Abstract
Background:
Community-acquired pneumonia (CAP) is a potentially life-threatening condition. The aim of this study is to investigate the stratified and prognostic value of admission lactate and severity scores (confusion, urea >7 mmol/L, respiratory rate ≥30/min, blood pressure <90 mm Hg systolic and/or ≤60 mm Hg diastolic, and age ≥65 years [CURB65], pneumonia severity index [PSI], sequential organ failure assessment [SOFA], qSOFA) in patients with CAP in emergency department.
Methods:
Adult patients diagnosed with CAP admitted between January 2017 and January 2019 were enrolled and divided into severe CAP (SCAP) group and nonSCAP (NSCAP) group according to international guidelines, death group, and survival group according to 28-day prognosis. Predicting performance of parameters above was compared using receiver operating characteristic curves and logistic regression model. Cox proportional hazard regression model was used to identify variables independently associated with 28-day mortality.
Results:
A total of 350 patients with CAP were enrolled. About 196 patients were classified as SCAP and 74 patients died after a 28-day follow-up. The levels of CURB65, PSI, SOFA, qSOFA, and admission lactate were higher in the SCAP group and death group. SOFA showed advantage in predicting SCAP, while qSOFA is superior in predicting 28-day mortality. The combination of SOFA and admission lactate outperformed other combinations in predicting SCAP, and the combination of qSOFA and lactate showed highest superiority over other combinations in predicting 28-day mortality.
Conclusion:
The SOFA is a valuable predictor for SCAP and qSOFA is superior in predicting 28-day mortality. Combination of qSOFA and admission lactate can improve the predicting performance of single qSOFA.
Keywords: community-acquired pneumonia, lactate, prognosis, sequential organ failure assessment
1. Introduction
Community-acquired pneumonia (CAP) has plagued humankind for centuries and has claimed numerous lives all over the world. CAP is a leading cause of hospitalization and death with heavy medical cost burden in the United States.[1] Typical symptoms of patients with CAP includes fever, a productive cough with purulent sputum, dyspnea, and chest pain. The most common pathogens comprise of virus, bacteria, fungi, etc. The severity of CAP varies from mild to life-threatening. Severity assessment and prognosis prediction are of vital significance, as early identification of severe CAP can screen patients who may require aggressive therapy and reduce mortality.
Nowadays, the majority of international guidelines recommend the use of severity evaluation tools to triage the treatment location of patients with CAP, and the most widely used scores of them are pneumonia severity index (PSI) score and confusion, urea >7 mmol/L, respiratory rate >30/min, low systolic (<90 mm Hg) or diastolic (≤60 mm Hg) blood pressure, and age ≥65 years old (CURB65) score.[2,3] The PSI score, a 20-parameter score with different weights, is complex and inconvenient to use.[3] Comparatively, the CURB65 score is comprised of 5 variables and is easier to remember.[2] However, the CURB65 score has lower sensitivity to identify patients with severe CAP (SCAP) with high risk of mortality.[2,4]
The Sepsis 3.0 Task Force excluded the need for systemic inflammatory response syndrome (SIRS) criteria and renewed the diagnostic criteria of sepsis, which was defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection.[5] Organ dysfunction was characterized by an increase in sequential organ failure assessment (SOFA) score of 2 points or more.[5] The bedside quick SOFA (qSOFA) score, which incorporated hypotension, altered mental status and tachypnea, has been utilized as a tool to screen sepsis outside the intensive care unit (ICU). Comparatively, SOFA showed superior prognostic accuracy for in-hospital mortality than qSOFA.[6,7] However, the clinical performance of SOFA and qSOFA in CAP has not yet been fully elucidated.
Serum lactate is an important predictor for mortality in patients with sepsis and hyperlactatemia on admission is an early biomarker of organ failure.[8] Moreover, a lactate level >2 mmol/L and a requirement of vasopressors to maintain a mean arterial blood pressure of 65 mm Hg in patients with sepsis were defined as septic shock.[5,8] CAP is one of the most important causes of sepsis and international guidelines recommend measurement of lactate level within 3 hours of presentation as part of sepsis bundle.[9,10] However, only few studies exist regarding the value of lactate level at admission in the stratification and prognosis prediction of patients with CAP.
The aim of the present study was to evaluate the stratified and prognostic prediction value of CAP severity scores (CURB65, PSI, SOFA, qSOFA) and lactate level at admission in patients with CAP in the emergency department (ED).
2. Methods
This was a retrospective and observational monocentric cohort study carried out in Beijing Chao-yang Hospital, Capital Medical University, which is a tertiary teaching hospital with approximately 250,000 annual ED visits. The Institutional Review Board and Medical Ethics Committee has approved this study. The requirement of written informed consents was waived because of the retrospective design of this study.
