Supplemental Digital Content is available in the text
Keywords: Crohn disease, inflammatory bowel disease, rosacea, ulcerative colitis
Abstract
Rosacea has been reported with several systemic comorbidities, but its relationship with inflammatory bowel disease (IBD) is unclear. Thus, our objective is to conduct a meta-analysis on the association of rosacea with IBD.
We conduct a meta-analysis and searched MEDLINE, CENTRAL, and Embase databases for case-controlled and cohort studies that assessed the association of rosacea with IBD from inception to July 2nd, 2018. Two authors independently selected studies, extracted data, and assessed the risk of bias of included studies. Disagreement was resolved by discussion. We performed random-effects model meta-analysis to obtain the pooled risk estimates for Crohn disease (CD) and ulcerative colitis (UC) in patients with rosacea.
We included three case-control and three cohort studies. The risk of bias of included studies was generally low. The meta-analysis on case-control studies showed marginally increased odds of CD (pooled odds ratio (OR) 1.30, 95% confidence interval (CI) 0.99–1.69) and a significantly increased odds of UC (pooled OR 1.64, 95% CI 1.43–1.89) in patients with rosacea. The meta-analysis on cohort studies demonstrated significant increased risk of CD (pooled hazard ratio (HR) 1.58, 95% CI 1.14–2.20) and UC (pooled HR 1.18, 95% CI 1.01–1.37) in patients with rosacea.
The evidence indicates an association of rosacea with IBD. If patients with rosacea suffer from prolonged abdominal pain, diarrhea, and bloody stool, referral to gastroenterologists may be considered.
1. Introduction
Rosacea is a prevalent chronic inflammatory disease characterized by flushing, persistent erythema, telangiectasia, inflammatory papules and pustules on the central face.[1] Rosacea was estimated to affect 5.46% of adults, especially those aged 45 to 60 years.[2] Although rosacea most commonly involves women after the age of 30,[3,4] both men and women may be affected.[2] The pathogenesis of rosacea is unclear and has been proposed to relate to dysregulation of neurovascular and neuroimmune communication.[5–7] Both innate and adaptive immune system activation have been implicated in the pathogenesis of rosacea.[6,8] In addition to genetic factors and fair skin types predisposition,[6,9] environmental factors including alcohol consumption, ultraviolet light, cold and hot may exacerbate rosacea. Microorganisms such as Demodex mites,[10,11]Bacillus oleronius,[12] and small intestinal bacterial overgrowth (SIBO)[13] have also been associated with rosacea. Various comorbidities of rosacea have been reported recently, including cardiovascular disease,[14] migraine,[15] and mood disorders.[16]
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of gastrointestinal tract which includes 2 main types: Crohn disease (CD) and ulcerative colitis (UC). UC is a relapsing non-transmural inflammatory disease restricted to the mucosa of the colon. By contrast, CD usually causes more persistent transmural inflammation of the gastrointestinal mucosa with skip lesions and can involve the entire gastrointestinal tract, resulting in sinus tracts, perforations, and fistulae.[17,18] The clinical manifestations of IBD include prolonged diarrhea with abdominal pain, weight loss, fatigue, fever, and bloody stool.[18] The prevalence of IBD is about 0.3% and appears to be lower in Asia and the Middle East.[19] Recently, a rising incidence of IBD has been found in newly industrialized countries.[19] Various risk factors for IBD have been proposed,[20] including age,[21] gender,[22,23] smoking,[24,25] obesity,[26,27] microorganisms,[28,29] and medications.[30,31] IBD usually needs long-term medical control and is associated with an increased mortality of affected patients.[32]
Although the pathogenesis remains unclear, rosacea and IBD, same as chronic inflammatory diseases, share some commonalities including genetic susceptibility, immunological features, microbiota, and trigger factors.[6,13,33] A few cases of simultaneous occurrence of rosacea and IBD have been reported.[34–38] However, conflicting results have been reported. The objective of this study was to systematically examine the evidence on the association of rosacea with IBD.
2. Methods
2.1. Literature search
We conducted a meta-analysis on the association of rosacea with IBD. Therefore, ethical approval was not necessary. The reporting of this study was in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline[39] and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guideline.[40] The protocol for this study has been registered with PROSPERO (CRD42018102922). The MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), and Embase databases were searched from inception to July 2nd, 2018. The search terms included rosacea, IBD, Crohn disease (CD), and ulcerative colitis (UC). The detailed search strategy is listed in the Supplement. We did not apply any language or geographic limitations.
