Supplemental Digital Content is available in the text
Keywords: cerebrospinal fluid, infant, lumbar puncture, reference values
Abstract
Cerebrospinal fluid (CSF) protein values decline over the first few months of life as the infant's blood-CSF barrier matures. However, published studies have not reported CSF protein reference values of Chinese infants and differ in the reported rate, timing, and magnitude of this decline. The objective of this study was to determine reference intervals for CSF protein using available data of children in southern China. This retrospective study included infants who had a lumbar puncture (LP) performed in the Department of Pediatrics of Southern Medical University Affiliated Maternal and Child Health Hospital of Foshan of an urban tertiary care children's hospital between January 1, 2008 and May 31, 2018. Infants with conditions suspected or known to cause elevated CSF protein concentrations were excluded. Of 3712 infants undergoing LP, 1043 (28.1%) met inclusion criteria. Results showed that there is an age-related decline in CSF protein concentration. The median CSF protein value was 62 mg/dL [interquartile range (IQR): 47–81 mg/dL] in infants aged 0 to 56 days (group 1). The 95th percentile values were 116 mg/dL for infants 0 to 28 days and 80 mg/dL for infants 29 to 56 days. The 95th percentile values by age category were as follows: ages 0 to 14 days, 117 mg/dL; ages 15 to 28 days, 107 mg/dL; ages 29 to 42 days, 96 mg/dL; and ages 43 to 56 days, 74 mg/dL. The median CSF protein value was 21 mg/dL (IQR: 16–31 mg/dL) in infants aged 2 months to <3 years (group 2). The 95th percentile values were 57 mg/dL for infants 2 to <6 months and 34 mg/dL for infants 6 to ≤24 months. The 95th percentile values by age category were as follows: ages 2 to <3 months, 66 mg/dL; ages 3 to <4 months, 52 mg/dL; ages 4 to <5 months, 53 mg/dL; and ages 5 to <6 months, 42 mg/dL. We quantify the age-related decline in CSF protein concentrations among infants 2 years of age and younger and provide age-specific reference values. The values reported here can be used to interpret the results of LP in infants ≤2 years of age.
1. Introduction
Lumbar puncture (LP) and cerebrospinal fluid (CSF) analysis is crucial for the diagnosis of bacterial meningitis. This process depends on having well-established reference (“normal”) values.[1] For ethical reasons, studies of CSF rarely use completely healthy children. In fact, from a clinical standpoint, healthy subjects may not represent the most desirable reference group, as clinical decisions often rely on distinguishing between a serious pathologic process and a benign self-limited condition, both of which may theoretically alter CSF parameters. Thus, the most clinically relevant reference group may vary depending on the specific clinical scenario.[2] To define accurate reference ranges for CSF parameters, researchers have attempted to use neonates and young infants undergoing LP during emergency department evaluation of fever to established reference (“normal”) values.[2–10] Although many previous studies provide CSF reference values for young infants, the reported age-specific values and rates of decline vary considerably and no previous study has included a large number of Chinese infants for analysis or stratified infants into narrowly defined age categories.[2–10] Our objectives were to develop age-specific reference standards for CSF protein concentrations in a population of neonates and infants who presented for medical care with an indication for LP and were subsequently found to have no condition associated with elevated or depressed CSF protein concentrations by using a larger data to improve the precision and generalizability of reference values.
2. Methods
2.1. Study design and setting
The retrospective study was conducted in the Department of Pediatrics of Foshan Women and Children Hospital Affiliated to Southern Medical University, China, a tertiary care hospital. The hospital admits a total of approximately 450,000 children annually. The Ethics Committee of Foshan Women and Children Hospital Affiliated to Southern Medical University approved this study with a waiver of informed consent. All experiments and methods were performed in accordance with the relevant guidelines and regulations.
