Abstract
Immune checkpoint inhibitors (ICIs) like cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA4) and programmed death cell protein 1 (anti-PD1) have revolutionized cancer treatment. As ICI use becomes widespread, more immune-related adverse events (irAE's) are being reported. Our aim was to investigate the frequency and nature of new irAE's as well as report the frequency of flare-ups of pre-existing autoimmune conditions occurring after ICI therapy.
We performed a retrospective chart review of all patients treated for cancer with anti-PD1 or anti-CTLA4 or combination therapy at our tertiary care center from January 2014 to April 2016. Demographic data, cancer type and stage, irAE's (new immune disorders and disease flares of pre-existing autoimmune disorders on ICI therapy), and drug treatment information were extracted.
We identified 220 patients treated with ICI therapy during the study period out of which 27% (60/220) developed irAE's. 11% in anti-CTLA4 group and 16% among anti-PD1 treated patients developed irAE's. IrAE's resulted in discontinuation of cancer therapy in 28% of those who developed irAE's. 21.4% had a flare of their autoimmune disease but only 1 required discontinuation of immunotherapy.
IrAE's are an important emerging clinical disease entity for specialists to be aware of. Our study shows that ICI's can be safely used in patients with pre-existing autoimmune conditions with close monitoring. However, there is still a large unmet need to have a better understanding of how to systematically evaluate and manage patients with irAE's as well as for identifying the predictors of irAE's.
Keywords: anti-CTLA4, anti-PD1, immune checkpoint inhibitors, immune-related adverse events, ipilimumab, nivolumab, pembrolizumab
1. Introduction
Immune checkpoint inhibitors (ICIs), cytotoxic T-lymphocyte associated protein 4 (anti-CTLA4) and programmed death cell protein 1 (anti-PD1), have ushered in a new era of hope in cancer treatment especially in advanced and metastatic disease, as demonstrated in clinical trials. Ipilimumab was the first anti-CTLA4 to be approved by the Food and Drug Administration (FDA) for treatment of late-stage metastatic melanoma. Pembrolizumab has earned FDA approval to treat metastatic melanoma, metastatic non-small cell lung cancer (NSCLC), metastatic head and neck squamous cell cancer, refractory classical Hodgkin lymphoma, metastatic urothelial carcinoma, microsatellite instability-high cancer, recurrent or metastatic gastric and cervical cancer, refractory or relapsed primary mediastinal B cell lymphoma, advanced hepatocellular and Merkel cell carcinoma, and renal cell cancer. FDA has approved nivolumab for treatment of metastatic melanoma, metastatic NSCLC and small cell lung cancer, metastatic renal cell cancer, classical Hodgkin's lymphoma, advanced head and neck squamous cell cancer, metastatic urothelial cancer, microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer, and patients with hepatocellular carcinoma who have been previously treated with sorafenib. The combination of nivolumab and ipilimumab has also been approved for the treatment of metastatic melanoma. Other ICI's that have been FDA approved include programmed death-ligand 1 inhibitors atezolizumab, avelumab, and durvalumab for various malignancies including urothelial cancer, NSCLC, and metastatic Merkel cell cancer.[1–4] Additional checkpoints such as OX40, Lymphocyte Activation Gene 3, TIGIT (T cell immunoreceptor with Ig and ITIM domains), TIM-3 (T cell immunoglobulin and mucin domain), and BLTA (B and T lymphocyte attenuator) are under investigation currently as potential immunotherapy targets.
Tumor cells evade the immune system by using several strategies that include expression of immunosuppressive molecules (PDL-1, PDL-2), immunosuppressive enzymes (indoleamine 2-3 dioxygenase), immunosuppressive cytokines (transforming growth factor-β, interleukin [IL]-4, IL-6, IL-10), and expansion of immunosuppressive cells (myeloid-derived suppressor cell, T-regulatory cells). The core principle of immunotherapy is to enhance the antitumor activity of cytotoxic- T cells by blocking these strategies.[5,6]
CTLA4 is an inhibitory receptor expressed on T cells, which upon interaction with its ligands on the dendritic cells and antigen-presenting cells leads to attenuation of immune response. Ipilimumab blocks the interaction of CTLA4 with its ligands and this leads to increased proliferation and activation of T cells including tumor-infiltrating T effector cells.[7] The activity of the effector T-cells in the tumor microenvironment is negatively regulated by PD1 receptor and its interaction with its ligands PDL1, PDL2 on the tumor cells. Pembrolizumab and nivolumab act by blocking the interaction of PD-1 receptor with its ligands and thereby restore the antitumor activity of the effector T cell. This allows the immune system to recognize and fight cancer cells.[7,8]
However, increased T cell activation and proliferation can also provoke powerful autoimmune reactions in other organ systems. The manufacturer on the drug labels lists immune-mediated side effects as black box warning. These side effects include but are not limited to autoimmune-mediated enterocolitis, hepatitis, dermatitis (including toxic epidermal necrolysis), neuropathies, and endocrinopathies (including hypophysitis, adrenal insufficiency [including adrenal crisis], and hyper- or hypothyroidism)[9–12] (Table 1).