2.2. Study selection
Studies were included if they met the following criteria:
-
1.
observational studies assessing the association of rosacea with IBD;
-
2.
study design being case-control or cohort studies; and
-
3.
the study subjects were humans.
Two authors (FW and CC) independently selected relevant studies by scanning the titles and abstracts of search results. The full text of potential studies was obtained and examined for eligibility by the 2 authors. After resolving disagreement by discussion, the 2 authors decided which studies to be included.
2.3. Data extraction and risk of bias assessment
We extracted the following data from the included studies: first author, publication year, study design, country, study settings, and risk estimates. The quantitative estimates included odds ratio (OR) for case-control studies and hazard ratio (HR) for cohort studies with 95% confidence intervals (CI). Two authors evaluated the risk of bias of the included studies by using the Newcastle-Ottawa Scale (NOS).[41] Disagreement was resolved by discussion. For case-control studies, we examined the risk of bias in the following 8 items, including adequacy of case definition, representativeness of cases, selection of controls, definition of controls, comparability of cases and controls, ascertainment of exposure, same method of ascertainment for cases and controls, and non-response rate. On the other hand, for cohort studies, we evaluated the following eight items, including the representativeness of exposed cohort, selection of non-exposed cohort, ascertainment of exposure, outcome of the interest not present at start of study, assessment of outcome, comparability of cohorts, follow-up duration, as well as adequacy of follow up of cohorts.
2.4. Statistical analysis
We conducted meta-analyses to calculate a pooled OR with 95% CI for case-control studies and a pooled HR with 95% CI for cohort studies. We assessed heterogeneity across the studies by using the I2 statistic. An I2 of >50% was considered substantial statistical heterogeneity.[42] We decided a priori to use the random-effects model for meta-analyses because we assumed clinical heterogeneity across the included studies. All the meta-analyses were conducted by using the Review Manager version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
3. Results
3.1. Characteristics of included studies
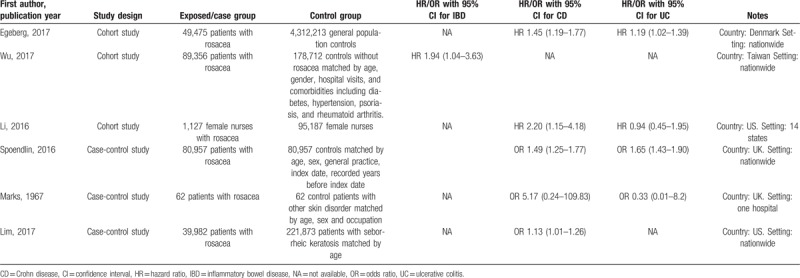
The process of identification and selection of relevant studies is shown in Figure 1. We obtained 198 records after searching the MEDLINE, CENTRAL, and Embase databases. After removing 6 duplicates, the titles and abstracts of the remaining 192 records were independently scanned by 2 authors, and 179 articles were excluded. After examining the full text, we included 3 cohort studies and 3 case-control studies with a total of 5,149,963 study subjects. Apart from one Asian study conducted in Taiwan,[43] the remaining 5 studies were carried out in the West.[44–48] The characteristics of the included cohort and case-control studies are reported in Table 1.
Figure 1.