2.2. Study participants
Children, who had an LP performed as part of their emergency evaluation between January 1, 2008 and May 31, 2018, were eligible for inclusion. To ensure accurate identification of all eligible children, clinical and laboratory data were obtained from the medical records of all patients with CSF testing performed. Data included age, sex, laboratory data [CSF white blood cell (WBC) counts, CSF glucose and protein concentrations, CSF clarity, character and color, and CSF Gram stain]. The medical records of all patients receiving CSF analysis were identified and reviewed retrospectively using standardized criteria for classifying patient diagnoses. Figure 1 outlines major exclusion criteria that were used to derive the reference group 1. Patients were excluded sequentially when missing any component of the CSF profile (i.e., WBC count, protein, or glucose); the LP was traumatic; pinocytosis (>50 WBCs/μL); prematurity (age at birth <37 weeks and age <1 year) or patients with confirmed diagnosis associated with increased protein level. In a traumatic LP, the presence of red blood cells in the CSF alters WBC counts and adjustment formulas cannot reliably approximate the actual values.[11–14] Confirmed diagnosis associated with increased protein level include serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis, or septic arthritis), congenital infection, seizure before presentation, static encephalopathy, hydrocephalus, viral central nervous system (CNS) infection, previous intracranial infection, stroke, demyelinating disease, or ventricular shunt.[15] Patients with acute or chronic systemic disease, for example, cancer and hematologic or rheumatologic disorders, were also excluded. CSF protein was analyzed on 2 different instruments over the course of the 10 years included in the study as follows: Beckman AU640, May 30, 2008 to December 5, 2012; Beckman AU5821, December 5, 2012 to date. In all cases, analyses were performed according to manufacturer's directions. One thousand forty-three (28.1%) patients were assigned to the reference group. For initial analysis of the age dependence of CSF protein concentrations, patients were first categorized according to the following age groups: 0 to younger than 14 days, 15 days to younger than 28 days, 29 days to younger than 42 days, 43 days to younger than 56 days; monthly for the remainder of the first and the second year (i.e., 2–3, >3–4, >4–5, ... >23–24 months), and annually (i.e., 2, >2–3, >3–4 years,...) thereafter. To facilitate the implementation of our results into clinical practice, age groups were subsequently condensed into 2 groups: group 1 (0–14, 15–28, 29–42, 43–56 days) and group 2 (2–<6, 6–<12, 12–<18, 18–<24, and 24–<36 months).
Figure 1.
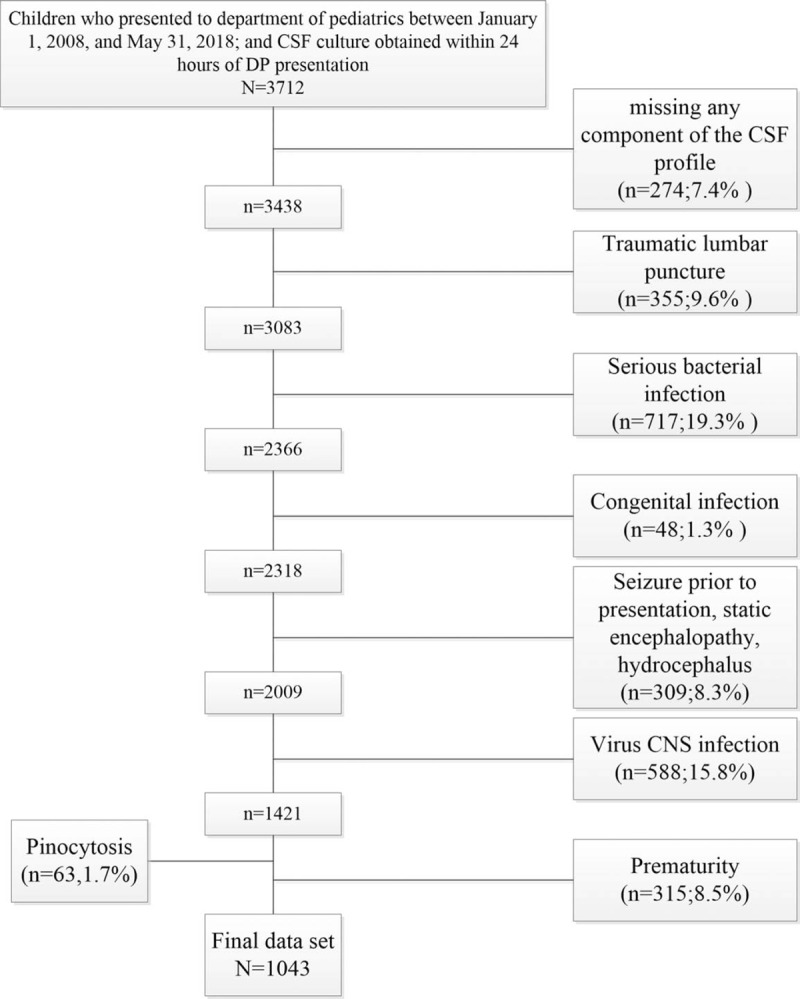
Flow chart of patient inclusion and exclusion criteria. CNS = central nervous system, CSF = cerebrospinal fluid.