Table 1.
Summary of irAE's reported with ICI's during phase 3 trials.
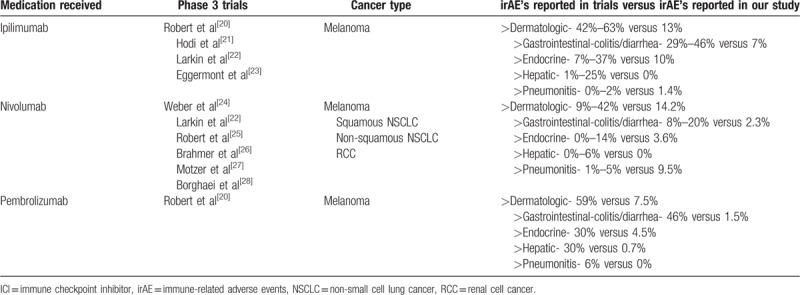
Because the earlier phase I, II, and III clinical trials of these ICIs have excluded patients with prior autoimmune diseases, the extent of immune-related adverse events (irAE's) may be underestimated. Indeed, as the use of ICIs becomes widespread in the oncology community, there have been several case reports and series of irAE's from ICIs, including rheumatologic irAE's.[13,14] In this article, we report a series of patients seen and evaluated at the University of Iowa Hospitals and Clinics (UIHC) in a 27-month period that has used anti-PD1 and/or anti-CTLA 4 as treatment for malignancy and have been reported to have irAE's, the treatments given, and the responses achieved. We also report on the frequency of flares of pre-existing autoimmune diseases in our cohort.
2. Methods
This was a retrospective study involving all patients treated for malignancy with pembrolizumab, nivolumab, ipilimumab, or combination therapy with nivolumab and ipilimumab at the UIHC from January 2014 to April 2016. Institutional review board (IRB) approval from the University of Iowa IRB board was obtained. After IRB approval, detailed manual chart review was performed on each patient, noting any new onset or worsening of autoimmune or autoinflammatory condition after starting treatment with one of the above medications. The conditions included: inflammatory arthritis; pneumonitis; hypophysitis; colitis; thyroiditis; optic neuritis; myasthenia gravis; and skin rashes. Thus, patients with a pre-existing autoimmune or inflammatory disorder that worsened after starting ICIs were included in the cohort. Demographic data including age, sex, smoking status, cancer type and stage, autoimmune diagnosis and symptoms, available labs and imaging, and treatment were all obtained on individual chart review.
3. Results
Out of a total of 220 patients who were treated with either anti-CTLA-4 (n = 69) or anti-PD1 (pembrolizumab = 67, nivolumab = 84), we identified 24 patients who developed irAE's on ipilimumab and 35 patients who developed irAE's in the anti-PD1 group (23 patients developed irAE's on nivolumab and 12 patients developed irAE's while taking pembrolizumab). All of the patients in the ipilimumab group were being treated for melanoma (Table 2), whereas in the anti-PD1 group, 14 patients had NSCLC, 12 patients had melanoma, 4 had renal cell carcinoma, and 1 patient each had bladder cancer, clear cell sarcoma, Hodgkin's lymphoma, gastric adenocarcinoma, and stage 4 squamous cell cancer with unknown primary (Table 3). Only 1 patient was treated with combination therapy with ipilimumab and nivolumab and the patient developed colitis leading to discontinuation of immunotherapy (Table 3). The average age in the ipilimumab group was 61 years with 17 males and 8 females while the average age in the anti-PD1 group was 62 years old, with 16 patients being male and 19 patients being female. irAE's that developed after anti-CTLA-4 and anti-PD-1 therapies differed in nature- with colitis and endocrine irAE's observed more commonly in anti-CTLA-4 group whereas pneumonitis and rheumatologic irAE's were more prevalent in the anti-PD-1 group. The severity of irAE's resulted in discontinuation of therapy in 28% of the patients who developed irAE's. The timing of irAE development with respect to ICI therapy was variable for each patient. Details about the features, diagnosis, and management of each type of irAE as well as pre-existing autoimmune conditions and effects of ICI's on them are provided below.