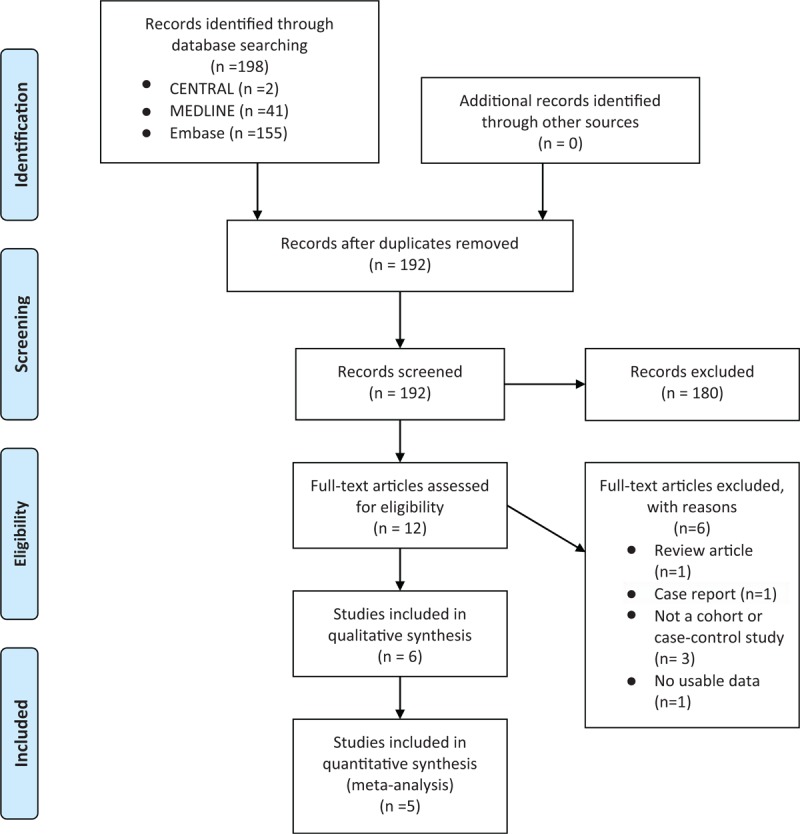
PRISMA study flow diagram.
Table 1.
Characteristics of included cohort and case-control studies.
3.2. Risk of bias of included studies
The risk of bias of included case-control and cohort studies are summarized in Figure 2A and B, respectively. None of our included studies were rated with a high risk of bias in any item of the NOS. All of the 3 cohort studies were as associated with unclear risk of bias in the ‘ascertainment of exposure’ item because rosacea was identified by using corresponding International Classification of Disease (ICD) codes or self-reports of clinicians’ diagnosis, lacking independent validation.[43–45] From the same reason, 2 included case-control studies were rated unclear risk in the ‘adequacy of case definition’ item.[46,48] Two case-control and 2 cohort studies were judged as unclear risk of bias in the ‘comparability’ of cases and controls or of cohorts because they only adjusted for 2 or 3 of the most important confounding factors of IBD, which include age, gender, smoking, and obesity.[43,44,46,47]
Figure 2.
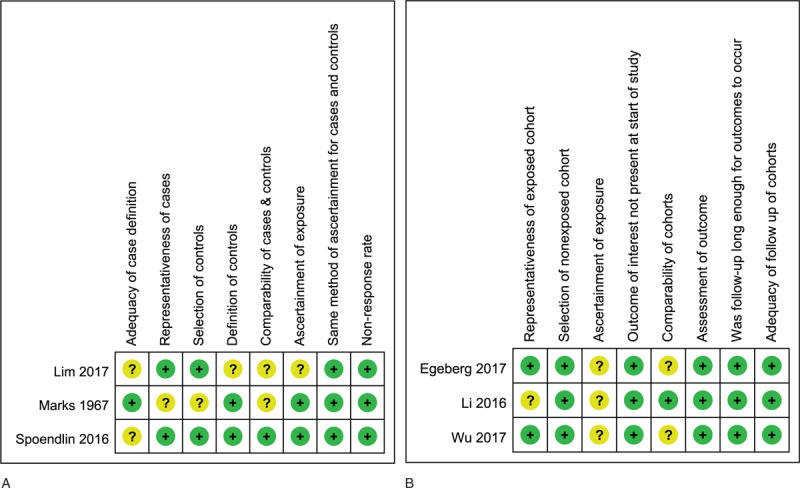
A. Risk of bias of included case-control studies. B. Risk of bias of included cohort studies.
3.3. Association of rosacea with IBD in case-control studies
All the included 3 case-control studies with a total of 423,893 subjects provided data regarding the association of rosacea with CD.[46–48] As shown in Figure 3A, the meta-analysis demonstrated marginally increased odds of prevalent CD in patients with rosacea (pooled OR 1.30, 95% CI 0.99–1.69). We identified substantial statistical heterogeneity across the three studies (I2 = 74%).
Figure 3.