The procedures of enrolling patients were as follows: Adult patients diagnosed with CAP[11] with available radiologic and laboratory data admitted between January 2017 and January 2019 were enrolled. CAP was diagnosed by acute onset of symptoms and presence of signs of lower respiratory tract infection initiated in the community, with new pulmonary infiltrates on chest radiography[11]; The medical records of all enrolled patients were collected and reviewed; Demographic characteristics including comorbidities, vital signs on admission of all enrolled patients on ED arrival were collected and recorded. Laboratory data on admission including full blood count, hemoglobin level, hemocrit, platelet level, albumin, hepatic function (aspartate aminotransferase, alanine aminotransferase, total bilirubin, direct bilirubin), renal function (creatinine, blood urea nitrogen [BUN]), electrolytes, and arterial blood gas including lactate level were assessed and collected. CURB65, PSI, SOFA and qSOFA scores for each patient were calculated according to international criteria and analyzed.
The following patients were excluded: patients with acquired immunodeficiency syndrome, active tuberculosis or metastatic tumor; patients finally diagnosed with pulmonary cancer, noninfectious interstitial lung disease, pulmonary edema, pulmonary embolism, and pulmonary vasculitis; patients with pregnancy; patients transferred from other hospitals, discharged from hospital within 10 days or diagnosed with hospital acquired pneumonia; and patients with incomplete clinical, laboratory, or radiographic records.
All enrolled patients were categorized into severe CAP (SCAP) group or nonsevere CAP (NSCAP) group according to consensus guidelines.[11] Patients with SCAP should meet the following criteria (≥1 major criteria or ≥3 minor criteria). Major criteria: invasive mechanical ventilation, and septic shock with the need for vasopressors. Minor criteria: respiratory rate ≥30 breaths/min, PaO2/FiO2 ratio ≤250, multilobar infiltrates, confusion/disorientation, uremia (BUN level ≥20 mg/dL), leukopenia (white blood cell count <4000 cells/mm3), thrombocytopenia (platelet count <100,000 cells/mm3), hypothermia (core temperature <36°C), and hypotension requiring aggressive fluid resuscitation. The 28-day mortality after admission was the primary end point. According to their 28-day prognosis, patients were also categorized into death group or survival group.
All analyses were performed using SPSS 22.0 statistical software package (SPSS Inc, Chicago, IL). Data with normal distribution were presented as mean ± standard deviation and compared using Student t test. Data with skewed distribution were presented as median (interquartile range) and compared using Mann–Whitney U nonparametric test. The categorical variables were described as percentages and compared using the Chi-squared test or Fisher exact test. The independent predictors of outcomes (SCAP or 28-day mortality) were determined by stepwise binary logistic regression analysis. Receiver operating characteristic (ROC) curves for each predictor were constructed and the area under the curve (AUC) was determined to assess their predictive values. Comparisons of each predictor were conducted using MedCalc 15.0 Software (Acacialaan, Ostend, Belgium). A Z test was used for comparing the AUCs between different curves. For comparison of the AUCs, Z = (A1 − A2)/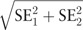 was used, the test values being Z0.05 = 1.96 and Z0.01 = 2.58. Based on the cutoff values, sensitivity, specificity, positive predictive value (PPV), and negative predictive value were also calculated. A Cox regression model was used to identify variables independently associated with mortality. Kaplan−Meier survival curve were drawn using cutoff values of each predictors. A 2-tailed value of P < .05 was considered statistically significant.
was used, the test values being Z0.05 = 1.96 and Z0.01 = 2.58. Based on the cutoff values, sensitivity, specificity, positive predictive value (PPV), and negative predictive value were also calculated. A Cox regression model was used to identify variables independently associated with mortality. Kaplan−Meier survival curve were drawn using cutoff values of each predictors. A 2-tailed value of P < .05 was considered statistically significant.
3. Results
During the study period, 437 patients with CAP were recruited and screened. Of them, 87 patients were excluded: Four patients were diagnosed with pulmonary tuberculosis, 3 patients with pulmonary embolism, 6 patients with lung cancer, and 3 patients with interstitial lung disease. Four patients were HIV-positive and 3 patients were with pregnancy. Twelve patients were transferred from other hospitals, 8 patients discharged within 10 days after admission, 24 patients were with incomplete medical records, and 22 patients were missing after 28-day follow-up (Fig. 1). A total of 350 sex ratio and age-matched patients were finally enrolled in our present study. Of them, 196 patients were categorized as SCAP and 154 patients as NSCAP, 74 patients died, and 276 patients survived after 28-day follow-up (Table 1). The total mortality rate was 21.1%. The mean age of the entire cohort enrolled was 78 (65–84) and 217 patients were male. Comorbidities of enrolled patients included chronic obstructive pulmonary disease (13.1%), cardiovascular disease (16.9%), cerebral-vascular disease (26.9%), diabetes (24.3%), chronic renal disease (9.1%), and hepatobilillary disease (8.0%). There was no significant difference between the SCAP and NSCAP group, or the death and survival group in age, sex ratio, and comorbidities (Table 1).
Figure 1.
