Abstract
Research on the relationship between iodine intake and thyroid cancer (TC) risk is limited, and the findings are inconclusive. The objective of this study was to provide emerging evidence for the association between iodine intake and TC risk in a Chinese population.
An ecological study of epidemiology is used to compare the iodine intake among populations with different TC incidence in Zhoushan, China. Incidence rates of TC were investigated and compared among four counties of the Zhoushan Islands from 2014 to 2018. Iodized salt consumption rate and the level of urinary iodine concentration (UIC) were analyzed for pupils and pregnant women from four counties.
During 2014 to 2018, a total of 2495 new cases of TC were diagnosed in Zhoushan Islands. The mean crude incidence rate of TC was 51.29 per 100,000 inhabitants, and the standardized (world population) incidence rate (SIR) was 31.34 per 100,000 population. Incidence rates (SIR and crude incidence rates) were significantly higher in women than in men (χ2 test, P < .05). Both male and female, the incidence of TC in Daishan County is higher than the other three counties of Zhoushan. Iodized salt consumption rate and median UIC in pupils and pregnant women in Daishan County was significantly lower than the other three counties (χ2 test and Kruskal–Wallis test, all P < .05). The population with high TC incidence has a lower iodized salt consumption and a lower level of UIC compare with the relative low TC incidence populations.
The low consumption of iodized salt with mild iodine deficiency may contribute to explain the exceptionally high incidence of TC in Daishan County. Further subtle designed studies are needed to provide additional insights into the epidemiology and etiology of TC and help identify the safe limit of iodine intake for prevention.
Keywords: ecological study, iodine, thyroid cancer, urinary iodine
1. Introduction
Thyroid cancer (TC) is the most common malignancy of the endocrine system, including four histotypes, that is, papillary, follicular, anaplastic, and medullary. Among them, papillary thyroid cancer (PTC) is the most common subtype accounting for about 60% to 80% of the total incidence, but anaplastic thyroid cancer accounts for a large portion of the mortality because of its poor prognosis.[1] In most countries, incidence rates of TC have been continually increasing over the past few decades, particularly in women.[2,3] The highest incidence rates were reported mostly in island countries or regions, such as Hawaii, Japan, Iceland, Philippine, Sicily, and Cyprus.[4] According to the data of International Agency for Research on Cancer (IARC) in 2012, the age-standardized (world population) incidence of TC was 6.1 per 100,000 in women and 1.9 per 100,000 in men worldwide, and there were increases with 29.8% and 26.0% compared with 2008, respectively.[5]
In past decades, evidence suggested that excessive screening and diagnostic testing is likely the major, but may not be the only, contributor to the rising incidence of TC.[6–8] Another suspected risk factors include radiation exposure during childhood (whether from nuclear accidents, natural radiation, or medical imaging),[9] genetic factors,[10] pre-existing thyroid disease,[11] hormonal and reproductive factors,[12] environmental pollutants,[13,14] a family history of TC or thyroid disorders,[15] and possibly, iodine intake.
As an essential trace element for thyroid function, iodine plays an indispensable role in development, growth, and metabolism throughout human life.[16] It was reported that both deficient and excessive iodine intakes are associated with the development of thyroid disease with following U-shaped curves.[17] Recent years, epidemiological studies have suggested that iodine intake is associated with TC risk, but the results remain considerable controversy.[18–20] Here, we performed an ecological comparison of epidemiology in Zhoushan, a coastal city of Zhejiang province of China, with regional differences in TC incidence, to provide a new clue of the possible relationship between iodine intake and TC risk.
2. Materials and methods
2.1. Study sites and source of TC patients
The present study was conducted in Zhoushan, which is a famous port city and the largest archipelago in China consisting of 1390 islands. The city covers four counties under its jurisdiction: Dinghai, Putuo, Daishan, and Shengsi, with a land area of 1440 km2 and a population of 1.1 million (Fig. 1). Daishan locates in the north of Zhoushan with a population of 185,035 inhabitants.
Figure 1.