Table 2.
Patient demographics, cancer type and stage, IrAE reported, and its treatment while on ipilimumab.
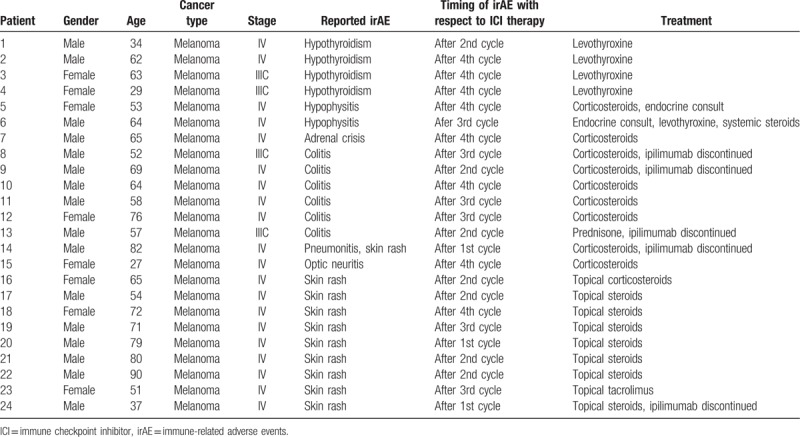
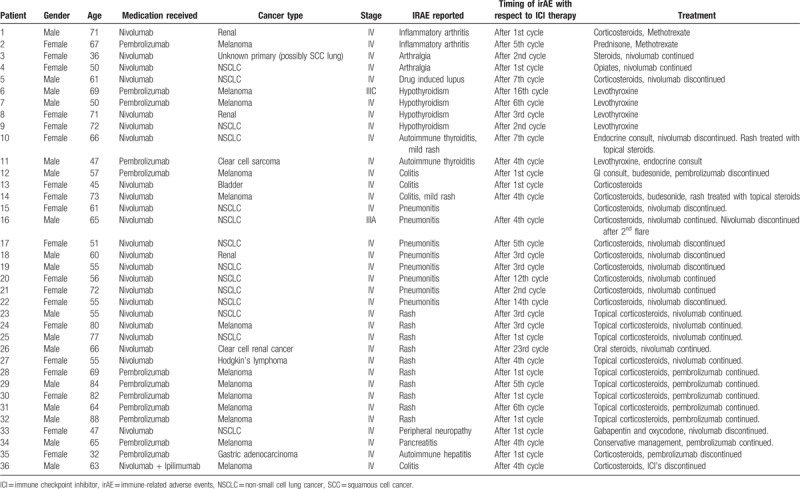
Table 3.
Patient demographics, cancer type and stage, IrAE reported, and its treatment while on anti-PD1 as well as combination chemotherapy.
3.1. Rheumatologic irAE's
3.1.1. Inflammatory arthritis and arthralgia
Two patients in the anti-PD1 group (n = 2/151; 1.3%) presented with new-onset polyarticular inflammatory arthritis during the course of their ICI therapy One patient was taking nivolumab and the other was taking pembrolizumab. Both patients were referred to rheumatology and were found to be rheumatoid factor and anti-cyclic citrullinated peptide negative with normal hand and foot x-rays. Both were treated with low dose oral prednisone (20 mg daily, subsequently tapered down over few weeks) and eventually methotrexate (20 mg/wk) with improvement in joint symptoms. Two patients on nivolumab were also noted to have arthralgia during their course of treatment, 1 was treated with steroid burst and taper with improvement in their symptoms and the other was treated with opiates. These 2 patients were not referred to rheumatology and were managed by their oncologist. All patients were continued on their cancer treatment. No arthritis/arthralgia was noticed in the ipilimumab group.
3.1.2. Drug-induced lupus
One patient in the anti-PD1 group (n = 1/151; 0.7%) developed suspected drug-induced lupus with hypersensitivity reaction and had to be admitted to the hospital. He was found to be SSA (sjogren syndrome type A antigen), SSB (sjogren syndrome type B antigen) positive and was treated with corticosteroids. His immune therapy was discontinued and the patient and family ultimately decided to proceed with hospice care after a prolonged hospitalization.
3.2. Endocrine irAE's
3.2.1. Thyroid disease
In the ipilimumab group, 6% (n = 4/69) developed new hypothyroidism and had to be started on levothyroxine. In the anti-PD1 group, 4% (n = 6/151) developed new thyroid disease during the course of their ICI therapy, out of which 4 developed hypothyroidism and 2 developed autoimmune thyroiditis. Cancer treatment with nivolumab was eventually stopped in 1 patient with autoimmune thyroiditis due to cancer progression. This patient's thyroid function eventually stabilized without treatment. The rest of the patients were started on levothyroxine. Cancer treatments in all of the patients were not changed based on the findings of thyroid disease.