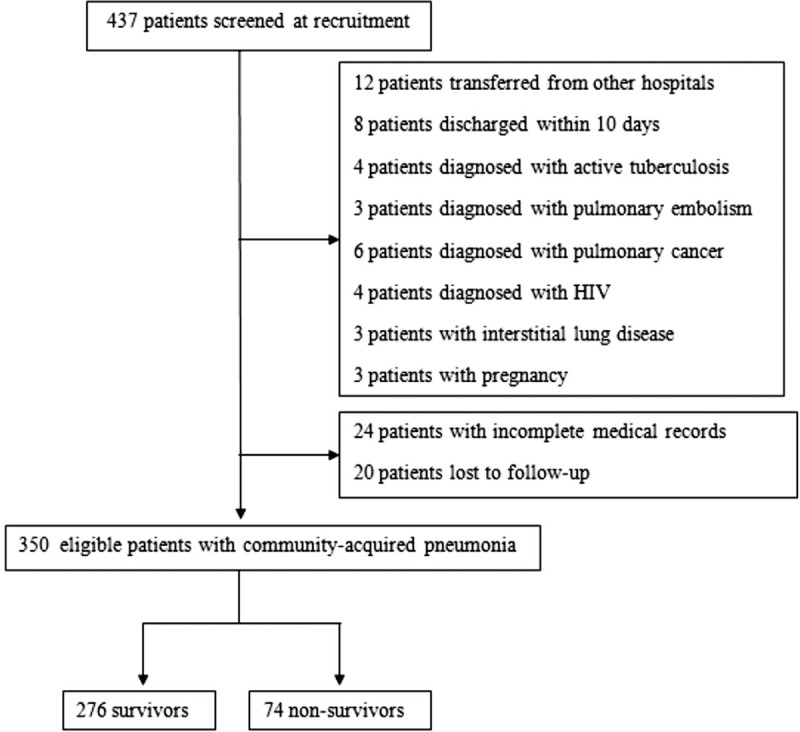
Flow chart of patients with community-acquired pneumonia enrolled in this study.
Table 1.
Baseline characteristics of enrolled patients with CAP in ED.
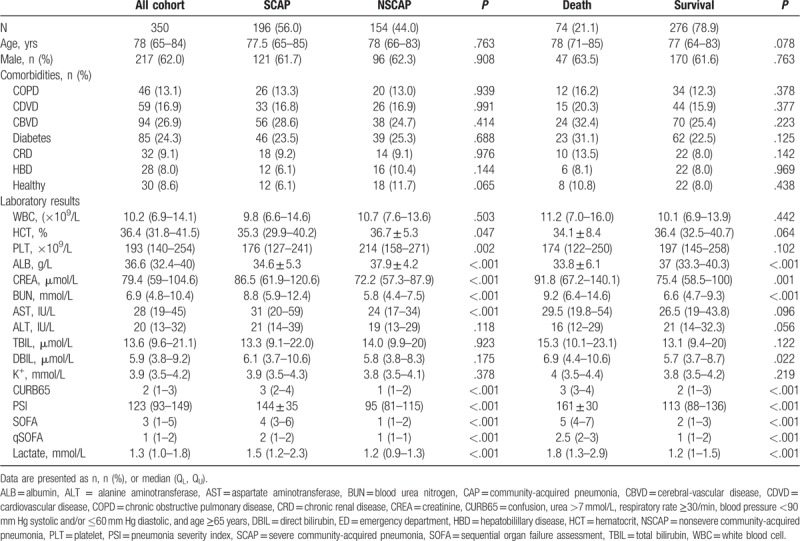
There were significant differences between either SCAP group and NSCAP group or the death and survival group in CURB65, PSI, SOFA, qSOFA, and lactate levels (P < .05) (Table 1). The CURB65, PSI, SOFA, qSOFA, and lactate levels were significantly higher in SCAP group and death group than that in NSCAP group and survival group, respectively.
In predicting SCAP, the AUROC of SOFA (0.871) was the highest among single predictors, followed by CURB65 (0.854), PSI (0.841), qSOFA (0.806), and lactate (0.716) (Table 2, Fig. 2A). Moreover, SOFA achieved the highest specificity and PPV, highlighting the superiority of SOFA. The AUROC of admission lactate was the least among single predictors in predicting SCAP. Pairwise comparisons among single predictors in predicting SCAP revealed that there was no significant difference between SOFA and CURB65 (P = .477), SOFA and PSI (P = .189), CURB65 and PSI (P = .459), while significant differences were found between SOFA and qSOFA (P = .0066), SOFA and lactate (P < .0001), CURB65 and lactate (P < .0001), PSI and lactate (P = .0001), qSOFA and lactate (P = .0055). Among the combinations of severity scores and lactate, the combination of SOFA + lactate achieved the highest AUROC (0.876) in predicting SCAP, followed by the combination of CURB65 + lactate (0.874), PSI + lactate (0.856), qSOFA + lactate (0.849) (Fig. 2B). Pairwise comparisons revealed no significant difference between SOFA + lactate and CURB65 + lactate (P = .920), SOFA + lactate and PSI + lactate (P = .334), SOFA + lactate and qSOFA + lactate (P = .148). However, the combination of SOFA + lactate did not show advantage compared with single SOFA (P = .404), while significant differences were found between CURB65 + lactate and single CURB65 (P = .031), PSI + lactate and single PSI (P = .029), qSOFA + lactate and single qSOFA (P = .004).