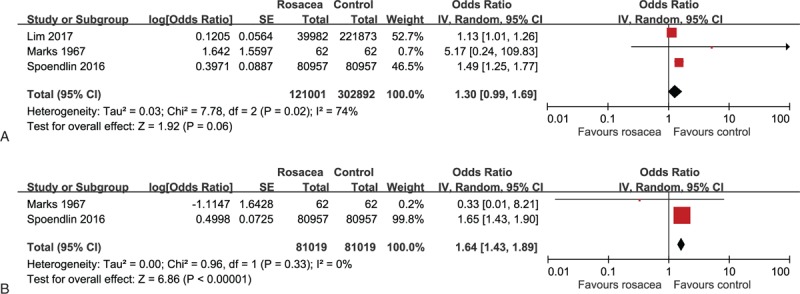
A. Forest plot for the association of rosacea with Crohn disease in case-control studies. B. Forest plot for the association of rosacea with ulcerative colitis in case-control studies.
As illustrated in Figure 3B, 2 out of the 3 included case-control studies contributed data to the association of rosacea with UC.[47,48] No statistical heterogeneity was found across the 2 studies (I2 = 0%). The meta-analysis found a significantly increased odds of prevalent UC in relation to rosacea (pooled OR 1.64, 95% CI 1.43–1.89).
3.4. Association of rosacea with IBD in cohort studies
Two included cohort studies with a total of 4,458,002 subjects supplied usable data on the HR estimates of CD and UC in patients with rosacea.[44,45] As displayed in Figure 4A, the meta-analysis of cohort studies demonstrated a significantly increased risk of incident CD in rosacea patients (pooled HR 1.58, 95% CI 1.14–2.20). Moreover, as shown in Figure 4B, the meta-analysis of cohort studies demonstrated a significantly increased risk of incident UC in rosacea patients (pooled HR 1.18, 95% CI 1.01–1.37). No statistical heterogeneity was detected across these studies for both CD and UC (I2 = 30% and 0%, respectively).
Figure 4.
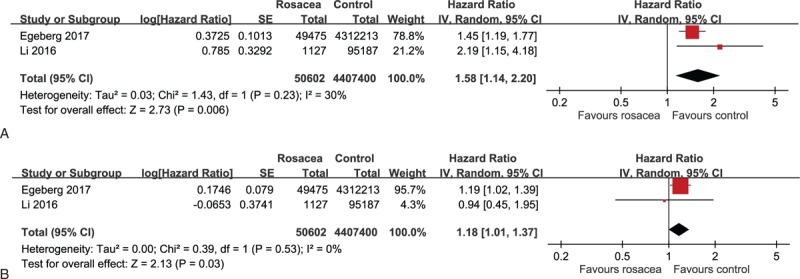
A. Forest plot for the association of rosacea with Crohn disease in cohort studies. B. Forest plot for the association of rosacea with ulcerative colitis in cohort studies.
The only Asian study included 268,068 subjects and showed significantly increased HR of incident IBD in rosacea (HR 1.94, 95% CI 1.04–3.63).[43] We contacted the authors for respective data on CD and UC; however, they decided not to conduct further calculations because of a very limited number of events (16 and 37 incident cases of IBD in the rosacea and reference cohorts, respectively).[43]
4. Discussion
Our study indicates patients with rosacea are associated with an increase in prevalent CD and UC. The evidence from case-control studies indicates 1.30-fold odds of prevalent CD and 1.64-fold odds of prevalent UC in rosacea patients when compared to controls. On the other hand, the evidence from cohort studies reveals a 1.58-fold risk of incident CD and a 1.18-fold risk of incident UC in rosacea patients when compared to controls. We identified statistical heterogeneity in the association of rosacea with Crohn disease (I2 = 74%; Fig. 3A); however, the direction of all the three case-control studies was consistent and indicated a positive association between the 2 diseases. Therefore, the evidence supports an association of rosacea with Crohn disease and the statistical heterogeneity merely represents variations between these studies.[49] Due to the lack of further detailed data from the included studies, we were unable to conduct a subgroup analysis to explore the origin of variations.