3.2.2. Hypophysitis/adrenal crisis
One patient in the ipilimumab group developed adrenal crisis and had to be admitted to the hospital and treated with intravenous steroids. Two patients in the same group (n = 2/69; 3%) developed hypophysitis. Endocrine was consulted, and patient was started on levothyroxine and oral steroids. Hypophysitis and adrenal crisis were not seen in patients in the anti-PD1 group.
3.3. Gastrointestinal irAE's
3.3.1. Colitis
Ten patients developed colitis. Six were treated with ipilimumab (n = 6/69; 7%), 3 (n = 3/151; 2%) were treated with anti-PD1 (2 were treated with nivolumab 1 was treated with pembrolizumab), and 1 was treated with combination chemotherapy with nivolumab and ipilimumab. Two patients were diagnosed based on symptoms and positron emission tomography scan findings of bowel wall thickening. One patient had colonoscopy with biopsy confirming moderate active colitis. Another patient was diagnosed and treated by a local gastroenterologist. All other patients were diagnosed based on computed tomography (CT) abdomen/pelvis findings of thickening of the colon. Five patients had their ICI's stopped after developing colitis (3 patients in the ipilimumab group, 1 patient on pembrolizumab, and 1 on combination therapy). The remainder of the patients were all treated with corticosteroids with improvement in symptoms and continued on their cancer treatment.
3.3.2. Autoimmune hepatitis
One patient (n = 1/151; 0.7%) in the anti-PD1 group (on pembrolizumab) developed autoimmune hepatitis and had to be hospitalized. Hepatology was consulted as well and her presentation was thought to be consistent with autoimmune hepatitis related to ICI therapy. She was started on high dose intravenous steroids and her immunotherapy was stopped. Her condition worsened despite steroids, and she ultimately decided to pursue palliative care. No cases of autoimmune hepatitis were observed in anti-CTLA 4 group.
3.3.3. Pancreatitis
One patient (n = 1/151; 0.7%) in the anti-PD1 group (on pembrolizumab) developed pancreatitis thought to be related to immune checkpoint inhibition. She responded well to conservative measures and did not require steroids. Pembrolizumab was safely continued in her case after her pancreatitis resolved. No cases of pancreatitis were observed in anti-CTLA 4 group.
3.4. Neurological irAE's
3.4.1. Peripheral neuropathy
One patient in the anti-PD1 group (n = 1/151; 0.7%) on nivolumab developed peripheral neuropathy in her hands and feet. She did not have a diagnosis of diabetes and her neuropathy was thought to be related to nivolumab use. She was started on gabapentin and oxycodone with partial relief and nivolumab was ultimately discontinued. No case of peripheral neuropathy was seen in ipilimumab group.
3.4.2. Optic neuritis
One patient (n = 1/69; 1.4%) in the ipilimumab group developed optic neuritis. Ophthalmology was consulted, and patient was started on high dose steroids with improvement in the symptoms. Ipilimumab was continued. There was no reported incidence of ocular side effects in the anti-PD1 group.
3.5. Respiratory system irAE's
3.5.1. Pneumonitis
Nine patients developed pneumonitis. Eight patients were from anti-PD1 group (n = 8/151; 5.3%) on nivolumab treatment and 1 (n = 1/69; 1.4%) was on ipilimumab. Eight patients were diagnosed based on symptoms and CT chest findings. One patient was diagnosed based on symptoms and a chest x-ray showing new ground-glass opacities. All 9 patients were treated with high dose corticosteroids. One patient went palliative upon hospital admission for pneumonitis. Nivolumab was discontinued in 6 patients and ipilimumab had to be stopped as well.
3.6. Dermatologic irAE's
3.6.1. Rash
Nine patients in the ipilimumab group (n = 9/69; 13%) reported skin rashes after being treated with ipilimumab. Eight patients were treated with topical corticosteroids and 1 patient was treated with topical tacrolimus with resolution of the rash. One of the patients who developed diffuse acneiform rash was switched to pembrolizumab, the rest were all continued on the same chemotherapy. Twelve patients in the PD1 group (n = 12/151; 8%) developed rash (7 on nivolumab and 5 on pembrolizumab) and were treated with topical steroids. Immunotherapy was continued in all of the PD1 patients.