Table 2.
Statistical data of ROC curve comparisons between CAP severity scores and admission lactate in predicting SCAP.
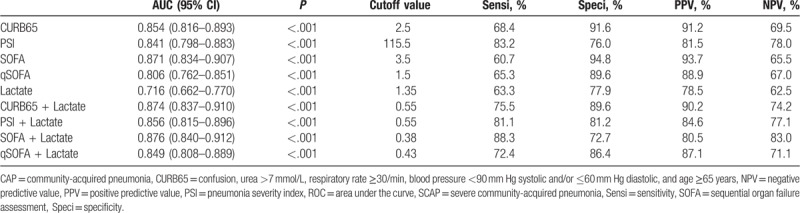
Figure 2.
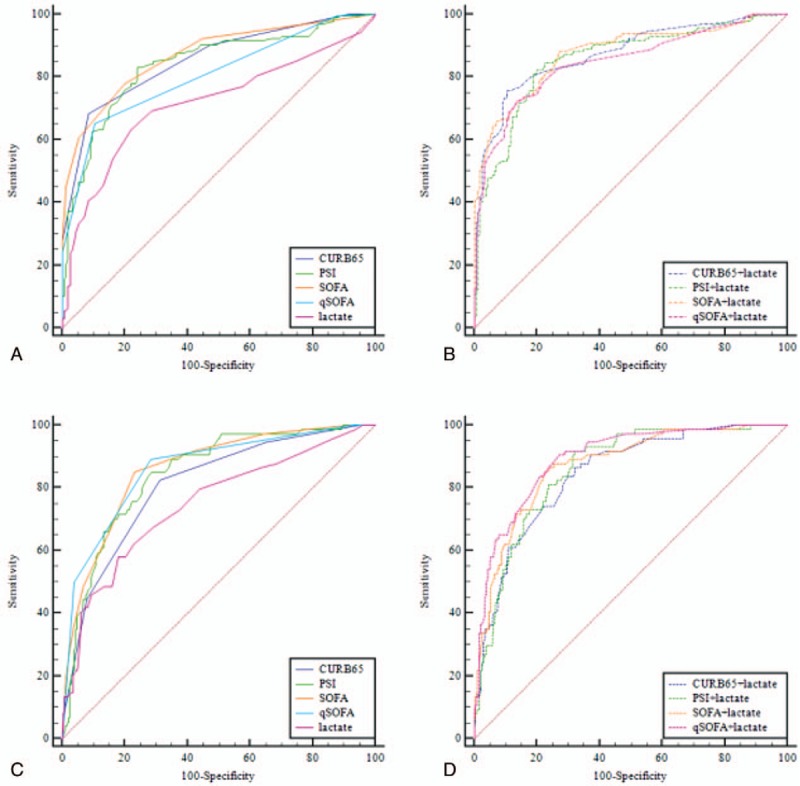
(A) Receiver operating characteristic (ROC) curve comparisons of different severity scores and admission lactate in predicting severe community acquired pneumonia (SCAP). (B) ROC curve comparisons of different combinations of severity scores and admission lactate in predicting SCAP. (C) ROC curve comparisons of different severity scores and admission lactate in predicting 28-day mortality. (D) ROC curve comparisons of different combinations of severity scores and admission lactate in predicting 28-day mortality.
In predicting 28-day mortality, the AUROC of qSOFA (0.861) was the highest among single predictors, followed by SOFA (0.860), PSI (0.845), CURB65 (0.808), and lactate (0.748) (Table 3, Fig. 2C). The AUROC of admission lactate was the least among single predictors in predicting 28-day mortality. Pairwise comparisons among single predictors revealed that there was no significant difference between qSOFA and SOFA (P = .967), qSOFA and PSI (P = .534), CURB65 and PSI (P = .139). Significant differences were found between qSOFA and CURB65 (P = .043), qSOFA and lactate (P = .0027), PSI and lactate (P = .008), SOFA and lactate (P = .001). Among the combinations of severity scores and lactate, the combination of qSOFA + lactate achieved the highest AUROC (0.893), followed by the combinations of SOFA + lactate (0.870), PSI + lactate (0.857), and CURB65 + lactate (0.844) (Fig. 2D). Pairwise comparisons among combinations of severity scores and lactate revealed that there was no significant difference between qSOFA + lactate and SOFA + lactate (P = .257), qSOFA + lactate and PSI + lactate (P = .084), CURB65 + lactate and PSI + lactate (P = .460), CURB65 + lactate and SOFA + lactate (P = .277), PSI + lactate and SOFA + lactate (P = .570), while significant difference was found between qSOFA + lactate and CURB65 + lactate (P = .011). Significant differences were also found between qSOFA + lactate and single qSOFA (P = .038), CURB65 + lactate and single CURB65 (P = .015), while there was no significant difference between the combination of SOFA + lactate and single SOFA (P = .230), PSI + lactate and single PSI (P = .076).
Table 3.