The mechanism underlying the association between rosacea and IBD is unclear. Rosacea and IBD are both chronic inflammatory diseases involving the interplay between genetic and immunological elements. Familial history has been reported as a risk factor, suggesting that genetic predisposition may be critical in the 2 diseases.[9,50] Rosacea was identified to involve human leukocyte antigen (HLA)-DRB1∗03:01, HLA-DQB1∗02:01, and HLA-DQA1∗05:01 as well as the intergenic single-nucleotide polymorphism between HLA-DRA and butyrophilin-like 2 (BTNL2).[51] BTNL2 possesses an immunomodulatory function by regulation of T cell activation and tolerance.[52] Interestingly, previous studies have detected strong overdominance of HLA-DRB1∗03:01 in UC,[53] and BTNL2 was linked to an increased risk for IBD.[54,55] Furthermore, glutathione S-transferases (GST) plays a major role in cellular defense against reactive oxygen species, providing photoprotection.[56] GSTT1 null genotype has been found significantly increased in rosacea patients[57] and was associated with increased susceptibility to CD and UC.[58] These common genetic factors may contribute to the association between rosacea and IBD.
Both rosacea and IBD are associated with alterations in innate and adaptive immunity.[6] Dysfunction of the innate immune system may contribute to the development of chronic inflammation and vascular abnormalities. For example, activation of macrophages and toll-like receptor 2, dysregulations of mast cells, fibroblasts, and production of reactive oxygen species, matrix metalloproteinases, tumor necrosis factor and interleukin-1β contribute to inflammation of rosacea and IBD.[6,59] As to adaptive immunity, T helper type 1 and 17 cells as well as B cells are pathogenic in both rosacea and IBD through production of interferon-gamma, tumor necrosis factor, interleukin-17, and multiple immunoglobulins.[6,59,60]
Rosacea and IBD share common risk factors. Obesity is a risk factor for IBD,[26,27] while a higher body-mass index has been associated with increased severity of rosacea in twins.[61] On the other hand, previous smoking status is a risk factor for rosacea.[9] Interestingly, active smoking appears to be associated with a reduced risk for rosacea, with risk increasing about 1 year after smoking cessation.[3,6,62] Similar influence of smoking was also noted in UC.[63] In the present study, only 1 included case-control studies and 2 included cohort studies had considered smoking as a confounding factor.[44,45,48] In these studies, only 1 case-control and one cohort studies had adjusted for body-mass index additionally.[45,48]
A significantly increased prevalence of SIBO has been found in patients with rosacea.[13] Remarkably higher SIBO has been observed in both CD and UC patients, and eradication of SIBO substantially improved clinical symptoms of IBD.[33,64,65] Higher SIBO has also been found in rosacea patients, and eradication of SIBO led to complete resolution of rosacea and maintained remission.[13] It has been speculated that SIBO increases intestinal permeability, with resultant translocation of bacterial components and proinflammatory cytokine into the blood flow and triggering inflammation of the skin.[13,66] The observed association between rosacea and IBD is compatible with the newly emerging concept of ‘skin-gut axis’, which proposed gastrointestinal dysbiosis affects the skin through complicated communications between immune, metabolic, and nervous systems.[67]
The present study has several limitations. First, we originally planned to assess publication bias, but the limited number of included studies prevented us from drawing a funnel plot. However, the total sample size was large and the risk of bias of included studies was generally low. Second, the included studies were primarily from Western countries, especially the US and UK. The only Asian study provided data on the association of rosacea with IBD but lacked respective data of CD and UC for meta-analysis.[43] More studies for rosacea patients in different ethnic groups are needed. Third, we did not find studies that examined the association between rosacea of different severity or phenotypes and IBD. Whether the strength of association differs among patients of different rosacea severity or phenotypes remains unclear.
In conclusion, our study demonstrates a modest association of rosacea with prevalent and incident IBD. Conversely, another study has shown that IBD is associated with an increase in rosacea.[68] Putting together the evidence, rosacea and IBD are related to each other. When rosacea patients suffer from chronic abdominal pain, prolonged diarrhea, and bloody stool, the possibility of comorbid IBD should be considered.
Author contributions
Conceptualization: Ching-Chi Chi.
Data curation: Fang-Ying Wang, Ching-Chi Chi.
Formal analysis: Fang-Ying Wang, Ching-Chi Chi.
Investigation: Fang-Ying Wang, Ching-Chi Chi.
Methodology: Ching-Chi Chi.
Project administration: Ching-Chi Chi.
Resources: Ching-Chi Chi.
Software: Fang-Ying Wang, Ching-Chi Chi.
Supervision: Ching-Chi Chi.
Validation: Ching-Chi Chi.
Writing – original draft: Fang-Ying Wang.
