide decision making for the revascularization strategy in patients with complex multivessel and LM disease.[19] The positive association of the MHR with the SYNTAX score in stable patients with CAD was identified in several studies.[20,21] As mentioned in previous studies,[20,41] hsCRP was correlated with MHR, which is confirmed in both females and males in our study. WBC count is also correlated with MHR both in males and females, indicating its inflammatory role in patients with CAD.
The association of elevated MHR with smoking was demonstrated in a recent study.[22] Moreover, the study group found a significantly higher MHR in males than in females, which was confirmed in our study. The smoking rate in females participating in the present study was impressively low, which may partially explain the lower MHR level in females.
Additionally, we identified that male sex remained a predictor of higher MHR levels in the multivariate logistic regression analysis after adjusting for confounding factors, showing the independent impact of sex on the MHR.
Gender stratification was supposed to be helpful in exploring the relationship between the MHR and CAD. Interestingly, an elevated MHR was associated with a trend toward a higher prevalence of CAD in both males and females, but the difference was not statistically significant (P = .078 and P = .058). A larger study population may be necessary. The prevalence of a high SYNTAX score was higher in the upper MHR tertile groups in both male and female stable patients with CAD. However, Pearson correlate analysis revealed a positive relationship between MHR and SYNTAX score in males but not females. The relationship was further confirmed by multivariate logistic regression analysis. The incidence of triple vessel disease and high SYNTAX score in females declined from the MHR Tertile 2 group to the Tertile 3 group, accompanied by an increased rate of ACEI/ARB and beta-blocker therapy, which may be explained by the protective role of ACEIs/ARBs and beta-blockers against atherosclerosis progression.[43] The ROC curve showed a predictive value of MHR for high SYNTAX score only in male stable patients with CAD. The application of MHR in predicting severe CAD may be restricted to males.
The present study has several limitations. First, this was a retrospective cross-sectionally designed study. Routinely used parameters reflecting inflammatory status in clinical practice, such as WBC, monocyte, and hsCRP levels, were collected and evaluated, and new biomarkers such as interleukin-6, pentraxin 3, and matrix metalloproteinase 9 were not analyzed. Second, due to the lack of follow-up data collected, the results of this study cannot provide any prognostically valuable information regarding MHR in patients with CAD. Third, the monocyte count and HDL-C level were analyzed by a single test; thus, the variation in this index may be neglected. HDL-C has been shown to have multiple subfractions, and high values are not always good. Drug-induced increases in HDL-C levels have not been proven to be automatically beneficial. This limitation may limit the value of the MHR. Further large studies are needed to investigate the relationship between MHR and CAD, including the extent and outcome prediction. Gender stratification may be helpful in these studies.
Author contributions
Data curation: Weifeng Xu, Haiwang Guan, Jingnan Pan.
Formal analysis: Da Gao, Zicheng Wang.
Investigation: Weifeng Xu, Haiwang Guan.
Software: Da Gao.
Supervision: Mahboob Alam, Jiangfang Lian, Jianqing Zhou.
Writing – original draft: Weifeng Xu.
Writing – review & editing: Mahboob Alam.
WEIFENG XU orcid: 0000-0002-7071-9403.
Footnotes
Abbreviations: ACEIs = angiotensin-converting enzyme inhibitors, ACS = acute coronary syndrome, ALB = albumin, ALT = alanine aminotransferase, AMI = acute myocardial infarction, ARBs = angiotensin receptor blockers, AST = aspartate aminotransferase, AUC = area under the curve, CABG = coronary artery bypass grafting, CAD = coronary artery disease, CAG = coronary angiography, CCBs = calcium channel blockers, CI = confidence interval, CVDs = cardiovascular diseases, DBP = diastolic blood pressure, DM = diabetes mellitus, FBG = fasting blood glucose, GGT = gamma-glutamyl transferase, HDL-C = high-density lipoprotein cholesterol, HGB = hemoglobin, HP = hypertension, hsCRP = high sensitivity C-reactive protein, LAD = left anterior descending artery, LCx = left circumflex artery, LDL-C = low-density lipoprotein cholesterol, LM = left main artery, LVEF = left ventricular ejection fraction, MHR = monocyte count to high-density lipoprotein cholesterol ratio, MON = monocyte, NSTE-ACS = non-ST segment elevated acute coronary syndrome, OR = odds ratio, PCI = percutaneous coronary intervention, PLT = platelet, pPCI = primary PCI, RCA = right coronary artery, ROC = receiver-operating characteristic, SBP = systolic blood pressure, Scr = serum creatinine, STEMI = ST segment elevated myocardial infarction, TBIL = total bilirubin, TC = total cholesterol, TG = lipid panel including triglyceride, UA = uric acid, WBC = white blood cell.