3.7. Pre-existing autoimmune conditions and effects of ICI's
Thirty-four patients in the anti-PD-1 group had pre-existing autoimmune conditions: hypothyroidism (n = 19), adrenal insufficiency (n = 1), rheumatoid arthritis (n = 4), psoriatic arthritis (n = 1), psoriasis (n = 2), 1 patient each with pemphigus and discoid lupus, immune-mediated thrombocytopenia (n = 2), myasthenia gravis (n = 2), and inflammatory bowel disease (n = 2). Eight patients (23.5%) had flares of their pre-existing autoimmune condition including 2 patients with hypothyroidism and 1 patient each with adrenal insufficiency, psoriatic arthritis, psoriasis, myasthenia gravis, and ulcerative colitis. They were treated with adjustment of their pharmacologic therapy for the underlying autoimmune disease and with addition of steroids in certain cases. Immunotherapy was continued in all except 1 patient who had recurrent ulcerative colitis flares (Fig. 1).
Figure 1.
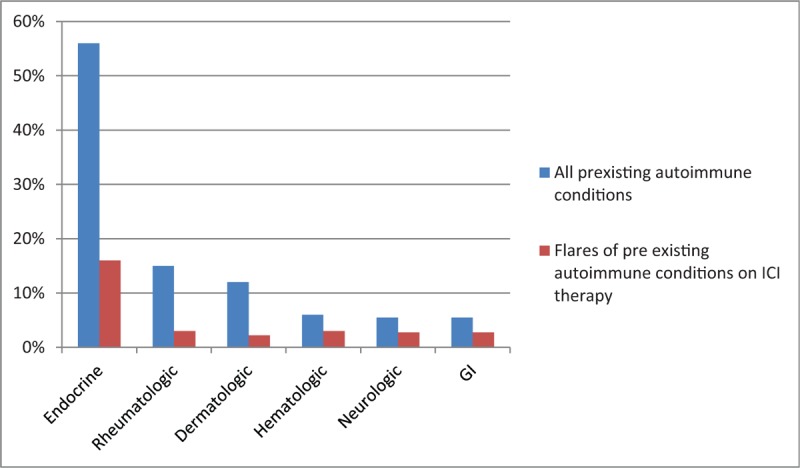
Pre-existing autoimmune conditions and flare-ups after PD1 therapy. PD1 = programmed death cell protein 1.
Twelve patients in the anti-CTLA-4 group had pre-existing autoimmune conditions: hypothyroidism (n = 9) and 1 patient each with celiac disease, primary biliary cirrhosis, and psoriasis. Only 1 person (8.3%) had a flare of autoimmune thyroiditis and required an increase in the pharmacologic therapy and ipilimumab was continued (Fig. 2).
Figure 2.
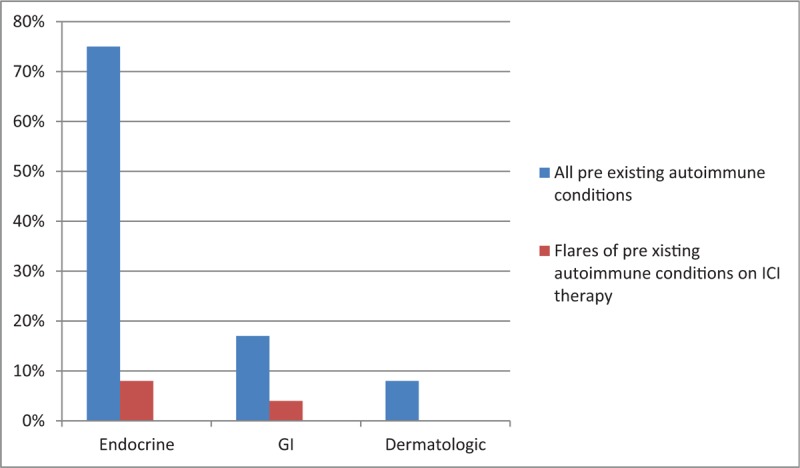
Pre-existing autoimmune conditions and flares-ups after CTLA-4 therapy. CTLA-4 = cytotoxic T-lymphocyte associated protein 4.
4. Discussion
ICIs-related adverse events including dermatologic, gastrointestinal/hepatic, pulmonary, and endocrine adverse events are well described in clinical trials and literature. However, as the use of ICI's becomes more prevalent, new and rare irAE's are coming to light including rheumatologic irAE's. Rheumatologic adverse events related to ICIs are a new class of emerging disease entity that have not been well described and categorized in clinical trials. There is paucity of detailed literature on rheumatologic irAE's and our current knowledge about them is based on limited number of clinical case reports and case series that have been published.[13–16] Clinical trials with ICIs have also excluded patients with pre-existing autoimmune conditions; however, literature suggests that ICI's may worsen or cause exacerbation of those conditions.[17,18] Also, there is very little literature published regarding safety of irAE's in pre-existing autoimmune conditions.[19] Here we present the results of our experience with these drugs in a tertiary care center; looking at the development of all irAE's including rheumatologic irAE's which have not been reported in phase 3 trials. Our case series also looks at the pre-existing autoimmune conditions and the effect of ICI's on them.