Writing – review & editing: Ching-Chi Chi.
Ching-Chi Chi orcid: 0000-0001-5699-0283.
Fang-Ying Wang orcid:0000-0002-8189-4944.
Supplementary Material
Footnotes
Abbreviations: BTNL2 = butyrophilin-like 2, CD = Crohn disease, CI = confidence interval, GST = glutathione S-transferases, HLA = human leukocyte antigen, HR = hazard ratio, IBD = inflammatory bowel disease, ICD = International Classification of Disease, MOOSE = Meta-analysis of Observational Studies in Epidemiology, NOS = Newcastle-Ottawa Scale, OR = odds ratio, SIBO = small intestinal bacterial overgrowth, UC = ulcerative colitis.
How to cite this article: Wang FY, Chi CC. Association of rosacea with inflammatory bowel disease. Medicine. 2019;98:41(e16448).
The authors have no funding and conflicts of interests to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol 2004;50:907–12. [DOI] [PubMed] [Google Scholar]
- [2].Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol 2018. [DOI] [PubMed] [Google Scholar]
- [3].Spoendlin J, Voegel JJ, Jick SS, et al. A study on the epidemiology of rosacea in the U.K. Br J Dermatol 2012;167:598–605. [DOI] [PubMed] [Google Scholar]
- [4].Augustin M, Herberger K, Hintzen S, et al. Prevalence of skin lesions and need for treatment in a cohort of 90 880 workers. Br J Dermatol V 165 2011;865–73. [DOI] [PubMed] [Google Scholar]
- [5].Schwab VD, Sulk M, Seeliger S, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. Br J Dermatol 2011;15:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Holmes AD, Spoendlin J, Chien AL, et al. Evidence-based update on rosacea comorbidities and their common physiologic pathways. J Am Acad Dermatol 2018;78:156–66. [DOI] [PubMed] [Google Scholar]
- [7].Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc 2011;15:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol 2015;135:2198–208. [DOI] [PubMed] [Google Scholar]
- [9].Abram K, Silm H, Maaroos HI, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol 2010;24:565–71. [DOI] [PubMed] [Google Scholar]
- [10].Moran EM, Foley R, Powell FC. Demodex and rosacea revisited. Clin Dermatol 2017;35:195–200. [DOI] [PubMed] [Google Scholar]
- [11].Turgut Erdemir A, Gurel MS, Koku Aksu AE, et al. Demodex mites in acne rosacea: reflectance confocal microscopic study. Australas J Dermatol 2017;58:e26–30. [DOI] [PubMed] [Google Scholar]
- [12].Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol 2007;157:474–81. [DOI] [PubMed] [Google Scholar]
- [13].Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol 2008;6:759–64. [DOI] [PubMed] [Google Scholar]
- [14].Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol 2015;73:249–54. [DOI] [PubMed] [Google Scholar]
- [15].Egeberg A, Ashina M, Gaist D, et al. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol V 76 2017;454–8. [DOI] [PubMed] [Google Scholar]
- [16].Egeberg A, Hansen PR, Gislason GH, et al. Patients with rosacea have increased risk of depression and anxiety disorders: a Danish nationwide cohort study. Dermatology (Basel, Switzerland) 2016;232:208–13. [DOI] [PubMed] [Google Scholar]
- [17].Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet (London, England) 2007;369:1641–57. [DOI] [PubMed] [Google Scholar]
- [18].Domenech E, Manosa M, Cabre E. An overview of the natural history of inflammatory bowel diseases. Dig Dis (Basel, Switzerland) 2014;32:320–7. [DOI] [PubMed] [Google Scholar]
- [19].Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (London, England) 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- [20].Ye Y, Pang Z, Chen W, et al. The epidemiology and risk factors of inflammatory bowel disease. Int J Clin Exp Med 2015;8:22529–42. [PMC free article] [PubMed] [Google Scholar]
- [21].Ekbom A, Helmick C, Zack M, et al. The epidemiology of inflammatory bowel disease: a large, population-based study in Sweden. Gastroenterology 1991;100:350–8. [DOI] [PubMed] [Google Scholar]
- [22].Munkholm P, Langholz E, Nielsen OH, et al. Incidence and prevalence of Crohn's disease in the county of Copenhagen, 1962-87: a sixfold increase in incidence. Scand J Gastroenterol 1992;27:609–14. [DOI] [PubMed] [Google Scholar]
