Abstract
Antral follicle count (AFC) has been widely investigated for the prediction of clinical pregnancy or live birth. This study discussed the effects of AFC quartile levels on pregnancy outcomes combined with female age, female cause of infertility, and ovarian response undergoing in vitro fertilization (IVF) treatment. At present, many research about AFC mainly discuss its impact on clinical practice at different thresholds, or the analyses of AFC with respect to assisted reproductive technology outcomes under using different ovarian stimulation protocols. Factors that include ovarian sensitivity index, female age, and infertility cause are all independent predictors of live birth undergoing IVF/intracytoplasmic sperm injection, while few researchers discussed influence of female-related factors for clinical outcomes in different AFC fields.
A total of 8269 infertile women who were stimulated with a long protocol with normal menstrual cycles were enrolled in the study, and patients were categorized into 4 groups based on AFC quartiles (1–8, 9–12, 13–17, and ≥18 antral follicles).
The clinical pregnancy rates increased in the 4 AFC groups (28.25% vs 35.38% vs 37.38% vs 40.13%), and there was a negative association between age and the 4 AFC groups. In addition, female cause of infertility like polycystic ovary syndrome, Tubal factor, and other causes had great significance on clinical outcome, and ovarian response in medium (9–16 oocytes retrieved) had the highest clinical pregnancy rate at AFC quartiles of 1 to 8, 9 to 12, 13 to 17, and ≥18 antral follicles.
This study concludes that the female-related parameters (female cause of infertility, female age, and ovarian response) combined with AFC can be useful to estimate the probability of clinical pregnancy.
Keywords: antral follicle count, clinical pregnancy rate, female age, female cause of infertility, in vitro fertilization, ovarian response
1. Introduction
In 21st century, many people are suffering from infertility on account of genetic and environmental factors.[1,2] With the development of in vitro fertilization (IVF) technology, more and more people who cannot conceive natural pregnancy choose IVF for solving their problem. Many clinical factors are used to evaluate the assisted reproductive technology (ART) outcomes or predict the effects of pregnancy outcomes. Body mass index (BMI),[3,4] age,[5] cause of infertility,[6] endocrine indicators (follicle-stimulating hormone [FSH], estradiol [E2], inhibin-B, antimüllerian hormone [AMH]),[7–12] antral follicle count (AFC),[13,14] ovarian parameters (ovarian volume, ovarian vascularity indices, oocytes retrieved),[15–18] treatment medication (letrozole, clomiphene),[19,20] and protocol (gonadotropin-releasing hormone [GnRH] agonist, GnRH antagonist)[21–23] have been proposed for the research of the success rate of IVF.
The AMH and AFC have been widely investigated for the prediction of clinical pregnancy or live birth.[24–27] Although AMH is a stronger predictor of ovarian response to gonadotropin therapy than AFC,[28] and it was more strongly correlated with oocyte yield than AFC in the GnRH-antagonist cohort.[14] AMH remains at a relatively constant level in the menstrual cycle and it is difficult to present the effect of minimal changes on pregnancy outcomes. Meanwhile, recent changes in the assay mean different cutoff values have been proposed.[29,30] In contrast, the ovarian AFC has relatively low intercycle variability and low to moderate interobserver variability. It is proved to be the best predictor of clinical practice.[31]
At present, many research about AFC mainly discuss its impact on clinical practice at different thresholds,[13,32,33] or the analyses of AFC with respect to ART outcomes under using different ovarian stimulation protocols.[31] Factors that include ovarian sensitivity index, female age, and infertility cause are all independent predictors of live birth undergoing IVF/intracytoplasmic sperm injection (ICSI),[6] while few researchers discussed influence of female-related factors for clinical outcomes in different AFC fields. Our study firstly analyzed the potential predictors that effected clinical outcomes, then discussed the effects of AFC quartile levels on pregnancy outcomes with female age, female cause of infertility, and ovarian response undergoing IVF treatment and created the logistic regression model to predict the possibility of clinical pregnancy.
2. Methods
2.1. Study population
A total of 8269 infertile women who were stimulated with a long protocol with normal menstrual cycles at the Reproductive Medicine Center of Tongji Hospital, China from January 2014 to August 2017 were enrolled in the study. Patients who undergoing their IVF/ICSI treatment used their own oocytes and the embryos transferred were fresh-embryo or frozen-embryo. The infertile women in the research only suffered from single cause, and women with combined cause of infertility were excluded. All data acquisition, data management, and data analyses were performed by the data Analysis Center of Tongji Hospital. The study was approved by the ethics committees at the Reproductive Medicine Center of Tongji Hospital, and informed consent was signed by the patients before participation.