Polyarthralgia with ipilimumab was reported by a phase I/II clinical trial as early as 2005.[29] The affected patient developed pain/swelling in fingers, wrists, ankles, and knees after the third dose of ipilimumab. The symptoms responded promptly to non-steroidal anti-inflammatory therapy. Arthralgia has also reported in the phase 3-study involving nivolumab in the treatment of melanoma[24,25] but no incidence of inflammatory arthritis was noted. Goldtsein et al reported 2 cases of polymyalgia rheumatica (PMR) and giant cell arteritis occurring in patients with malignant melanoma treated with ipilimumab.[15] The first patient presented with typical symptoms 1 week after finishing a course of ipilimumab therapy, while the second one developed symptoms 10 weeks after completion of ipilimumab. Both patients had a good response to corticosteroids. Cappelli et al reported 13 patients who received ipilimumab and/or nivolumab and developed autoimmune rheumatic side effects.[13] Four out of 13 patients developed sicca syndrome and 9 developed inflammatory arthritis out of which 4 had inflammation and synovitis proven by imaging and 4 had inflammation confirmed by synovial fluid analysis. All of the patients were treated with corticosteroids and in addition, 2 patients were also administered methotrexate and anti-Tumor necrosis factor therapy for inflammatory arthritis induced by ICI's. Calabrese et al reported 13 cases of rheumatologic irAE's on ICI including inflammatory arthritis, myositis, sicca symptoms, and PMR like symptoms. All patients required therapy with corticosteroids and 3 patients required treatment with biologic agents in addition to steroids.[14] There have been case reports of dermatomyositis and orbital myositis occurring after ipilimumab therapy.[16,30] In our case series, we identified a total of 2 patients (1.3%) who developed inflammatory arthritis in the anti-PD1 group; however, no cases were found in the ipilimumab group. Both of our patients had good response to corticosteroids and methotrexate and did not require additional disease-modifying antirheumatic drugs (DMARD) or biologic therapy. Kostine et al published a single-center prospective cohort study in 2018 evaluating rheumatic irAE's in all cancer patients receiving ICI therapy.[31] Out of 524 patients who received ICI therapy, 7 patients developed inflammatory arthritis mimicking rheumatoid arthritis, 11 patients developed PMR, 2 patients developed psoriatic arthritis, and 2 patients were reported to have noninflammatory musculoskeletal conditions. Majority were treated with glucocorticoids, couple of patients required methotrexate and the rest were treated with nonsteroidal anti-inflammatory drugs. ICI was continued in all but 1 patient. More recently, Richter et al published the largest cohort of patients who developed rheumatologic irAE's including inflammatory arthritis, connective tissue disease, vasculitis and inflammatory myopathy on ICI therapy.[32] Patients were treated mainly with corticosteroids and DMARDS like methotrexate had to be used in addition to corticosteroids in a few patients. Greater than 90% of patients who developed these irAE's were able to continue their ICI therapy. However, no guidelines exist regarding treatment of rheumatologic irAE's related to ICI's. Although Naidoo et al recently proposed an algorithm for treatment of inflammatory arthritis related to immune checkpoint blockade,[33] it is based on experience with a limited number of patients and more data is needed to establish standard guidelines for early recognition, evaluation, and treatment of rheumatologic irAE's.