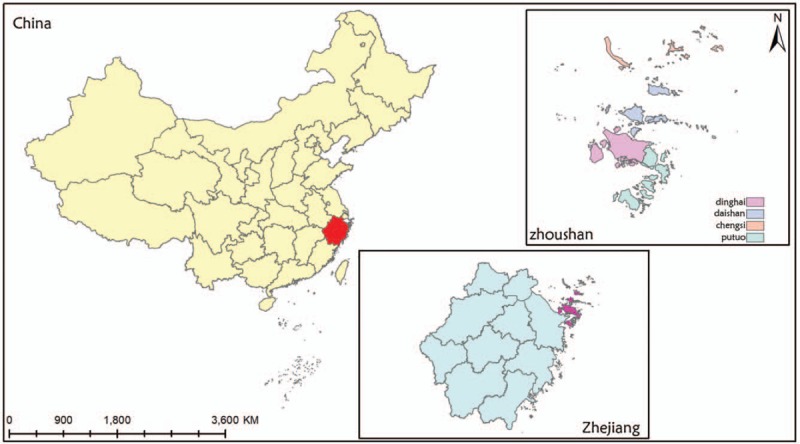
The location of four counties in Zhoushan Islands, Zhejiang province, China.
In this study, TC patients are all new cases clinically diagnosed in the year from 2014 to 2018. Data of the TC patients were obtained from China's cancer surveillance system. Age and sex structure of patients and the structure of histopathologic types in the available data were also calculated. The incidence is presented as crude and world population standardized rates per 100,000 population. The number of inhabitants was based on the sixth census in 2010 of the Zhoushan Bureau of Statistics. Age- and sex-standardized incidence rates were calculated using population estimates from the World Standard Population (WSP) of the 2001 census. All analyses were approved by the ethics committees of Zhoushan Municipal Center for Disease Control and Prevention (ZSCDC).
2.2. Monitoring of iodized salt consumption rate
During 2014 to 2018, the iodine nutrition status of primary school children and pregnant women in four counties was monitored by Zhoushan Municipal Center for Disease Control and Prevention (ZSCDC) every year.
Each monitoring county was divided into five sampling areas according to the geographical location (east, west, south, north, and center), and one township/street was randomly selected in each sampling area. One primary school was selected from each township/street, and 42 non-resident students aged 8 to 10 were selected from each primary school (14 students in each age group, half male and half female). If the sample is <42, it can be made up at a nearby school. A total of 21 pregnant women (7 in early, middle, and late pregnancy) were selected from the above-sampled township/street.
Total of 30 g of domestic edible salt was collected from each family of the student and pregnant women for detecting the content of salt iodine by “Determination of iodine in general test method for the salt industry” (GB/T 13025.7–2012). The standard for qualified iodized salt is the determination of iodine content (in terms of iodine elements) in edible salt within the range of 18 to 33 mg/kg according to the method of Chinese “edible salt iodine content” (GB 26878–2011).
Qualified iodine salt consumption rate = A/B∗100%.
-
1.
The number samples of salt with an iodine content in 18 to 33 mg/kg
-
2.
The number of samples tested
2.3. Detection of UIC
More than 90% of iodine consumed is excreted in the urine, making urine iodine concentration (UIC) a good biomarker of recent iodine intake.[21] The median UIC is an excellent indicator for the iodine status of a population.[21] We measured UIC of children and pregnant women in four counties from 2014 to 2018. Five milliliters of a random urine sample from the above children and pregnant women was collected to detect the level of urine iodine. In order to avoid the dilution of urine caused by excessive drinking water, the urine samples of pregnant women were collected at a time with gynecological B-ultrasound examination was conducted. Urine iodine content was determined by arsenic and cerium catalytic spectrophotometric method for iodine in urine (WS/T 107–2006). Based on the recommendations from WHO, iodine nutrition status was categorized into four groups: insufficient (children: UIC < 100 μg/L, pregnant women: UIC < 150 μg/L), adequate (children: UIC 100–199 μg/L, pregnant women: UIC 150–249 μg/L), more than adequate (children: UIC 200–299 μg/L, pregnant women: UIC 250–349 μg/L), and excessive (children: UIC ≥ 300 μg/L, pregnant women: UIC ≥ 350 μg/L).[15]
2.4. Statistical analysis
UIC is expressed as median (25th, 75th percentiles), and differences among four counties were compared using the Kruskal–Wallis test. Group comparisons of categorical variables were performed using the χ2 test. All statistical analyses were performed using the SPSS Statistics 19.0 software package (Chicago, IL). A P value of <.05 was considered statistically significant.