All data were prospectively collected for the purpose of investigating the clinical pregnancy outcome undergoing IVF/ICSI treatment. Patient characteristics like age, duration of infertility, type of infertility, and female cause of infertility were self-reported and most of them were supported by medical inquiry. Meanwhile, female height and weight were achieved by medical measuring for calculating BMI. Other indicators, including E2 and progesterone at day 1 of stimulation, AFC, doses of FSH, insemination method, and the number of oocytes retrieved were also obtained by professional medical technician.
2.2. Laboratory procedure for ovarian stimulation protocol
The protocol for ovarian stimulation was the standard long GnRH-agonist protocol.[33,34] This involved downregulation with a GnRH agonist beginning in the mid-luteal phase of the menstrual cycle 7 days before the earliest expected date of menstruation. Successful ovarian suppression was confirmed 2 weeks later through ultrasound evidence of a thin endometrium, measuring <5 mm at the junction of the upper 3rd and lower two-thirds in the longitudinal plane, absent ovarian activity, and a serum E2 level <200 pmol/L.[33] Gonadotrophin stimulation was initiated with either recombinant FSH or urine highly purified FSH with or without human menopausal gonadotropin. The initial dose ranged from 150 to 300 IU/d. The starting dose of gonadotropin that is used for ovarian stimulation was determined on the basis of age, BMI, and AFC as well as basal FSH or AMH levels.[34] This dose was prescribed for 5 days and the patient then scanned to assess the initial response. The subsequent daily dose of FSH was only adjusted at this stage if it was thought there was a risk of poor or exaggerated response.[33]
Subsequent ovarian response was monitored through serum E2, progesterone (P), LH assessments, and serial transvaginal ultrasound examinations. Gonadotrophin doses were adjusted when needed. The objective follicles were defined as follicles between 14 and 26 mm, with 200 to 300 pg/mL estradiol production.[34] There were at least 3 follicles measuring R18 mm in diameter, 6000 to 10,000 units of human chorionic gonadotropin was administered. Oocytes were retrieved 36 hours later. The total number of oocytes retrieved was recorded, including the number of mature and immature oocytes. Subjects who did not develop at least 3 leading follicles measuring R14 mm in diameter after 12 days of gonadotropin treatment were advised to discontinue treatment or convert to IUI, depending on other clinical factors including their tubal status and their partner's semen quality, and these cycles were counted as cancelled cycles.[33] The type of insemination includes IVF, ICSI, 50% IVF + 50% ICSI, and early rescue ICSI. Except for early rescue ICSI, evidence for fertilization was assessed ∼18 hours after insemination. Fertilization was initially checked 6 hours, after IVF insemination, and if the oocytes failed to fertilize, ICSI was done immediately for early rescue ICSI.[34] One or 2 normally cleaved embryos, as set out by the Human Fertility Embryology Authority policy, were transferred into the uterus under ultrasound guidance 3 or 5 days after oocyte retrieval, and urine pregnancy test was performed 14 or 12 days later to determine the outcome. If the test was positive (biochemical pregnancy), a transvaginal ultrasound was arranged 3 weeks later to confirm a clinical pregnancy, defined as presence of an intrauterine gestation with evidence of cardiac activity. All pregnancies were followed up to delivery and the outcome recorded.[33]
2.3. Outcome measures
The primary outcome was clinical pregnancy, which was defined as pregnancy phenomenon that clinically considered. The number of oocytes retrieved was also recorded to measure the status of ovarian response.
2.4. Statistical analysis
Patients were categorized into 4 groups according to AFC quartiles: 1 to 8, 9 to 12, 13 to 17, and ≥18. The characteristics of clinical pregnancy and no clinical pregnancy patients were compared. Descriptive statistics of the continuous variables are presented as means and the standard deviations, and the categorical variables are presented as number and percentage. Student t test was performed for evaluating the statistical relations between the subgroups, and the Chi-squared test was used to evaluate the significance of the proportion of the categorical variables. When the patients were divided into 4 groups according to AFC quartiles, 1-way analysis of variance and Chi-squared test were used to ascertain the significant differences among these groups. Multivariate logistic regression model was used to analyze the effect of the potential predictors on clinical pregnancy because it is widely used in most studies and proved to be effective in predicting IVF outcome of clinical practice research.[35,36] The 2-tailed value of P < .05 was considered statistically significant. All the statistical analysis was carried out using R language.