Statistical data of ROC curve comparisons between CAP severity scores and admission lactate in predicting 28-day mortality.

Binary logistic regression analysis revealed that CURB65 (OR = 3.268), SOFA (OR = 2.079), and qSOFA (OR = 2.185) entered the regression model and were independent predictors for SCAP, and PSI (OR = 1.016), SOFA (OR = 1.343), and qSOFA (OR = 3.729) were independent predictors for 28-day mortality (Table 4). Cox regression analysis revealed that PSI, SOFA, and qSOFA independently associated with mortality of patients with CAP, and the adjusted hazard ratios are summarized in Table 5. Kaplan–Meier survival curves using cutoff values of each predictors are described in Figure 3 (all P < .001).
Table 4.
Independent predictors by multivariate binary logistic regression analysis.
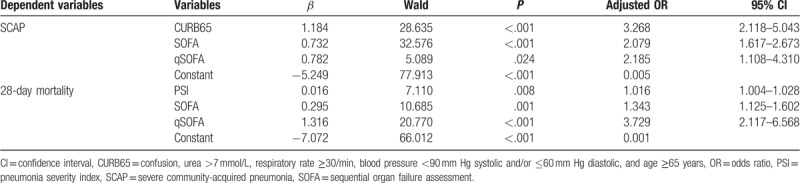
Table 5.
Independent predictors for 28-day mortality of patients with community-acquired pneumonia in emergency department.

Figure 3.
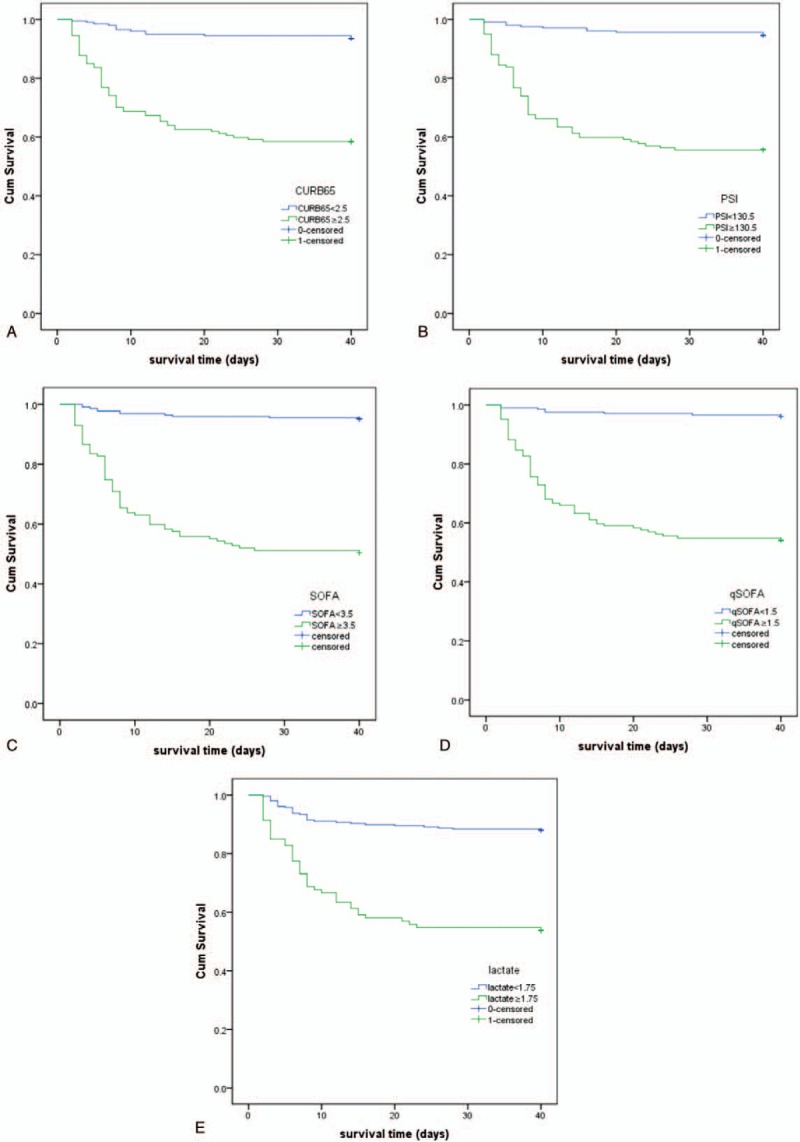
(A) Kaplan–Meier survival curve for patients with community-acquired pneumonia (CAP) using cutoff value of CURB65 score (P < .001, censored at 40 days). (B) Kaplan–Meier survival curve for patients with CAP using cutoff value of pneumonia severity index (PSI) score (P < .001, censored at 40 days). (C) Kaplan–Meier survival curve for patients with CAP using cutoff value of sequential organ failure assessment (SOFA) score (P < .001, censored at 40 days). (D) Kaplan–Meier survival curve for patients with CAP using cutoff value of qSOFA score (P < .001, censored at 40 days). (E) Kaplan–Meier survival curve for patients with CAP using cutoff value of admission lactate (P < .001, censored at 40 days).