3. Results
3.1. Basic characteristics of TC patients
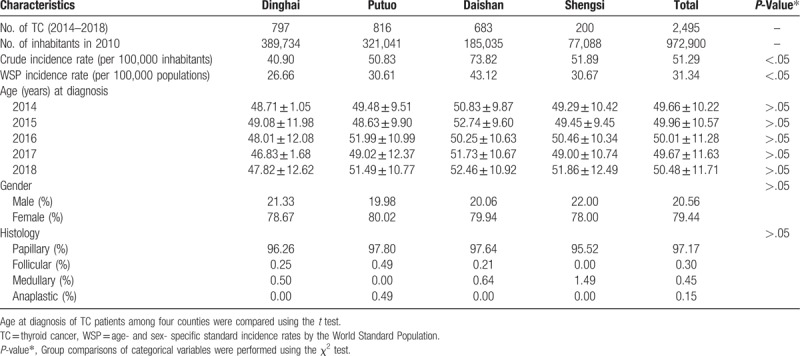
From 2014 to 2018, a total of 2495 new TC patients were diagnosed in the Zhoushan Islands. Among the patients, 1982 (79.44%) were women, and 513 (20.56%) were men. The median age was 48 years and ranged from 18 to 75 years. Ages at diagnosis did not significantly change with different counties and years from 2014 to 2018 (Kruskal–Wallis test, P > .05). Papillary thyroid cancer is the most common TC, accounting for more than 97% of cases in the Zhoushan Islands. The histology types of TC were not significantly different among the four counties (Fisher test, P > .05). The Basic characteristics of the TC patients are shown in Table 1.
Table 1.
Characteristics of TC patients in four counties of Zhoushan Islands in the 2014 to 2018 period.
3.2. TC incidence in Zhoushan Islands
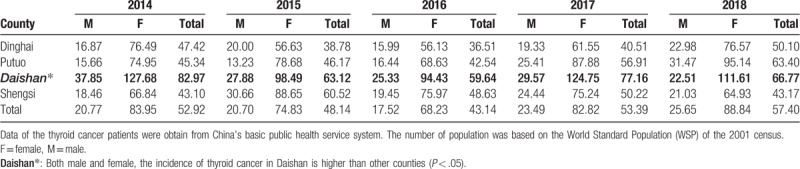
The crude TC incidence rate in Zhoushan from 2014 to 2018 was 51.29 per 100,000 inhabitants, and the age- and sex-adjusted incidence rates by WSP was 31.34 per 100,000 population. The standardized incidence rates of TC in four counties of Zhoushan were observed during the study period (Table 2). In all study years, the rates were significantly higher in women than in men (χ2 test, P < .001). Interestingly, the incidence rates of TC in Daishan County were significantly higher than the other three counties from 2014 to 2018 (χ2 test, P < .001).
Table 2.
The standardized (world population) incidence rate (SIR) of thyroid cancer (per 100,000 population) in four counties of Zhoushan City in the 2014 to 2018 period.
3.3. Iodized salt consumption rate in Zhoushan
In Zhoushan, interventions for iodized salt to prevent iodine deficiency diseases (IDDs) were introduced in 1995; however, residents’ consumption rate of iodized salt is not high in Daishan County. During 2014 to 2018, iodized salt consumption rate in Daishan County was significantly lower than the other three counties for both children and pregnant women (Fig. 2).
Figure 2.
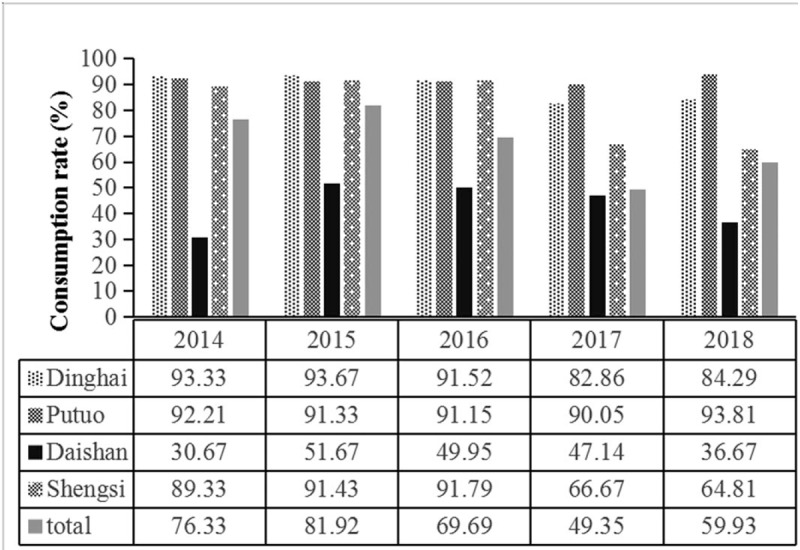
The consumption rate of iodized salt in four counties of Zhoushan Islands during 2014 to 2018.