3. Results
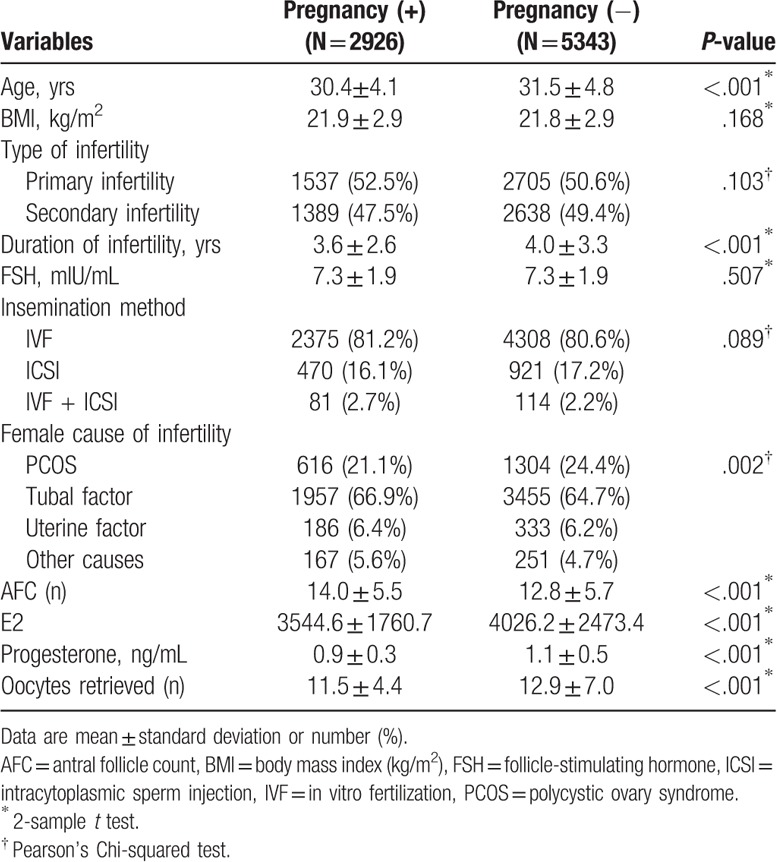
A total of 8269 patients with long protocol IVF/ICSI cycles were recruited and 2926 of these women (35.39%) succeeded in clinical pregnancy. Descriptive statistics of potential predictors in pregnant and nonpregnant women are presented in Table 1. The average age of the women who succeeded in pregnancy is younger than those with no pregnancy (30.4 vs 31.5), while the average AFC of the women who succeed in pregnancy is higher than those with no pregnancy (14.0 vs 12.8). There were no significant differences in BMI and FSH between women with pregnancy and women without pregnancy.
Table 1.
Descriptive statistics of potential predictors in pregnant and nonpregnant women (N = 8269).
Multivariate logistic regression analysis with potential predictors such as female age, BMI, type of infertility, FSH, female cause of infertility, AFC, and so on in the model showed that only female cause of infertility (Tubal factor: odds ratio [OR] 1.2; 95% confidence interval [CI] 1.07–1.34), AFC (OR 1.04; 95% CI 1.03–1.04), age (OR 0.95; 95% CI 0.94–0.96), duration of infertility (OR 0.96; 95% CI 0.95–0.98), E2 (OR 0.99; 95% CI 0.99–0.99), progesterone (OR 0.25; 95% CI 0.22–0.28), and oocytes retrieved (OR 0.96; 95% CI 0.96–0.97) is significantly predictive of clinical pregnancy.
The distribution of clinical pregnancy rate at each AFC is presented in Figure 1. Many studies have grouped AFC, and there was no uniform categorization of AFC.[13,32,33] Patients were categorized into 4 groups based on AFC quartiles,[33] predictors that have a statistically significant impact on clinical pregnancy, and clinical outcomes are compared in Table 2.
Figure 1.

The cumulative probability of clinical pregnancy following in vitro fertilization treatment at each antral follicle count (AFC) thresholds.
Table 2.