4. Discussion
Our study is among the very few researches that explored the stratified and prognostic value of CURB65, PSI, SOFA, qSOFA, and admission lactate level at the same time. Our study revealed that among single predictors, SOFA demonstrated the highest AUROC compared with CURB65, PSI, qSOFA, and lactate in predicting SCAP, while qSOFA showed superiority in predicting 28-day mortality, though SOFA and qSOFA did not show absolute superiority among further multiple pairwise comparisons. These may be owing to the relatively small sample size and single center design, and we need more large-cohort in-depth researches to explore these results. The admission lactate level demonstrated least superiority in predicting either SCAP or 28-day mortality among single predictors. Moreover, among the combinations of severity scores and lactate, the combination of SOFA + lactate outperformed other combinations in predicting SCAP, while the combination of qSOFA + lactate demonstrated advantage over other combinations in predicting 28-day mortality. Our results highlighted the superiority of SOFA and qSOFA in the risk stratification and prognosis prediction value in patients with CAP in ED. Physicians in ED could utilize these tools to assist routine clinical practice to enhance their awareness of CAP and reduce mortality.
The CAP, a respiratory tract infectious disease and a common presentation to ED, will remain a major source of morbidity and mortality in the elderly in western countries for years to come due to demographic changes.[12] Optimum management of patients with CAP is of vital importance to reduce mortality. CAP usually presents with a wide spectrum of diseases from mild and self-limiting to life-threatening and sometimes fatal.[13] Severity assessment strategy is recommended and it is crucial for selection of appropriate treatment location, initial empirical antibiotic agents as well as adjective and supportive therapies.[14] Various different kinds of CAP scoring systems exist and they can be utilized to evaluate and provide support for clinical diagnosis and treatment, while physicians should take clinical judgment and experience into consideration to avoid overestimation and underestimation of severity of CAP.[13,14]
The most common used severity assessment tools for CAP are PSI score and CURB65 score. The PSI score, developed in 1997 and comprising of 20 variables including demographics, comorbidities, and clinical variables, categorized patients into 5 risk classes.[3] Classes I and II are at low risk and recommended for outpatient treatment, patients in class III without obvious desaturation can also be treated in outpatient, while patients in classes IV and V are suggested to be managed as inpatients in the majority of patients.[3,12] An observational study by Renaud et al showed that PSI-using could reduce outpatients treatment rate and the mortality rates were lower in ED which used the PSI.[12,15] Moreover, implementation of PSI resulted in an significant increase of patients treated in the community without an increase of mortality and hospital readmission.[12,16] Nonetheless, in viewing of the crowdedness of EDs, PSI is relatively cumbersome and inconvenient to use, requiring 20 variables with different weights. In addition, PSI underestimate severity of young patients with CAP and was not advised to guide ICU admission,[17,18] and the underlying health conditions may strongly influence mortality based on severity in elderly patients.[19] Study by Zhang et al showed that PSI performed better than CURB65 for mortality prediction, while its discriminative power decreased with advancing age.[20] However, in our study, the predicting performance for 28-day mortality of PSI was similar to qSOFA and CURB65.
The CURB65 score comprised of 5 variables and categorized patients into 3 risk groups scores 0 to 1: low risk of 30-day mortality; score 2: intermediate risk of 30-day mortality; scores 3 to 5: high risk of 30-day mortality.[2,12] It was primarily designed to predict mortality and screen low-risk patients with CAP suitable for ambulatory management.[2,21] The CURB65 score has been extensively validated and performed similarly to the PSI score in predicting 30-day mortality of patients with CAP,[13] though previous study revealed that CURB65 may be more suitable for identifying high risk patients, while PSI had advantage in the identification of low risk patients.[22] The simplicity of calculation of CURB65 showed superiority over other complex scoring systems in ED.[21] In view of the simplicity, the CRB65 score, which excluded the only laboratory urea criteria, was recommended in outpatient use and has gained widespread acceptance. Previous data reported no obvious difference in predictive accuracy for 30-day mortality between PSI, CURB65, and CRB65.[22]
The sepsis 3.0 Task Force has updated the definition of sepsis, excluding the need for SIRS criteria.[5] However, the clinical implication of SOFA and qSOFA in CAP has not been fully elucidated in detail.[23] Ranzani et al demonstrated that qSOFA outperformed SIRS and performed better clinical usefulness as prompt tools for patients with CAP in ED.[23] Kim et al reported an AUC of 0.83, 0.81, 0.86, and 0.77 in mortality prediction for SOFA, qSOFA, PSI, and CURB65, respectively.[24] Tokioka et al proved that the prognostic performance of qSOFA for in-hospital mortality and ICU admission was not significantly different from those of PSI and CURB65.[6] A meta-analysis by Jiang et al analyzed 6 studies which enrolled 17,868 patients, and results indicated that a qSOFA score ≥2 is strongly associated with mortality, while the low sensitivity of qSOFA limit the early identification of mortality in patients with pneumonia.[25] Another research by Song et al analyzed 443 patients and reported an AUROC of 0.72 for qSOFA in mortality prediction.[8] They also concluded that qSOFA and lactate is useful and practical in the early prediction of in-hospital mortality among patients with CAP in ED.[8] The predicting performance of qSOFA in our research coincided with previous studies.[6,8,24] However, the mortality rate of our cohort was higher (21.1%), this may due to that the patients enrolled in our group were with higher age.