- [23].Loftus EV, Jr, Silverstein MD, Sandborn WJ, et al. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gut 2000;46:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mahid SS, Minor KS, Soto RE, et al. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clinic Proc 2006;81:1462–71. [DOI] [PubMed] [Google Scholar]
- [25].To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn's disease. Aliment Pharmacol Ther V 43 2016;549–61. [DOI] [PubMed] [Google Scholar]
- [26].Blain A, Cattan S, Beaugerie L, et al. Crohn's disease clinical course and severity in obese patients. Clin Nutr (Edinburgh, Scotland) 2002;21:51–7. [DOI] [PubMed] [Google Scholar]
- [27].Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis 2011;17:2162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gradel KO, Nielsen HL, Schonheyder HC, et al. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology 2009;137:495–501. [DOI] [PubMed] [Google Scholar]
- [29].Luther J, Dave M, Higgins PD, et al. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis 2010;16:1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ungaro R, Bernstein CN, Gearry R, et al. Antibiotics associated with increased risk of new-onset Crohn's disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol 2014;109:1728–38. [DOI] [PubMed] [Google Scholar]
- [31].Felder JB, Korelitz BI, Rajapakse R, et al. Effects of nonsteroidal antiinflammatory drugs on inflammatory bowel disease: a case-control study. Am J Gastroenterol 2000;95:1949–54. [DOI] [PubMed] [Google Scholar]
- [32].Duricova D, Pedersen N, Elkjaer M, et al. Overall and cause-specific mortality in Crohn's disease: a meta-analysis of population-based studies. Inflamm Bowel Dis 2010;16:347–53. [DOI] [PubMed] [Google Scholar]
- [33].Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000;95:3503–6. [DOI] [PubMed] [Google Scholar]
- [34].Kosmidou M, Gaitanis G, Nomikos K, et al. Severe rosacea in a patient on infliximab for ulcerative colitis: pathophysiological considerations. Acta Derm-Venereologica 2009;89:522–3. [DOI] [PubMed] [Google Scholar]
- [35].Romiti R, Jansen T, Heldwein W, et al. Rosacea fulminans in a patient with Crohn's disease: a case report and review of the literature. Acta Derm-Venereologica 2000;80:127–9. [PubMed] [Google Scholar]
- [36].Tsuchiya S, Ichioka S, Tajima S. Rhinophyma-like hypertrophy of the nose caused by chronic facial pyoderma in a patient with Crohn's disease. J Plast Surg Hand Surg 2014;48:344–6. [DOI] [PubMed] [Google Scholar]
- [37].van Steensel MA, Badeloe S, Winnepenninckx V, et al. Granulomatous rosacea and Crohn's disease in a patient homozygous for the Crohn-associated NOD2/CARD15 polymorphism R702W. Exp Dermatol 2008;17:1057–8. [DOI] [PubMed] [Google Scholar]
- [38].Wakabayashi M, Fujimoto N, Uenishi T, et al. A case of acne fulminans in a patient with ulcerative colitis successfully treated with prednisolone and diaminodiphenylsulfone: a literature review of acne fulminans, rosacea fulminans and neutrophilic dermatoses occurring in the setting of inflammatory bowel disease. Dermatology (Basel, Switzerland) 2011;222:231–5. [DOI] [PubMed] [Google Scholar]
- [39].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [41].Wells GA SB, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. 2006. [Google Scholar]
- [42].Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011. [Google Scholar]
- [43].Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: Results from a nationwide cohort study in Taiwan. J Am Acad Dermatol 2017;76:911–7. [DOI] [PubMed] [Google Scholar]
- [44].Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol 2017;176:100–6. [DOI] [PubMed] [Google Scholar]
- [45].Li WQ, Cho E, Khalili H, et al. Rosacea, use of tetracycline, and risk of incident inflammatory bowel disease in women. Clin Gastroenterol Hepatol 2016;14:e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lim H, Fischer A, Kendall J, et al. Rosacea associated with increased prevalence of gastrointestinal disorders in absence of systemic antibiotics. J Invest Dermatol 2017;137:S30. [Google Scholar]