Predictors and clinical outcome according to antral follicle count (AFC) quartiles.

There was a negative association between age and AFC (33.4 vs 31.5 vs 30.4 vs 29.2). However, the number of oocytes retrieved increased with AFC (8.1 vs 11.4 vs 14.0 vs 15.9), as did the clinical pregnancy rates. At AFC quartiles of 1 to 8, 9 to 12, 13 to 17, and ≥18 antral follicles, the mean clinical pregnancy rates were 28.25%, 35.38%, 37.38%, and 40.13%, respectively. There was significant difference in progesterone among the patient groups.
Table 3 summarizes the statistics from the logistic regression analysis of AFC quartiles in clinical pregnancy. The category AFC 1 to 8 was used as a reference. After adjustment for age, duration of infertility, female cause of infertility, progesterone, E2, and oocytes retrieved, the adjusted ORs for clinical pregnancy increased from 1.39 (1.22–1.59) in the 9 to 12 category to 1.52 (1.33–1.73) in the 13 to 17 category, and reached 1.70 (1.49–1.94) in the ≥18 category. It indicates that higher AFC increased clinical pregnancy rate, which was consistent with the previous study.[13,33] Logistic regression analysis showed that female cause of infertility, age, and oocytes retrieved have significant impact on pregnancy. Further study has been conducted to investigate the relationship between AFC levels and these parameters.
Table 3.
Logistic regression analysis of AFC quartiles in clinical pregnancy.

Based on the potential indicators involved in the logistic regression model, we concluded the model with some statistically significant relevant predictors, which were AFC, duration of infertility, oocytes retrieved, female cause of infertility, age, E2, and progesterone.
The ROC curve is used to evaluate the performance of the prediction model, and the AUC of each model is compared. The AUC is the area under the ROC curve, which reflects the prediction performance of the model, and the greater the value, the better the performance of the model. The results are presented in Table 4.
Table 4.
The AUC of the prediction model with the potential indicators.

From the value of the AUC we can see, model c is higher than the other, which means the model has the best performance, so the predictors of the final model including AFC, E2, P, DOI, age, oocytes, and cause, and the logistic regression model can be used to predict the possibility of clinical pregnancy, here we randomly selected the data of 20 patients, the predicted results are presented in Table 5.
Table 5.
Predictive of clinical pregnancy with the data of 20 patients.

We predict the possibility of clinical pregnancy at different AFC thresholds combined with female cause of infertility, female age, and ovarian response in Figures 2–4.
Figure 2.

Prediction of clinical pregnancy rate at different antral follicle count (AFC) thresholds combined with female cause of infertility, and female cause of infertility was polycystic ovary syndrome (PCOS), tubal factor, uterine factor, and other causes. The antral follicle count groups were 1 to 8, 9 to 12, 13 to 17, and ≥18 antral follicles, the value of duration of infertility, oocytes retrieved, age, estradiol (E2), and progesterone took the mean.
Figure 4.

Prediction of clinical pregnancy at different antral follicle count (AFC) thresholds combined with ovarian response. Three ovarian response groups were low (1–8 oocytes retrieved), medium (9–16 oocytes retrieved), and high (≥17 oocytes retrieved), the antral follicle count groups were 1 to 8, 9 to 12, 13 to 17, and ≥18 antral follicles, the value of duration of infertility, age, estradiol (E2), and progesterone took the mean.
Cause of infertility has proved to be predictive of clinical pregnancy,[6] female cause of infertility considered in the research were mainly polycystic ovary syndrome (PCOS),[37] tubal factor, uterine factor, and other causes, the predictors AFC, duration of infertility, oocytes retrieved, female cause of infertility, age, E2, and progesterone were involved in the logistic regression model, and the result are illustrated in Figure 2. From the result we can conclude that the possibility of clinical pregnancy in tubal factor was 1.2 times higher than the odds in PCOS (OR 1.20; 95% CI 1.07–1.34) (Table 3), and the clinical pregnancy rate of PCOS was the lowest in the female cause of infertility among the AFC groups (Fig. 2), which indicated that PCOS had a great impact on infertility and its clinical research value attracted a lot of scholars.[13,20,32,35,36]
Regarding female age, AFC, duration of infertility, oocytes retrieved, age, E2, and progesterone were incorporated into the prediction model, female cause of infertility was excluded in the model to eliminate its effect on outcome, and the result are illustrated in Figure 3. Five years as a classification level for ease of interpretation, and it was here subgrouped into 6 age groups; 20 to 25, 26 to 30, 31 to 35, 36 to 40, 41 to 45, and >45 years.