3.4. UIC in children in Zhoushan Islands
A total of 840 urine samples from children aged 8 to 10 years in 4 counties annually within the study period, with a median UIC of 152.83, 161.80, 124.90, and 145.60 μg/L in Dinghai, Putuo, Daishan, and Shengsi, respectively. The median UIC of children in Daishan County was significantly lower than those in the other three counties (Kruskal–Wallis test, P < .05) (Fig. 3A). According to the iodine nutrition status as recommended by WHO, only 35% of subjects were in the adequate range (UIC 100–199 μg/L). More than half of the children (57%) belonged to the category of insufficient iodine intake (UIC < 100 μg/L). The rates for children with excessive iodine intake (UIC ≥ 300 μg/L) and more than adequate iodine intake (UIC 200–299 μg/L) were 8% and 5%, respectively (Fig. 3B).
Figure 3.
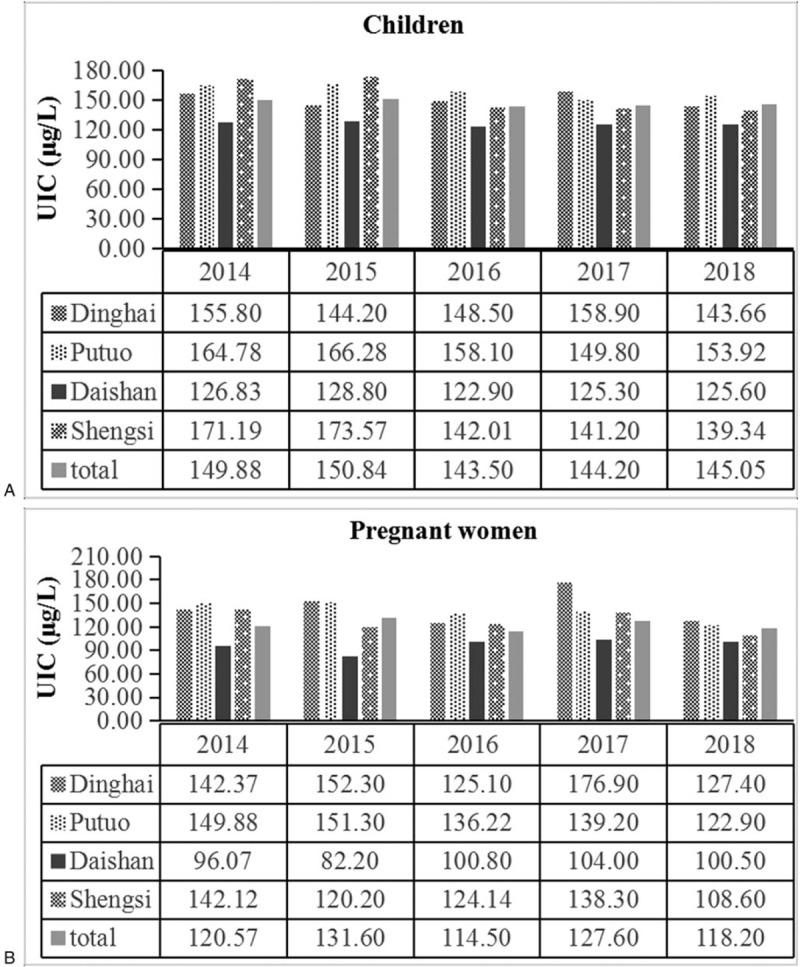
The median UIC of children (A) and pregnant women (B) in four counties of Zhoushan City during 2014 to 2018.
3.5. UIC in pregnant women in Zhoushan
A total of 420 urine samples from pregnant women were also monitored in 4 counties annually, with a median UIC of 146.07, 142.20, 96.80, and 124.00 μg/L, respectively. Similarly, the median UIC of pregnant women in Daishan County was significantly lower than those in the other three counties (Kruskal–Wallis test, P < .05) (Fig. 4A). Based on the iodine nutrition status recommended by WHO for pregnant women, only 21% of subjects were in the adequate range (UIC 150–249 μg/L). More than half of them (74%) belonged to the category of insufficient iodine intake (UIC < 150 μg/L). The rates for patients with excessive iodine intake (UIC ≥ 500 μg/L) and more than adequate iodine intake (UIC 250–499 μg/L) were 0% and 5%, respectively (Fig. 4B).