How to cite this article: Xu W, Guan H, Gao D, Pan J, Wang Z, Alam M, Lian J, Zhou J. Sex-specific association of monocyte count to high-density lipoprotein ratio with SYNTAX score in patients with suspected stable coronary artery disease. Medicine. 2019;98:41(e17536).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Global Burden of Disease Study Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roth GA, Huffman MD, Moran AE, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015;132:1667–78. [DOI] [PubMed] [Google Scholar]
- [3].Zalokar JB, Richard JL, Claude JR. Leukocyte count, smoking, and myocardial infarction. N Engl J Med 1981;304:465–8. [DOI] [PubMed] [Google Scholar]
- [4].Prentice RL, Szatrowski TP, Fujikura T, et al. Leukocyte counts and coronary heart disease in a Japanese cohort. Am J Epidemiol 1982;116:496–509. [DOI] [PubMed] [Google Scholar]
- [5].Grimm RH, Neaton JD, Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA 1985;254:1932–7. [PubMed] [Google Scholar]
- [6].Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005;45:1638–43. [DOI] [PubMed] [Google Scholar]
- [7].Afiune NA, Mansur AP, Avakian SD, et al. Monocytosis is an independent risk marker for coronary artery disease. Arq Bras Cardiol 2006;86:240–4. [DOI] [PubMed] [Google Scholar]
- [8].Lassale C, Curtis A, Abete I, et al. Elements of the complete blood count associated with cardiovascular disease incidence: findings from the EPIC-NL cohort study. Sci Rep 2018;8:3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Olivares R, Ducimetiere P, Claude JR. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol 1993;137:49–53. [DOI] [PubMed] [Google Scholar]
- [10].Ansell BJ, Navab M, Watson KE, et al. Anti-inflammatory properties of HDL. Rev Endocr Metab Disord 2004;5:351–8. [DOI] [PubMed] [Google Scholar]
- [11].Mineo C, Deguchi H, Griffin JH, et al. Endothelial and antithrombotic actions of HDL. Circ Res 2006;98:1352–64. [DOI] [PubMed] [Google Scholar]
- [12].Nofer JR, van der Giet M, Tolle M, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest 2004;113:569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Platt DE, Ghassibe-Sabbagh M, Youhanna S, et al. Circulating lipid levels and risk of coronary artery disease in a large group of patients undergoing coronary angiography. J Thromb Thrombolysis 2015;39:15–22. [DOI] [PubMed] [Google Scholar]
- [14].Hafiane A, Genest J. High density lipoproteins: measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin 2015;3:175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kanbay M, Solak Y, Unal HU, et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol 2014;46:1619–25. [DOI] [PubMed] [Google Scholar]
- [16].Cetin MS, Cetin EHO, Kalender E, et al. Monocyte to HDL cholesterol ratio predicts coronary artery disease severity and future major cardiovascular adverse events in acute coronary syndrome. Heart Lung Circ 2016;25:1077–86. [DOI] [PubMed] [Google Scholar]
- [17].Cicek G, Kundi H, Bozbay M, et al. The relationship between admission monocyte HDL-C ratio with short-term and long-term mortality among STEMI patients treated with successful primary PCI. Coron Artery Dis 2016;27:176–84. [DOI] [PubMed] [Google Scholar]
- [18].Cagdas M, Karakoyun S, Yesin M, et al. The association between monocyte HDL-C ratio and SYNTAX score and SYNTAX score II in STEMI patients treated with primary PCI. Acta Cardiol Sin 2018;34:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219–27. [PubMed] [Google Scholar]
- [20].Akboga MK, Balci KG, Maden O, et al. Usefulness of monocyte to HDL-cholesterol ratio to predict high SYNTAX score in patients with stable coronary artery disease. Biomark Med 2016;10:375–83. [DOI] [PubMed] [Google Scholar]
- [21].Kundi H, Kiziltunc E, Cetin M, et al. Association of monocyte/HDL-C ratio with SYNTAX scores in patients with stable coronary artery disease. Herz 2016;41:523–9. [DOI] [PubMed] [Google Scholar]
- [22].Yilmaz M, Kayancicek H. A new inflammatory marker: elevated monocyte to HDL cholesterol ratio associated with smoking. J Clin Med 2018;7: pii: E76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang CH, Hsieh MJ, Chen CC, et al. SYNTAX score: an independent predictor of long-term cardiac mortality in patients with acute ST-elevation myocardial infarction. Coron Artery Dis 2012;23:445–9. [DOI] [PubMed] [Google Scholar]