Serum lactate has been a useful biomarker and predictor for perfusion status and risk stratification of patients with sepsis.[26] Elevation of serum lactate is a manifestation of organ dysfunction and higher lactate level is associated with higher hospital mortalities and longer ED stay.[27] Few previous studies have investigated the value of lactate in the risk stratification and prognosis prediction in patients with CAP. Song et al retrospectively analyzed 443 patients with CAP, and results indicated that the AUROC of qSOFA with lactate was not significantly different from SOFA (0.828 vs 0.845, P = .509), and qSOFA with lactate is a useful and practical tool for prediction of in-hospital mortality of patients with CAP.[8] Another study by Frenzen et al enrolled 303 patients with CAP, while results revealed that the admission lactate predicted poor prognosis independent of other prognostic parameters and the combination of lactate and CRB/CURB65 outperformed CRB/CURB65 alone.[9] Gwak et al reported that the initial lactate level is independently associated with mortality in hospitalized patients with CAP, while laboratory parameters of PSI were not.[28] A similar study by Demirel also concluded that the lactate level, the PSI and CURB65 are good predictors for in-hospital mortality in patients with pneumonia.[29]
Severity assessment tools can help physicians recognize SCAP and take timely interventional measures. Regrettably, limitations still exist for these severity assessment tools.[30] Firstly, previous researches did not exclude patients with requirements to withhold life-sustaining equipments, which could compromise the validity of their findings.[30] In addition, local culture and resources may exert influence on the decision for ICU admission.[30,31] Moreover, these tools are not perfect enough yet, and patients with good prognosis may be admitted to an source-limited ICU, while others at risk of death may be neglected.[30] These may finally exert influence on treatment of patients with CAP, thus leading to different prognoses.
There are some limitations needed to be addressed. Firstly, the relatively small sample size and the retrospective study design may result in selection bias and limit the generalizability of our results for external validity. Secondly, our research only enrolled patients with CAP in ED. These patients are with older age and more complications, which could have higher mortality rate and may influence the final results. Last but not least, some of our enrolled patients have influenza coinfection and we did not analyze them separately. This may also exert influence on our results. We hope to explore the predictive value of these predictors on influenza CAP in our further in-depth researches.
5. Conclusion
In conclusion, we analyzed the stratified and prognostic prediction value of CURB65, PSI, SOFA, qSOFA, and lactate at the same time in ED of our solitary center. We found that SOFA is superior to other predictors in predicting SCAP, while qSOFA is superior in predicting 28-day mortality, and the combination of qSOFA and lactate could improve the predicting performance of single qSOFA. More multicenter studies with larger sample size are needed to validate our results.
Author contributions
Conceptualization: Haijiang Zhou, Tianfei Lan, Shubin Guo.
Data curation: Haijiang Zhou, Tianfei Lan.
Formal analysis: Haijiang Zhou, Tianfei Lan, Shubin Guo.
Investigation: Haijiang Zhou, Tianfei Lan.
Methodology: Haijiang Zhou, Tianfei Lan, Shubin Guo.
Software: Haijiang Zhou, Tianfei Lan.
Supervision: Shubin Guo.
Validation: Haijiang Zhou, Tianfei Lan, Shubin Guo.
Visualization: Haijiang Zhou, Tianfei Lan.
Writing – original draft: Haijiang Zhou, Tianfei Lan.
Writing – review & editing: Shubin Guo.
Footnotes
Abbreviations: AUC = area under the curve, AUROC = area under the receiver operating characteristics curve, BUN = blood urea nitrogen, CAP = community-acquired pneumonia, CURB65 = confusion, urea >7mmol/L, respiratory rate ≥30/min, blood pressure <90mm Hg systolic and/or ≤60mm Hg diastolic, and age ≥65 years, ED = emergency department, ICU = intensive care unit, NSCAP = nonsevere community acquired pneumonia, PPV = positive predictive value, PSI = pneumonia severity index, ROC = receiver operating characteristics, SCAP = severe community-acquired pneumonia, SOFA = sequential organ failure assessment.
How to cite this article: Zhou H, Lan T, Guo S. Stratified and prognostic value of admission lactate and severity scores in patients with community-acquired pneumonia in emergency department. Medicine. 2019;98:41(e17479).