Figure 4.
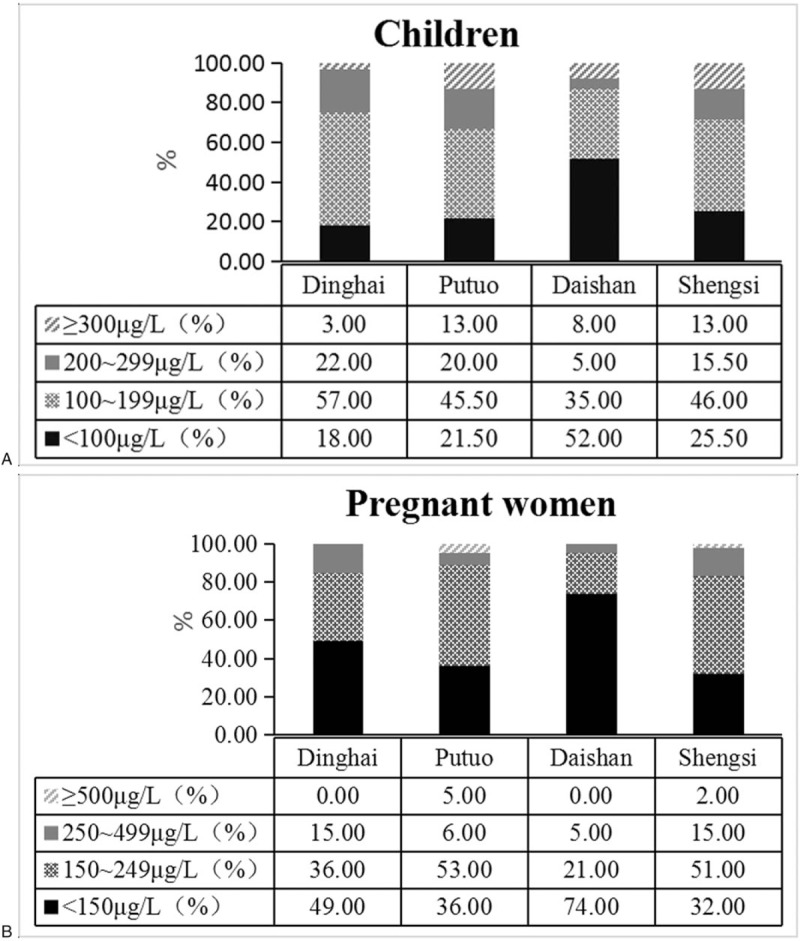
Distribution of children (A) and pregnant women (B) according to iodine nutrition status based on WHO recommendations in four counties of Zhoushan City during 2014 to 2018.
4. Discussion
Ecological study of epidemiology analyzes the relationship between exposure factors and diseases by describing the exposure situation of certain factors and the frequency of disease occurrence or death in different populations. The most significant advantage of the ecological study is that it can provide etiological clues for further study of diseases with unknown etiology.[22]
In the present study, we explore the relationship between iodine intake and TC risk by mean of an ecological comparison. The results showed that Daishan County has the highest TC incidence compared with the other three counties during the period of 2014 to 2018. We then compared the iodine intake among different TC incidence regions indicating that the population of Daishan County has the lowest iodized salt consumption rate and the median UIC compared with other three counties. We speculate that the high incidence of TC in Daishan County is likely to be the consequence of the relatively low consumption of iodized salt in this area where has abundant resources of salt.
The main reason for the low consumption of iodized salt in Daishan is that the county has rich resources of salt with nearly 30,000 mu salt pans, the residents could produce salt privately or obtain it through friends. On the other hand, due to their life-long dietary intake of iodine-rich foods such as seaweeds, most residents believe that Zhoushan is an iodine-replete area, and thus do not pay attention to the intake of iodized salt.