2.3. Statistical analysis
Statistical analysis was performed using the SPSS Statistics 19.0 for Windows (SPSS Inc, Chicago, IL) program. Categorical data were described using frequencies and percentages, and continuous variables were described by using mean, median, interquartile range (IQR), and 90th and 95th percentile values. Linear regression was used to determine the associations between age and CSF protein concentrations. Because the relationship between CSF protein values and age appeared to be nonlinear for children aged <36 months, exponential curve analysis was performed using logarithmically transformed CSF protein values as the dependent variable in group 2 (Table 1). Figures are created using SPSS. The distribution of residuals is normal. Kruskal-Wallis H tests were subsequently used to compare the distribution of CSF protein concentrations amongst the predefined age categories to facilitate implementation of our results into clinical practice: group 1 (0–14, 15–28, 29–42, and 43–56 days) and group 2 (2–<6, –<12, 12–<18, 18–<24, and 24–<36 months). Two months also include 57, 58, 59, and 60 days.
Table 1.
Results of regression analysis for cerebrospinal fluid protein concentrations in infants.

3. Results
3.1. Study cohort
A total of 3712 children presented to the department of pediatrics and had a CSF culture obtained within 24 hours of presentation. Of these, 2669 (71.9%) met sequential exclusion criteria as follows: missing any component of the CSF profile (274); traumatic LP (n = 355); serious bacterial infection (n = 717); congenital infection (n = 48); seizure before presentation, static encephalopathy, and hydrocephalus (n = 309); virus CNS infection (n = 588); pinocytosis (n = 63); and prematurity (n = 315). The remaining 1043 (28.1%) subjects were included in the final analysis (Fig. 1). The median patient age was 13 days (IQR: 3–16 days) in group 1 (0–56 days); 74.5% (n = 307) were 28 days or younger, 66.0% (n = 272) were boys. The median patient age was 7 months (IQR: 3–14 months) in group 2 (2–<24 months); 46.2% (n = 231) were 6 months or younger, 58.6% (n = 293) were boys. The median patient age was 3 years (IQR: 2–5 years) in group 3 (2–≤9 years); 46.2% (n = 123) were 6 months or younger, 68.0% (n = 123) were boys. The CSF protein concentrations are shown in Tables 2 and 3. The other parameters are shown in Supplemental Tables S1 and S2.
Table 2.
Cerebrospinal fluid protein concentrations in infants aged 2 years and younger∗.
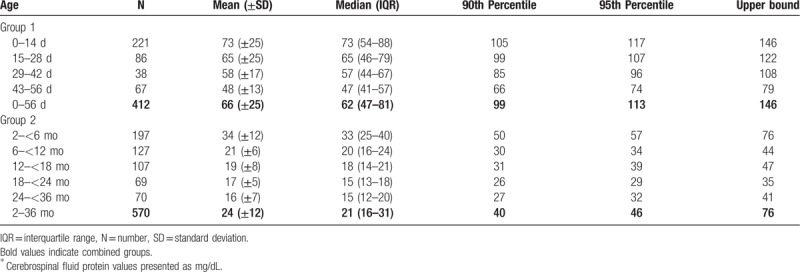
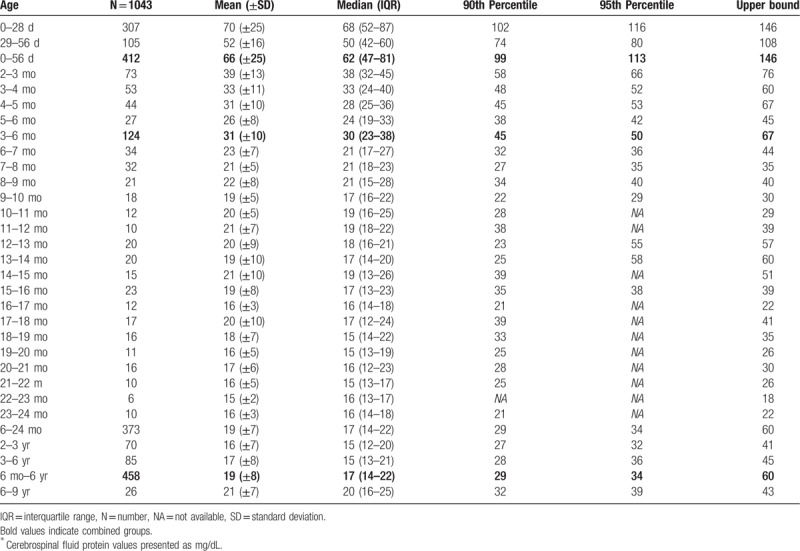
Table 3.