Figure 3.

Prediction of clinical pregnancy at different antral follicle count (AFC) thresholds combined with female age, female age was divided into 6 groups: 20 to 25, 26 to 30, 31 to 35, 36 to 40, 41 to 45, and >45 years. The antral follicle count groups were 1 to 8, 9 to 12, 13 to 17, and ≥18 antral follicles, the value of duration of infertility, oocytes retrieved, estradiol (E2), and progesterone took the mean.
Women aged in 20 to 25 years had the highest clinical rate among the AFC groups, the odds of clinical pregnancy in 36 to 40 years was 0.6 times lower than the odds in 20 to 25 years (OR 0.58; 95% CI 0.48–0.70), and women aged in 41 to 45 years was 0.2 times lower than those in 20 to 25 years (OR 0.19; 95% CI 0.12–0.28), the clinical pregnancy rate of women in 41 to 45 years in groups 3 and 4 (AFC 13–17 and AFC ≥18) were 10.3% and 11%, while the clinical pregnancy rate of women <35 years was higher than 40%, furthermore, women higher than 45 years almost cannot be pregnant in all AFC groups.
Ovarian response measured in the study by the number of oocytes retrieved. The prediction model was built with AFC, duration of infertility, oocytes retrieved, age, E2, and progesterone, female cause of infertility was excluded in the model to eliminate its effect on outcome, and the results are illustrated in Figure 4. According to retrieved oocytes number, 3 groups were formed to identify low (1–8 oocytes retrieved), medium (9–16 oocytes retrieved), and high (≥17 oocytes retrieved).
The odds of clinical pregnancy in high group was 0.63 times lower than the odds in low group (OR 0.63; 95% CI 0.55–0.73), and the clinical pregnancy rate in low and high ovarian response (1–8 oocytes retrieved and ≥17 oocytes retrieved) in 13 to 17 AFC group were 30.6% and 25.7%. Previous study showed that the adjusted ORs for fresh embryo live birth per started cycle increased from 1.823 (1.395–2.381) in the 6–10 oocyte category to 2.142 (1.609–2.851) in the 11 to 15 oocyte category. However, it decreased to 1.918 (1.376–2.672) in the ≥16 oocyte category.[34]
4. Discussion
This study discussed strong associations between AFC and clinical outcome undergoing IVF treatment in China, and developed a prediction model to estimate the probability of clinical pregnancy under female-related parameters, measured by female cause of infertility, female age, and ovarian response. Previous studies on AFC for clinical outcome undergoing IVF treatment indicated that AFC was a effective predictor of live birth and a higher live-birth rate occurred as AFC increased within a certain range.[13,33] Our conclusion was consistent with these conclusions.
The innovation of this study is that the data were more comprehensive without no deliberately deleted, including patients with poor physical condition (BMI range from 14.7 to 37, age range from 21 to 48), and cause of infertility is combined with AFC to discuss the clinical outcome. Previous studies aimed to estimate the probability of live birth or to predict ovarian response at different AFC cutoff levels without considering female-related parameters, especially different female causes of infertility. Cause of infertility was qualified as an independent predictor in many studies. PCOS, Tubal factor, Uterine factor, and other causes were considered in the present research. The PCOS is commonly characterized by menstrual abnormalities and clinical or biochemical features of hyperandrogenism.[38] PCOS had a great impact on infertility and it was found to be the lowest clinical pregnancy rate among female cause of infertility in the present study. The previous papers evaluated the proportion of PCOS-related infertility among all patients and found that PCOS was the main cause of infertility in women aged 18 to 35 years,[32] and women with PCOS maintain a stable oocyte count and live-birth rate across the age range of 22 to 41 years during IVF, compared with women with tubal factor infertility, who experience a significant decline with increasing age.[39] However, it must be emphasized that the conclusion we concluded only can be extrapolated to the ultrasound finding “polycystic ovaries.” Male factors that were nonsignificant as predictors were excluded in the research. The probable reason is that assisted reproductive technology has an effective treatment on male infertility.