HZ and TL are both first authors and they contributed equally to this article.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997;336:243–50. [DOI] [PubMed] [Google Scholar]
- [4].Ahn JH, Choi EY. Expanded A-DROP score: a new scoring system for the prediction of mortality in hospitalized patients with community-acquired pneumonia. Sci Rep 2018;8:14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tokioka F, Okamoto H, Yamazaki A, et al. The prognostic performance of qSOFA for community-acquired pneumonia. J Intensive Care 2018;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA 2017;317:290–300. [DOI] [PubMed] [Google Scholar]
- [8].Song H, Moon HG, Kim SH. Efficacy of quick sequential organ failure assessment with lactate concentration for predicting mortality in patients with community-acquired pneumonia in the emergency department. Clin Exp Emerg Med 2019;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Frenzen FS, Kutschan U, Meiswinkel N, et al. Admission lactate predicts poor prognosis independently of the CRB/CURB-65 scores in community-acquired pneumonia. Clin Microbiol Infect 2018;24:306.e1–6. [DOI] [PubMed] [Google Scholar]
- [10].Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- [11].Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44Suppl 2:S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chalmers JD, Rutherford J. Can we use severity assessment tools to increase outpatient management of community-acquiredpneumonia? Eur J Intern Med 2012;23:398–406. [DOI] [PubMed] [Google Scholar]
- [13].Lim WS, Baudouin SV, George RC, et al. British Thoracic Society guidelines for the management of community-acquired pneumonia in adults: update 2009. Thorax 2009;64:iii1–55. [DOI] [PubMed] [Google Scholar]
- [14].Cao B, Huang Y, She DY, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J 2018;12:1320–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Renaud B, Coma E, Labarere J, et al. Routine use of the Pneumonia Severity Index for guiding the site-of-treatment decision of patients with pneumonia in the emergency department: a multi-center, prospective, observational, controlled cohort study. Clin Infect Dis 2007;44:41–9. [DOI] [PubMed] [Google Scholar]
- [16].Chalmers JD, Akram AR, Hill AT. Increasing outpatient treatment of mild community-acquired pneumonia: systematic review and meta-analysis. Eur Respir J 2011;37:858–64. [DOI] [PubMed] [Google Scholar]
- [17].Chalmers JD, Singanayagam A, Hill AT. Predicting the need for mechanical ventilation and/or inotropic support for young adults admitted to the hospital with community-acquired pneumonia. Clin Infect Dis 2008;47:1571–4. [DOI] [PubMed] [Google Scholar]
- [18].Chalmers JD, Mandal P, Singanayagam A, et al. Severity assessment tools to guide ICU admission in community-acquired pneumonia: systematic review and meta-analysis. Intensive Care Med 2011;37:1409–20. [DOI] [PubMed] [Google Scholar]
- [19].Hamaguchi S, Suzuki M, Sasaki K, et al. Adult Pneumonia Study Group – Japan. Six underlying health conditions strongly influence mortality based on pneumonia severity in an ageing population of Japan: a prospective cohort study. BMC Pulm Med 2018;18:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang ZX, Yong Y, Tan WC, et al. Prognostic factors for mortality due to pneumonia among adults from different age groups in Singapore and mortality predictions based on PSI and CURB-65. Singapore Med J 2018;59:190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen YX, Li CS. Lactate on emergency department arrival as a predictor of mortality and site-of-care in pneumonia patients: a cohort study. Thorax 2015;70:404–10. [DOI] [PubMed] [Google Scholar]
- [22].Chalmers JD, Singanayagam A, Akram AR, et al. Severity assessment tools for predicting mortality in hospitalized patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 2010;65:878–83. [DOI] [PubMed] [Google Scholar]
- [23].Ranzani OT, Prina E, Menéndez R, et al. New sepsis definition (sepsis-3) and community-acquired pneumonia mortality. A validation and clinical decision-making study. Am J Respir Crit Care Med 2017;196:1287–97. [DOI] [PubMed] [Google Scholar]
- [24].Kim MW, Lim JY, Oh SH. Mortality prediction using serum biomarkers and various clinical risk scales in community-acquired pneumonia. Scand J Clin Lab Invest 2017;77:486–92. [DOI] [PubMed] [Google Scholar]
- [25].Jiang J, Yang J, Jin Y, et al. Role of qSOFA in predicting mortality of pneumonia: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009;37:1670–7. [DOI] [PubMed] [Google Scholar]
- [27].Bou Chebl R, El Khuri C, Shami A, et al. Serum lactate is an independent predictor of hospital mortality in critically ill patients in the emergency department: a retrospective study. Scand J Trauma Resusc Emerg Med 2017;25:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gwak MH, Jo S, Jeong T, et al. Initial serum lactate level is associated with inpatient mortality in patients with community-acquired pneumonia. Am J Emerg Med 2015;33:685–90. [DOI] [PubMed] [Google Scholar]
- [29].Demirel B. Lactate levels and pneumonia severity index are good predictors of in-hospital mortality in pneumonia. Clin Respir J 2018;12:991–5. [DOI] [PubMed] [Google Scholar]
- [30].Phua J, Dean NC, Guo Q, et al. Severe community-acquired pneumonia: timely management measures in the first 24 hours. Crit Care 2016;20:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wunsch H, Angus DC, Harrison DA, et al. Comparison of medical admissions to intensive care units in the United States and United Kingdom. Am J Respir Crit Care Med 2011;183:1666–73. [DOI] [PubMed] [Google Scholar]