Cerebrospinal fluid protein concentrations in infants monthly∗.
3.2. Age dependence of CSF protein concentrations for infants 56 days or younger
The median CSF protein value was 62 mg/dL (interquartile range: 47–81 mg/dL) in group 1. There was an age-related decline in CSF protein concentration (Fig. 2A). The median CSF protein concentrations declined from a high value of 75.0 mg/dL at 0 to 1 week to a low value of 47.0 mg/dL at 8 weeks of age. CSF protein concentrations were higher for infants ≤28 days of age than for infants 29 to 60 days of age (P < .05, Kruskal-Wallis test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 116 mg/dL) for infants ≤28 days of age and 50 mg/dL (95th percentile value, 80 mg/dL) for infants 29 to 56 days. CSF protein concentrations by 2-week age intervals are shown in Table 2. The 95th percentile CSF protein concentrations were as follows: ages 0 to 14 days, 117 mg/dL; ages 15 to 28 days, 107 mg/dL; ages 29 to 42 days, 96 mg/dL; and ages 43 to 56 days, 74 mg/dL (Table 2). CSF protein concentration decreased across each age interval when compared with infants in the next highest age category [P < .05 for all pairwise comparisons except for group (15–28 days) versus group (29–42 days), Kruskal-Wallis test] (Fig. 2B).
Figure 2.
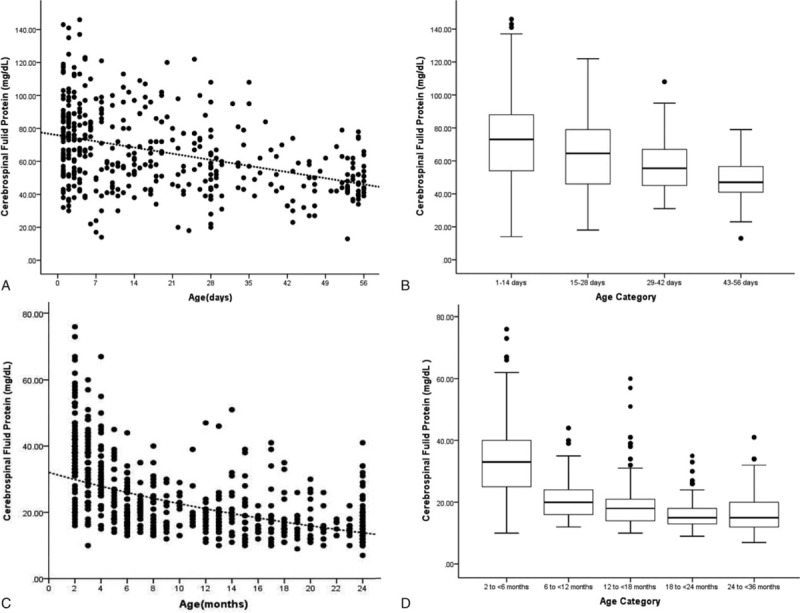
A, Relationship of cerebrospinal fluid protein concentrations and age ≤56 days; a linear regression line shows the decline in protein concentrations with age. C, Relationship of cerebrospinal fluid protein concentrations and age 2 to <36 months; linear regression line shows that the cerebrospinal fluid protein concentrations decreased as the age increased. Each black circle represents data from one infant. B, Boxplot showing variation in cerebrospinal fluid protein concentrations by age category (0–14, 15–28, 29–42, and 43–56 days). D, Boxplot showing variation in cerebrospinal fluid protein concentrations by age category (2–<6, 6–<12, 12–<18, 18–<24, and 24–<36 months); the line in the middle of the box denotes the median value. The ends of the boxes represent the interquartile range (i.e., 25th and 75th percentile) values. The whiskers extend 1.5 times the interquartile range values and the circles denote extreme outlying values.