- [47].Marks R, Beard RJ, Clark ML, et al. Gastrointestinal observations in rosacea. Lancet (London, England) 1967;1:739–43. [DOI] [PubMed] [Google Scholar]
- [48].Spoendlin J, Karatas G, Furlano RI, et al. Rosacea in patients with Ulcerative Colitis and Crohn's disease: a population-based case-control study. Inflamm Bowel Dis 2016;22:680–7. [DOI] [PubMed] [Google Scholar]
- [49].Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. Inflamm Bowel Dis 2011;64:1294–302. [DOI] [PubMed] [Google Scholar]
- [50].Childers RE, Eluri S, Vazquez C, et al. Family history of inflammatory bowel disease among patients with ulcerative colitis: a systematic review and meta-analysis. J Crohn's Colitis 2014;8:1480–97. [DOI] [PubMed] [Google Scholar]
- [51].Chang ALS, Raber I, Xu J, et al. Assessment of the genetic basis of rosacea by genome-wide association study. J Invest Dermatol 2015;135:1548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nguyen T, Liu XK, Zhang Y, et al. BTNL2, a butyrophilin-like molecule that functions to inhibit T cell activation. J Immunol 2006;176:7354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Goyette P, Boucher G, Mallon D, et al. High density mapping of the MHC identifies a shared role for HLA-DRB1∗01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat Genet 2015;47:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 2011;43:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Prescott NJ, Lehne B, Stone K, et al. Pooled sequencing of 531 genes in inflammatory bowel disease identifies an associated rare variant in BTNL2 and implicates other immune related genes. PLoS Genet 2015;11:e1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Seo KI, Cho KH, Park KC, et al. Change of glutathione S-transferases in the skin by ultraviolet B irradiation. J Dermatol Sci 1996;13:153–60. [DOI] [PubMed] [Google Scholar]
- [57].2006;Yazici AC, Tamer L, Ikizoglu G, et al. GSTM1 and Photoimmunol Photomed. 22:208–10. [DOI] [PubMed] [Google Scholar]
- [58].Senhaji N, Kassogue Y, Fahimi M, et al. Genetic polymorphisms of multidrug resistance gene-1 (MDR1/ABCB1) and glutathione S-transferase gene and the risk of inflammatory bowel disease among Moroccan patients. Mediators inflamm 20152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Choy MC, Visvanathan K, De Cruz P. An overview of the innate and adaptive immune system in inflammatory bowel disease. Inflamm Bowel Dis 2017;23:2–13. [DOI] [PubMed] [Google Scholar]
- [60].Yadav V, Varum F, Bravo R, et al. Inflammatory bowel disease: exploring gut pathophysiology for novel therapeutic targets. Transl Res 2016;176:38–68. [DOI] [PubMed] [Google Scholar]
- [61].Aldrich N, Gerstenblith M, Fu P, et al. Genetic vs environmental factors that correlate with rosacea: a cohort-based survey of twins. JAMA Dermatol 2015;151:1213–9. [DOI] [PubMed] [Google Scholar]
- [62].Breton AL, Truchetet F, Veran Y, et al. Prevalence analysis of smoking in rosacea. J Eur Acad Dermatol Venereol 2011;25:1112–3. [DOI] [PubMed] [Google Scholar]
- [63].Higuchi LM, Khalili H, Chan AT, et al. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol 2012;107:1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rana SV, Sharma S, Malik A, et al. Small intestinal bacterial overgrowth and orocecal transit time in patients of inflammatory bowel disease. Dig Dis Sci 2013;58:2594–8. [DOI] [PubMed] [Google Scholar]
- [65].Castiglione F, Del Vecchio Blanco G, Rispo A, et al. Orocecal transit time and bacterial overgrowth in patients with Crohn's disease. J Clin Gastroenterol 2000;31:63–6. [DOI] [PubMed] [Google Scholar]
- [66].Riordan S, McIver C, Thomas D, et al. Luminal bacteria and small-intestinal permeability. Scand J Gastroenterol 1997;32:556–63. [DOI] [PubMed] [Google Scholar]
- [67].O’Neill CA, Monteleone G, McLaughlin JT, et al. The gut-skin axis in health and disease: a paradigm with therapeutic implications. BioEssays 2016;38:1167–76. [DOI] [PubMed] [Google Scholar]
- [68].Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: A population-based cross-sectional study. J Am Acad Dermatol 2017;76:40–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