Various epidemiological studies have compared the incidence and risk of TC in iodine-deficient and iodine sufficient areas. A study on Sicily compared two areas with different iodine intake, and a statistically higher TC frequency was observed in areas with insufficient iodine intake than in the control area.[23] The same authors also showed the TC frequency in patients with a dysfunctional node in areas with insufficient iodine intake was twice as high as that in iodine sufficient areas.[24]
Other epidemiological studies have also attempted to link iodine intake with TC risk. Kim et al studied on an iodine-replete area in Korea suggested that patients with TC were more likely to be distributed in UIC < 300 μg/L and UIC ≥ 2500 μg/L comparing those with benign thyroid nodules.[25] A countrywide case–control study by Truong et al indicates that the possible interaction between consumption of cruciferous vegetables and low intake of dietary iodine may contribute to explain the exceptionally high incidence of TC among Melanesian women.[26] A meta-analysis demonstrated that a higher iodine intake (≥300 mg/day) and high consumption of saltwater fish and shellfish were protective factors for TC.[27] Also, animal experiments on the rat thyroid models have demonstrated that both iodine deficiency and iodine excess significantly increase thyroid tumorigenesis.[22,28] Iodine deficiency has been reported to cause the aberrant proliferation of thyrocytes and increase their sensitivity to ionizing radiation.[29]
As ecological research, this study takes the “collection” of individuals in different situations as the observation and analysis unit, without considering the existing confounding factors and other reasons, which may cause the results are inconsistent with the real situation.[22] The standardized incidence rates of TC were compared in four counties in present study. However, when compared the iodine intake among four counties, the median UIC was only investigated in children and pregnant women, who may not represent the UIC situation of the whole population in each county. From 2009 to 2010, a total of 3578 urban and rural residents covering lactation women, adults, children, and pregnant women were surveyed in Dinghai, Putuo, and Daishan counties. The results showed that the consumption of iodized salt and UIC of Daishan County was lower than other counties in Zhoushan (data not shown).
In conclusion, we observed that relatively low iodine intakes might contribute TC risk in Zhoushan Islands. It provides support for the explanation of the exceptionally high TC incidence in Daishan County. Further subtle designed case–control and cohort studies are needed to clarify the relationships between iodine intake and TC risk. Besides, new studies on the potential underlying mechanisms are still needed to provide additional insights into the etiology of TC and help identify the safe limit of iodine intake for prevention.
Acknowledgments
We thanks to Dr. Hang-Xiao (Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China) for proofreading the manuscript.
Author contributions
Conceptualization: Yong-Li Zhang, Peng Li.
Data curation: Peng Li.
Formal analysis: Peng Li, Qi Lin.
Investigation: Jing-Ping Yi, Zhi-Ya Liu, Min Zhang, Yan Chen.
Methodology: Peng Li.
Project administration: Yong-Li Zhang.
Resources: Yong-Li Zhang.
Supervision: Yong-Li Zhang.
Writing-original draft: Peng Li.
Writing-review & editing: Yong-Li Zhang, Peng Li.
Data curation: Peng Li.
Formal analysis: Peng Li, Qi Lin.
Investigation: Zhi-Ya Liu, Jing-Ping Yi, Yan Chen, Min Zhang, Qi Lin.
Validation: Yong-Li Zhang.
Writing – original draft: Peng Li.
Writing – review & editing: Peng Li, Yong-Li Zhang.
Footnotes
Abbreviations: IDD = iodine deficiency diseases, PTC = papillary thyroid cancer, TC = thyroid cancer, UIC = urinary iodine concentration, WSP = World Standard Population.
How to cite this article: Zhang YL, Li P, Liu ZY, Yi JP, Chen Y, Zhang M, Lin Q. Does relatively low iodine intake contribute to thyroid cancer? An ecological comparison of epidemiology. Medicine. 2019;98:41(e17539).