3.3. Age dependence of CSF protein concentrations for infants younger than 36 months
The median CSF protein value was 21 mg/dL (interquartile range: 16–31 mg/dL) in group 2. Exponential curve of CSF protein values versus age are shown in Figure 2C, and no other predictor variables was used. In this group, the CSF total protein concentrations fell rapidly with a maximal decrease during 2 to <6 months of life. Thereafter, the decline in CSF protein was less pronounced in the 6 to <18 months age range. Over an average 18 to <36 months age range, a plateau was reached. The 95th percentile values were 57 mg/dL for infants 2 to <6 months and 34 mg/dL for infants 6 to ≤24 months. The 95th percentile values by age category were as follows: ages 2 to <3 months, 66 mg/dL; ages 3 to <4 months, 52 mg/dL; ages 4 to <5 months, 53 mg/dL; and ages 5 to <6 months, 42 mg/dL. As summarized in Table 2, patients between ages 2 and 6 months had a higher mean concentrations, accompanied by greater variability of protein values, compared with older patients (P < .001, Kruskal-Wallis test). The patients’ mean CSF protein concentrations dropped rapidly during the first 6 months of life, reaching a minimum value of <26 mg/dL. After dividing the data into the 5 age groups of 2 to younger than 6 months, 6 to younger than 12 months, 12 to younger than 18 months, 18 to younger than 24 months, and 24 to younger than 36 months (Table 2), multiple comparisons statistical analysis found the first 2 groups to be statistically significantly (P < .001, Kruskal-Wallis test) different from each other. The group (24–36 months) was statistically significantly (P < .001, Kruskal-Wallis test) different from the first and second age group (2–<6 and 6–<12 months), but was not statistically different from the other 2 groups (12–<18 and 18–<24 months) by nonparametric testing (Fig. 2D).
4. Discussion
Currently there is no consensus on the use of age-specific reference values for CSF protein concentrations in Chinese children. The reference values for CSF protein concentrations for children used in children's hospitals in China are adult reference values. Because of the higher permeability of the immature barrier of neonate and young infants, their CSF has higher protein content than that of children and adults. In addition, maturation of the barrier gradually increases after birth, reaching its maximum at the third month of life.[16] This was shown by the age distribution of the values in the reference population, with a maximal decrease during the first 6 months of life.[4,10] Therefore, CSF protein concentrations measurement has little value in diagnosing neurological diseases in this age category. Although several previous studies provide age-specific reference values for CSF protein concentrations, the reported values vary considerably and no reference values were reported for Chinese infants.[2–10]
In this study, we established age-specific reference values for CSF protein concentrations from presumptively uninfected infants by using sequential, stringent exclusion criteria. We were further able to quantify the age-related decline in CSF protein concentrations over the first 2 months of life and the first 2 years of life. We determined that infants 0 to 28 days of age have higher CSF protein concentrations than infants 29 to 60 days of age. The patients’ mean CSF protein concentrations dropped rapidly between the third and sixth month of life and reached a minimum of <25 mg/dL by approximately 6 months. Thereafter, the decline in CSF protein was less pronounced and from 3 to 9 years, a slight increase was observed for CSF protein concentrations.