Other irAE's including dermatologic, gastrointestinal, and endocrine irAE's have been well categorized in clinical trials. During a phase 3 trial of ipilimumab from 2010, dermatological manifestations including rash and pruritus were the most commonly reported side effects seen with ipilimumab monotherapy. Other dermatologic side effects that have been reported are toxic epidermal necrolysis, Sweet's syndrome, and vitiligo.[21,34] In our study, a total of 21 patients from among 220 (9.5%) treated with ICI's developed dermatological side effects. Gastrointestinal side effects such as diarrhea are also commonly seen with ipilimumab.[21] However, incidence of colitis is rare at approximately 5% in patients treated with ipilimumab and has been reported to be between 3% and 5% in the anti-PD1 group in published reports[20,21,26,35] which is comparable to incidence of colitis seen in our study. Other commonly seen irAE's are endocrine side effects including adrenal crisis, hypo or hyperthyroidism, autoimmune thyroiditis, and hypophysitis.[36] Hepatitis, pneumonitis, myocarditis, central and peripheral nervous system side effects including neuropathies, hematological side effects including thrombocytopenia, ocular side effects like uveitis and renal side effects although rare have also been reported.[21,25,37–41] In our case series, the incidence of new hypothyroidism was 6% in the anti-CTLA4 group and 4% in the anti-PD1 group. The incidence of hypophysitis and adrenal crisis was found to be 1.4% and 3% in the anti-CTLA4 group; however, no cases were found in the anti-PD1 group. 1.4% developed pneumonitis in the anti-CTLA4 group while 5.3% were reported to have pneumonitis in the anti-PD1 group. Rare irAE's such as optic neuritis, autoimmune pancreatitis, peripheral neuropathy, and autoimmune hepatitis were also observed in our group.
Clinical trials with ICI's have excluded patients with pre-existing autoimmune conditions. Our study also evaluates patients with pre-existing autoimmune diseases and the effects of ICI's on them. In our study 21.4% of patients with autoimmune conditions had a flare of their pre-existing conditions requiring either corticosteroids and/or increase in pharmacotherapy. Out of these patients, only 1 required discontinuation of immunotherapy. One of our patients who received combination chemotherapy had past diagnosis of pulmonary sarcoidosis which remained stable on immunotherapy. Leonardii et al recently published a study about safety of PD1 inhibitors in patients with pre-existing autoimmune conditions and it was shown that 23% of the patients developed flares of their pre-existing autoimmune conditions but immunotherapy discontinuation due to the same was very infrequent[19] which is comparable to our findings. This suggests that ICI's may be safely used in patients with previously existing autoimmune conditions under close supervision.
Our study has numerous strengths including detailed chart review, providing information about all the different categories of irAE's that the patients developed on ICI's and analyzing the effects of ICI's on pre-existing autoimmune diseases. However, there are certain limitations as well. This study is retrospective in nature and is a single-center experience only. Since our patients were only followed for a limited period of time, we were unable to assess the impact of discontinuation of immunotherapy on mortality of the patients. Furthermore, few of the patients were noted to have mild arthralgia and mild numbness and tingling on chart review but were never referred to specialists. Also, patients may not have reported mild symptoms like self-limiting diarrhea, rash, arthralgias, and so on. So, there is a real possibility of under reporting of irAE's in our study. The incidence of irAE's may also be lower in our study due to the fact that only 1 patient was on combination immunotherapy during our study duration (since combination therapy was approved near the end of our study period in October 2015) and it is well known that patients on combination immunotherapy have higher incidence of irAE's.[42]
5. Conclusion
irAE's are an important emerging disease entity for practicing specialists to consider and be aware of. As the use of ICI's becomes more prevalent and widespread, we are becoming aware of more irAE's that have not been reported before. Although algorithms for management and treatment exist for most of the common irAE's reported like colitis, hypophysitis, stepwise approach, and management of rheumatologic irAE's as well some of the rare irAE's like optic neuritis and autoimmune myocarditis still remains an area shrouded by mystery. There is still a large unmet need to have a better understanding of how to systematically evaluate and manage patients with these irAE's as well as for identifying the predictors of irAE's in patients treated with ICI therapies. Furthermore, additional research is needed to evaluate whether discontinuation of ICI's due to irAE's has any impact on patient mortality and morbidity from oncologic standpoint and whether one can maintain more patients on ICI treatment by earlier referral to specialist for appropriate intervention of irAE's. Also, our study showed that very few patients with pre-existing autoimmune conditions had flares of their conditions and majority of these flares could be managed with pharmacologic therapy including steroids without discontinuation of ICI's which is very encouraging. These patients should be closely monitored by specialists and moving forward, collaborative effort between oncologists and specialists will be required to recognize and treat the irAE's and flares of pre-existing autoimmune conditions early to avoid long term debilitating complications to the patients.
Author contributions
Conceptualization: Namrata Singh.
Data curation: Aneet Kaur, Taylor Doberstein, Rachana Amberker.
Formal analysis: Aneet Kaur, Taylor Doberstein, Elizabeth Field.
Investigation: Namrata Singh.
Methodology: Namrata Singh.
Project administration: Namrata Singh.
Resources: Namrata Singh.
Supervision: Elizabeth Field, Namrata Singh.
Writing – original draft: Aneet Kaur, Taylor Doberstein, Rachana Amberker.
Writing – review and editing: Aneet Kaur, Rohan Garje, Elizabeth Field, Namrata Singh, Rachana Amberker.