- [24].Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med 1976;295:369–77. [DOI] [PubMed] [Google Scholar]
- [25].Castelli WP. Cholesterol and lipids in the risk of coronary artery disease--the Framingham heart study. Can J Cardiol 1988;4:5A–10A. [PubMed] [Google Scholar]
- [26].Romm PA, Green CE, Reagan K, et al. Relation of serum lipoprotein cholesterol levels to presence and severity of angiographic coronary artery disease. Am J Cardiol 1991;67:479–83. [DOI] [PubMed] [Google Scholar]
- [27].Tornvall P, Karpe F, Proudler A, et al. High-density lipoprotein: relations to metabolic parameters and severity of coronary artery disease. Metabolism 1996;45:1375–82. [DOI] [PubMed] [Google Scholar]
- [28].Syvanne M, Pajunen P, Kahri J, et al. Determinants of the severity and extent of coronary artery disease in patients with type-2 diabetes and in nondiabetic subjects. Coron Artery Dis 2001;12:99–106. [DOI] [PubMed] [Google Scholar]
- [29].Kosuge K, Sasaki H, Ikarashi T, et al. Risk factors for severe coronary artery disease - a case-control study of patients who have undergone coronary artery bypass grafting. J Atheroscler Thromb 2006;13:62–7. [DOI] [PubMed] [Google Scholar]
- [30].Hu W, Guo ZG, Chen J, et al. Correlation between cardiovascular risk factors and the severity of coronary artery lesions in female patients. Nan Fang Yi Ke Da Xue Xue Bao 2009;29:307–9. [PubMed] [Google Scholar]
- [31].Alber HF, Wanitschek MM, de Waha S, et al. High-density lipoprotein cholesterol, C-reactive protein, and prevalence and severity of coronary artery disease in 5641 consecutive patients undergoing coronary angiography. Eur J Clin Invest 2008;38:372–80. [DOI] [PubMed] [Google Scholar]
- [32].Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135–43. [DOI] [PubMed] [Google Scholar]
- [33].Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–74. [DOI] [PubMed] [Google Scholar]
- [34].Natarajan R, Cai Q. Monocyte retention in the pathology of atherosclerosis. Future Cardiol 2005;1:331–40. [DOI] [PubMed] [Google Scholar]
- [35].Meuret G, Bammert J, Hoffmann G. Kinetics of human monocytopoiesis. Blood 1974;44:801–16. [PubMed] [Google Scholar]
- [36].Ikata J, Wakatsuki T, Oishi Y, et al. Leukocyte counts and concentrations of soluble adhesion molecules as predictors of coronary atherosclerosis. Coron Artery Dis 2000;11:445–9. [DOI] [PubMed] [Google Scholar]
- [37].Kocaman SA, Sahinarslan A, Cemri M, et al. Independent relationship of serum uric acid levels with leukocytes and coronary atherosclerotic burden. Nutr Metab Cardiovasc Dis 2009;19:729–35. [DOI] [PubMed] [Google Scholar]
- [38].Ganda A, Magnusson M, Yvan-Charvet L, et al. Mild renal dysfunction and metabolites tied to low HDL cholesterol are associated with monocytosis and atherosclerosis. Circulation 2013;127:988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ucar FM. A potential marker of bare metal stent restenosis: monocyte count-to-HDL cholesterol ratio. BMC Cardiovasc Disord 2016;16:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tok D, Turak O, Yayla C, et al. Monocyte to HDL ratio in prediction of BMS restenosis in subjects with stable and unstable angina pectoris. Biomark Med 2016;10:853–60. [DOI] [PubMed] [Google Scholar]
- [41].Canpolat U, Cetin EH, Cetin S, et al. Association of monocyte-to-HDL cholesterol ratio with slow coronary flow is linked to systemic inflammation. Clin Appl Thromb Hemost 2016;22:476–82. [DOI] [PubMed] [Google Scholar]
- [42].Cetin EH, Cetin MS, Canpolat U, et al. Monocyte/HDL-cholesterol ratio predicts the definite stent thrombosis after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Biomark Med 2015;9:967–77. [DOI] [PubMed] [Google Scholar]
- [43].Iqbal J, Zhang YJ, Holmes DR, et al. Optimal medical therapy improves clinical outcomes in patients undergoing revascularization with percutaneous coronary intervention or coronary artery bypass grafting: insights from the synergy between percutaneous coronary intervention with TAXUS and cardiac surgery (SYNTAX) trial at the 5-year follow-up. Circulation 2015;131:1269–77. [DOI] [PubMed] [Google Scholar]