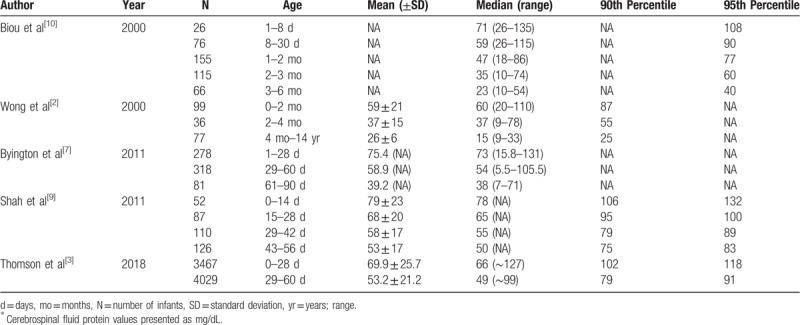
As shown in Table 4, the CSF protein concentrations values provided in our study are generally comparable to previously published reference values. In a single-center analysis of 1074 infants, Biou et al[10] defined the upper limit of CSF protein reference intervals as the 95th percentile. On the basis, the upper limit values for CSF protein are 108 mg/dL for infants ≤28 days and 77 mg/dL for infants 1 to 2 months of age, which were marginally lower than our 95th percentile values of 116 and 80 mg/dL, respectively. By comparison, Wong et al[2] reported an even lower range of up to 100 mg/dL for infants 0 to 28 days in a single-center analysis of 906 infants. In addition, Byington et al[7] defined the upper limits at 131 mg/dL for infants 0 to 28 days and 106 mg/dL for infants 29 to 60 days of age, which were marginally lower than our upper limit values of 146 and 108 mg/dL, respectively. In a single-center analysis of 375 infants, Shah et al[9] defined normative CSF protein concentrations using 95th percentile values (0–14 days: 132 mg/dL; 15–28 days: 101 mg/dL; 29–42 days: 89 mg/dL; 43–56 days: 82 mg/dL), which were similar with our 95th percentile values of 117, 107, 96, and 74 mg/dL, respectively. In a multicenter analysis of 7766 infants, Thomson et al[3] defined normative CSF protein concentrations using 95th percentile values (0–28 days: 118 mg/dL; 29–60 days: 91 mg/dL). We included infants up to 56 days of age; our 95th percentile values for CSF protein concentrations are similar for infants 0 to 28 days: 116 mg/dL and lower for infants 29 to 56 days: 80 mg/dL. Furthermore, Wong et al[2] reported that cerebrospinal fluid protein concentration decreased rapidly to a lowest point by 6 months and remained low throughout childhood, rarely exceeding 30 mg/dL and, finally, increasing in adolescence toward adult values. Biou et al[10] defined the upper limits at 60 mg/dL for infants 2 to 3 months of age, 40 mg/dL for infants 3 to 6 months of age, and 32 mg/dL for infants older than 6 months. On this basis, our upper limits for CSF protein values are 66, 50, and 34 mg/dL, respectively. We included infants aged 2 to 24 months, the patients’ mean CSF protein concentrations dropped rapidly between the second and sixth month of life and reached a minimum of <36 mg/dL by approximately 6 months and the CSF protein concentrations was rarely >34 mg/dL (95th) for infants older than 6 months in our study. Thus, based on our data and previous studies, CSF protein levels >116 mg/dL in neonates, 80 mg/dL in infants 29 to 56 days, 0.57 mg/dL in infants 2 to 6 months, and 0.34 mg/dL in infants older than 6 months should raise concerns about a pathologic process.
Table 4.
Summary of prior studies reporting age-specific cerebrospinal fluid protein concentrations∗.
One of the limitations in our study must be addressed. The infants in this study received LP for a clinical indication (e.g., fever or other concern for infection). Therefore, our findings do not represent normal values per se but reasonable reference standards to facilitate the interpretation of CSF parameters for infants who require LP as part of initial evaluation in the department of pediatrics.
In conclusion, the age-dependent reference values presented in this study will be used to provide guidance to clinicians when interpreting the CSF parameters of infants in southern China.
Author contributions
Conceptualization: Guohua Chen.
Data curation: Deqin Jia, Jieming Dai, Xing Zhou, Zhoulian Qin, Liang Chen, Jieyan Zhang.
Investigation: Zhigang Liu.
Methodology: Guohua Chen, Xiaocheng He.
Project administration: Zhigang Liu, Xingguang Ye.
Resources: Deqin Jia, Jieming Dai, Xing Zhou, Zhoulian Qin, Liang Chen, Jieyan Zhang.