Footnotes
Abbreviations: anti-CTLA4 = cytotoxic T-lymphocyte associated protein 4, anti-PD1 = programmed death cell protein 1, ICI = immune checkpoint inhibitor, irAE's = immune-related adverse events.
How to cite this article: Kaur A, Doberstein T, Amberker RR, Garje R, Field EH, Singh N. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Medicine. 2019;98:41(e17348).
This work was supported by Grant IRG-15-176-41 from the American Cancer Society, administered through The Holden Comprehensive Cancer Center at The University of Iowa.
The authors have no conflicts of interest to disclose.
References
- [1].Sidaway P. Urological cancer: atezolizumab: an alternative to cisplatin? Nat Rev Clin Oncol 2017;14:139. [DOI] [PubMed] [Google Scholar]
- [2].Rittmeyerm A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma. JAMA Oncol 2018;4:e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Antonia SJ, Villegas A, Daniel D. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- [5].Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schreiber RD, Old LJ, Smyth MJ. Cancer immuno editing: integrating immunity's roles in cancer suppression and promotion. Science (New York, NY) 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- [7].Park J, Kwon M, Shin EC. Immune checkpoint inhibitors for cancer treatment. Arch Pharm Res 2016;39:1577–87. [DOI] [PubMed] [Google Scholar]
- [8].Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yervoy Package Insert. Available at: https://packageinserts.bms.com/pi/pi_yervoy.pdf Accessed 10, 2015. [Google Scholar]
- [10].Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs 2016;30:571–84. [DOI] [PubMed] [Google Scholar]
- [12].Demlova R, Valik D, Obermannova R, et al. The safety of therapeutic monoclonal antibodies: implications for cancer therapy including immuno-checkpoint inhibitors. Physiol Res 2016;65Supplementum 4:S455–62. [DOI] [PubMed] [Google Scholar]
- [13].Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Calabrese C, Kirchner E, Kontzias A, et al. Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open 2017;3:e000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheumat 2014;66:768–9. [DOI] [PubMed] [Google Scholar]
- [16].Lecouflet M, Verschoore M, Giard C, et al. Orbital myositis associated with ipilimumab. Ann Dermatol Venereol 2013;140:448–51. [DOI] [PubMed] [Google Scholar]
- [17].Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016;2:234–40. [DOI] [PubMed] [Google Scholar]
- [18].Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–76. [DOI] [PubMed] [Google Scholar]
- [19].Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 2018;36:1905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- [21].Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522–30. [DOI] [PubMed] [Google Scholar]
- [24].Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375–84. [DOI] [PubMed] [Google Scholar]
- [25].Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- [26].Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol 2005;12:1005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book 2015;76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- [31].Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer—clinical aspects and relationship with tumor response: a single-center prospective cohort study. Ann Rheum Dis 2018;77:393–8. [DOI] [PubMed] [Google Scholar]
- [32].Richter M, Crawson C, Kottschade L, et al. Rheumatic syndromes associated with immune checkpoint inhibitors: a single- center cohort of sixty- one patients. Arthritis Rheumatol 2019;71:468–75. [DOI] [PubMed] [Google Scholar]
- [33].Naidoo J, Cappelli LC, Forde PM, et al. Inflammatory arthritis: a newly recognized adverse event of immune checkpoint blockade. Oncologist 2017;22:627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pintova S, Sidhu H, Friedlander PA, et al. Sweet's syndrome in a patient with metastatic melanoma after ipilimumab therapy. Melanoma Res 2013;23:498–501. [DOI] [PubMed] [Google Scholar]
- [35].Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Torino F, Corsello SM, Salvatori R. Endocrinological side-effects of immune checkpoint inhibitors. Curr Opin Oncol 2016;28:278–87. [DOI] [PubMed] [Google Scholar]
- [37].Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicenter, phase 2, dose-ranging study. Lancet Oncol 2010;11:155–64. [DOI] [PubMed] [Google Scholar]
- [38].Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Izzedine H, Gueutin V, Gharbi C, et al. Kidney injuries related to ipilimumab. Investig New Drugs 2014;32:769–73. [DOI] [PubMed] [Google Scholar]
- [40].Kopecky J, Trojanova P, Kubecek O, et al. Treatment possibilities of ipilimumab-induced thrombocytopenia – case study and literature review. Jpn J Clin Oncol 2015;45:381–4. [DOI] [PubMed] [Google Scholar]
- [41].Robinson MR, Chan CC, Yang JC, et al. Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. J Immunother 2004;27:478–9. [DOI] [PubMed] [Google Scholar]
- [42].Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]