Y-LZ and PL contributed equally to this work and should be considered as co-first authors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Burman KD. Is poorly differentiated thyroid cancer poorly characterized? J Clin Endocr Metab 2014;99:1167–9. [DOI] [PubMed] [Google Scholar]
- [2].Truong T, Rougier Y, Dubourdieu D, et al. Time trends and geographic variations for thyroid cancer in New Caledonia, a very high incidence area (1985-1999). Eur J Cancer Prev 2007;16:62–70. [DOI] [PubMed] [Google Scholar]
- [3].Ballivet S, Salmi LR, Dubourdieu D, et al. Incidence of thyroid cancer in New Caledonia, South Pacific, during 1985-1992. Am J Epidemiol 1995;141:741–6. [DOI] [PubMed] [Google Scholar]
- [4].Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- [5].La Vecchia C, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer 2015;136:2187–95. [DOI] [PubMed] [Google Scholar]
- [6].Morris LGT, Sikora AG, Tosteson TD, et al. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid 2013;23:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid 2014;24:472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic”- screening and overdiagnosis. N Engl J Med 2014;371:1765–7. [DOI] [PubMed] [Google Scholar]
- [9].Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 1995;141:259–77. [PubMed] [Google Scholar]
- [10].Adjadj E, Schlumberger M, de Vathaire F. Germ-line DNA polymorphisms and susceptibility to differentiated thyroid cancer. Lancet Oncol 2009;10:181–90. [DOI] [PubMed] [Google Scholar]
- [11].Dal Maso L, Bosetti C, La Vecchia C, et al. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control 2009;20:75–86. [DOI] [PubMed] [Google Scholar]
- [12].Zhang Y, Guo GL, Han X, et al. Do polybrominated diphenyl ethers (PBDEs) increase the risk of thyroid cancer? Biosci Hypotheses 2008;1:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- [14].Franceschi S, Preston-Martin S, Dal Maso L, et al. A pooled analysis of case-control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes Control 1999;10:583–95. [DOI] [PubMed] [Google Scholar]
- [15].WHO/UNICEF/ICCIDD. Assessment of iodine deficiency disorders and monitoring their elimination, a guide for program managers. 3rd ednGeneva: World Health Organization; 2007. [Google Scholar]
- [16].Laurberg P, Bulow Pedersen I, Knudsen N, et al. Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid 2001;11:457–69. [DOI] [PubMed] [Google Scholar]
- [17].Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res 2015;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Horn-Ross PL, Morris JS, Lee M, et al. Iodine and thyroid cancer risk among women in a multiethnic population: the Bay Area Thyroid Cancer Study. Cancer Epidemiol Biomarkers Prev 2001;10:979–85. [PubMed] [Google Scholar]
- [19].Cléro É, Doyon F, Chungue V, et al. Dietary iodine and thyroid cancer risk in French Polynesia: a case-control study. Thyroid 2012;22:422–9. [DOI] [PubMed] [Google Scholar]
- [20].Sun HQ, Xiao GX, Guo Y, et al. Statistical analysis of epidemiological ecology. Chin J Health Statist 2014;31:352–6. [Google Scholar]
- [21].Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future [J]. Nutr Rev 2012;70:553–70. [DOI] [PubMed] [Google Scholar]
- [22].Kanno J, Onodera H, Furuta K, et al. Tumor-promoting effects of both iodine deficiency and iodine excess in the rat thyroid. Toxicol Pathol 1992;20:226–35. [DOI] [PubMed] [Google Scholar]
- [23].Croatian National Cancer Registry. Croatian Health Service Yearbook 2001. 2002;Zagreb: Croatian National Institute of Public Health, 314. [Google Scholar]
- [24].Belfiore A, La ROSA GL, La PORTA GA, et al. Cancer risk in patients with cold nodules: relevance of iodine intake, sex, age and multinodularity. Am J Med 1992;93:363–9. [DOI] [PubMed] [Google Scholar]
- [25].Kim HJ, Kim NK, Park HK, et al. Strong association of relatively low and extremely excessive iodine intakes with thyroid cancer in an iodine-replete area. Eur J Nutr 2017;56:965–71. [DOI] [PubMed] [Google Scholar]
- [26].Truong T, Baron-Dubourdieu D, Rougier Y, et al. Role of dietary iodine and cruciferous vegetables in thyroid cancer: a countrywide case-control study in New Caledonia. Cancer Causes Control 2010;21:1183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cao LZ, Peng XD, Xie JP, et al. The relationship between iodine intake and the risk of thyroid cancer: a meta-analysis. Medicine (Baltimore) 2017;96:e6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boltze C, Brabant G, Dralle H, et al. Radiation-induced thyroid carcinogenesis as a function of time and dietary iodine supply: an in vivo model of tumorigenesis in the rat. Endocrinology 2002;143:2584–92. [DOI] [PubMed] [Google Scholar]
- [29].Liu XH, Chen GG, Vlantis AC, et al. Iodine mediated mechanisms and thyroid carcinoma. Crit Rev Clin Lab Sci 2009;46:302–18. [DOI] [PubMed] [Google Scholar]