Software: Guohua Chen, Xiaocheng He.
Writing – original draft: Ruipin Wan.
Writing – review and editing: Xingguang Ye.
Supplementary Material
Footnotes
Abbreviations: CNS = central nervous system, CSF = cerebrospinal fluid, IQR = interquartile range, LP = lumbar puncture, WBC = white blood cell.
How to cite this article: Liu Z, Jia D, Dai J, Zhou X, Qin Z, Chen L, Zhang J, Chen G, He X, Wan R, Ye X. Age-specific reference values for cerebrospinal fluid protein concentrations in children in southern China. Medicine. 2019;98:41(e17500).
Availability of data and material: The datasets analyzed in the current study are available from the corresponding author on reasonable request.
ZL, DJ, JD, and XZ contributed equally to this work.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Nigrovic L, Kuppermann N, Macias C, et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA 2007;297:52–60. [DOI] [PubMed] [Google Scholar]
- [2].Wong M, Schlaggar BL, Buller RS, et al. Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values. Arch Pediatr Adolesc Med 2000;154:827–31. [DOI] [PubMed] [Google Scholar]
- [3].Thomson J, Sucharew H, Cruz AT, et al. Cerebrospinal fluid reference values for young infants undergoing lumbar puncture. Pediatrics 2018;141:pii: e20173405. [DOI] [PubMed] [Google Scholar]
- [4].Kahlmann V, Roodbol J, Van Leeuwen N, et al. Validated age-specific reference values for CSF total protein levels in children. Eur J Paediatr Neurol 2017;21:654–60. [DOI] [PubMed] [Google Scholar]
- [5].Ahmed A, Hickey SM, Ehrett S, et al. Cerebrospinal fluid values in the term neonate. Pediatr Infect Dis J 1996;15:298–303. [DOI] [PubMed] [Google Scholar]
- [6].Bonadio WA, Stanco L, Bruce R, et al. Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks. Pediatr Infect Dis J 1992;11:589–91. [DOI] [PubMed] [Google Scholar]
- [7].Byington CL, Kendrick J, Sheng X. Normative cerebrospinal fluid profiles in febrile infants. J Pediatr 2011;158:130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chadwick SL, Wilson JW, Levin JE, et al. Cerebrospinal fluid characteristics of infants who present to the emergency department with fever: establishing normal values by week of age. Pediatr Infect Dis J 2011;30:e63–7. [DOI] [PubMed] [Google Scholar]
- [9].Shah SS, Ebberson J, Kestenbaum LA, et al. Age-specific reference values for cerebrospinal fluid protein concentration in neonates and young infants. J Hosp Med 2011;6:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Biou D, Benoist JF, Nguyen-Thi C, et al. Cerebrospinal fluid protein concentrations in children: age-related values in patients without disorders of the central nervous system. Clin Chem 2000;46:399–403. [PubMed] [Google Scholar]
- [11].Rubenstein J, Yogev R. What represents pleocytosis in blood-contaminated (“traumatic tap”) cerebrospinal fluid in children? J Pediatr 1985;107:249–51. [DOI] [PubMed] [Google Scholar]
- [12].Mayefsky J, Roghmann K. Determination of leukocytosis in traumatic spinal tap specimens. Am J Med 1987;82:1175–81. [DOI] [PubMed] [Google Scholar]
- [13].Bonadio W, Smith D, Goddard S, et al. Distinguishing cerebrospinal fluid abnormalities in children with bacterial meningitis and traumatic lumbar puncture. J Infect Dis 1990;162:251–4. [DOI] [PubMed] [Google Scholar]
- [14].Greenberg R, Smith P, Cotten C, et al. Traumatic lumbar punctures in neonates: test performance of the cerebrospinal fluid white blood cell count. Pediatr Infect Dis J 2008;27:1047–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Carraccio C, Blotny K, Fisher M. Cerebrospinal fluid analysis in systemically ill children without central nervous system disease. Pediatrics 1995;96(pt 1):48–51. [PubMed] [Google Scholar]
- [16].Statz A, Felgenhauer K. Development of the blood-CSF barrier. Dev Med Child Neurol 1983;25:152–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