Female age is a strong predictor of clinical pregnancy after IVF/ICSI, and it was negatively correlated with live birth as a predictor included in the prediction model for pregnancy or live birth in previous papers.[6,40,41] The women with pregnant in our study were significantly younger than those without pregnant, and women more than 45 years almost cannot be pregnant. We guess this possible reason is that the body part of the ovarian function are in the aging and degradation, it is easy to lead to abnormal chromosomes of the egg, the older, the more impact on the egg caused by the environment pollution, these are the factors that lead to infertility.[5] However, it worth to be mentioned that the effect of age on AFC and clinical pregnancy need to be long-term follow-up, the patient who got infertility when they were young, while they succeeded in pregnancy by assisted reproduction technology and did not seek further treatments, these patients may have more severe infertility as they became older. So the older women definitely may have lower probability in clinical pregnancy or live birth.
Ovarian response category was built for streamlining research into meaningful conclusions, and helpful for explanation in clinical practice.[42] While there was no uniform categorization of ovarian response, ovarian response was often measured by the number of oocytes retrieved in most papers,[27,34,43] 3 groups low, medium and high ovarian response were divided in our study on the basis of the purpose of the research objective. AFC was sensitive for the prediction of ovarian response, especially in poor ovarian response or moderate and severe OHSS.[33] Conclusions were different among studies about the relationship between AFC and ovarian response. It can be a effective predictor only in those three or fewer oocytes retrieved, and it had no significance in predicting live-birth rate,[31] compared to nontreatment, lower AFC with large (5–10 mm) antral follicles were the only ovarian parameter associated with oral contraception pretreatment.[44] Our result inferred that low ovarian response (9–16 oocytes retrieved) had higher clinical pregnancy rate than high ovarian response (1–8 oocytes retrieved). It is normal that the conclusions were different for experimental conditions and experimental parameters. Previous papers demonstrated that fresh embryo live-birth rate in 0 to 5 oocytes retrieved was higher than in >16 oocytes retrieved, which was consistent with our result.
There are some limitations in our study. First, ovarian stimulation protocol we used was long GnRH agonist, it may minimize the interference of individual gonadotropin doses. While it narrowed the scope of the conclusions, and it cannot be extrapolated to other protocols like short GnRH agonist and GnRH antagonist, the clinical pregnancy rate increased with AFC in a given stimulation protocol. However, different stimulation protocols changed the basal factors levels, and it may affect the impact of AFC on clinical outcome. Second, ovarian reserve markers included in the paper are FSH and AFC, a lack of other basal markers like LH and AMH levels, these indicators excluded for the reason that we were neglected to record or record missing before data collection. Furthermore, the clinical outcome in our paper is not comprehensive, we just recorded the number of oocytes retrieved and clinical pregnancy, pregnancy loss, live birth, and cumulative live-birth rate are what we need to consider in further study.
In conclusion, the results of this study show a strong association between AFC and clinical pregnancy after IVF/ICSI treatment, and AFC thresholds are proved to be effectively in prediction of clinical outcome. The female-related parameters such as female cause of infertility, female age, and ovarian response are combined with AFC levels to estimate the probability of clinical pregnancy, and the results are shown to give guidance for couples who get infertility and seek for assisted reproductive treatment. Moreover, it can provide valuable reference for the choice of the ovarian stimulation protocols.
Author contributions
Data curation: Cheng Hu, Yudi Geng, Lei Jin.
Formal analysis: Jianwu Xiong.
Funding acquisition: ShuJie Liao.
Methodology: Jianwu Xiong, Haiting Tu.
Resources: Haiting Tu, Wulin Pan, wei pan, Tingjuan Lu.
Software: Wulin Pan.
Writing – original draft: ShuJie Liao.
Writing – review & editing: wei pan, Lei Jin.
Footnotes
Abbreviations: AFC = antral follicle count, AMH = antimüllerian hormone, ART = assisted reproductive technology, AUC = area under the curve, BMI = body mass index, DOI = duration of infertility, E2 = estradiol, FSH = follicle-stimulating hormone, GnRH = gonadotropin-releasing hormone, IVF = in vitro fertilization, LH = luteinizing hormone, P = progesterone, PCOS = polycystic ovary syndrome, ROC = receiver-operating characteristic.
How to cite this article: Liao S, Xiong J, Tu H, Hu C, Pan W, Geng Y, Pan W, Lu T, Jin L. Prediction of in vitro fertilization outcome at different antral follicle count thresholds combined with female age, female cause of infertility, and ovarian response in a prospective cohort of 8269 women. Medicine. 2019;98:41(e17470).
This study is supported by the National Natural Science Foundation of China (NSFC) (grant no. 81672085, 71871169, U1933120, 81372804, 71373188, U1333115, 30901586). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Daumler D, Chan P, Lo KC, et al. Men's knowledge of their own fertility: a population-based survey examining the awareness of factors that are associated with, male infertility. Hum Reprod 2016;31:2781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lalitha C, Sayee R, Apoorva D. Environmental/occupational factors and seasonality of birth-male infertility. Laryngoscope 2014;35:367–1367. [Google Scholar]
- [3].Cardozo ER, Karmon AE, Gold J, et al. Reproductive outcomes in oocyte donation cycles are associated with donor BMI. Hum Reprod 2016;31:385–92. [DOI] [PubMed] [Google Scholar]
- [4].Parker K, Wong B, Link B, et al. Does body mass index (BMI) affect IVF outcomes? Fertil Steril 2011;96:S124–124. [Google Scholar]
- [5].Dougall KM, Beyene Y, Nachtigall RD. Age shock: misperceptions of the impact of age on fertility before and after IVF in women who conceived after age 40. Hum Reprod 2013;28:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vaegter KK, Lakic TG, Olovsson M, et al. Which factors are most predictive for live birth after in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments? Analysis of 100 prospectively recorded variables in 8,400 IVF/ICSI single-embryo transfers. Fertil Steril 2017;107:641–8.e2. [DOI] [PubMed] [Google Scholar]
- [7].Tan RR, Pu DH, Liu LP, et al. Comparisons of inhibin B versus antimüllerian hormone in poor ovarian responders undergoing in vitro fertilization. Fertil Steril 2011;96:905–11. [DOI] [PubMed] [Google Scholar]
- [8].Giorgetti C, Vanden MF, De RC, et al. Multivariate analysis identifies the estradiol level at ovulation triggering as an independent predictor of the first trimester pregnancy-associated plasma protein-A level in IVF/ICSI pregnancies. Hum Reprod 2013;28:2636–42. [DOI] [PubMed] [Google Scholar]
- [9].Moolenaar LM, Mohiuddin S, Munro DM, et al. High live birth rate in the subsequent IVF cycle after first-cycle poor response among women with mean age 35 and normal FSH. Reprod Biomed Online 2013;27:362–6. [DOI] [PubMed] [Google Scholar]
- [10].Mohiyiddeen L, Newman WG, Mcburney H, et al. Follicle-stimulating hormone receptor gene polymorphisms are not associated with ovarian reserve markers. Fertil Steril 2012;97:677–81. [DOI] [PubMed] [Google Scholar]
- [11].Andersen AN, Witjes H, Gordon K, et al. Predictive factors of ovarian response and clinical outcome after IVF/ICSI following a rFSH/GnRH antagonist protocol with or without oral contraceptive pre-treatment. Hum Reprod 2011;26:3413–23. [DOI] [PubMed] [Google Scholar]
- [12].Silverberg K, Minter T, Silverberg K, et al. Both AMH and day 3 FSH levels predict IVF stimulation outcome regardless of patient age; day 3 estradiol levels are not predictive. Fertil Steril 2012;98:S273–1273. [Google Scholar]
- [13].Holte J, Brodin T, Berglund L, et al. Antral follicle counts are strongly associated with live-birth rates after assisted reproduction, with superior treatment outcome in women with polycystic ovaries. Fertil Steril 2011;96:594–9. [DOI] [PubMed] [Google Scholar]
- [14].Nelson SM, Klein BM, Arce JC. Comparison of antimüllerian hormone levels and antral follicle count as predictor of ovarian response to controlled ovarian stimulation in good-prognosis patients at individual fertility clinics in two multicenter trials. Fertil Steril 2015;103:923–30. [DOI] [PubMed] [Google Scholar]
- [15].Nallapeta S, Sharma V. Relationship of different progesterone preparations on ovarian volumes in women undergoing IVF/ICSI treatment. Fertil Steril 2013;100:S60–160. [Google Scholar]
- [16].Polyzos NP, Drakopoulos P, Tournaye H. Modified natural cycle IVF for poor ovarian responders: rethink before concluding. Hum Reprod 2016;31:221–2. [DOI] [PubMed] [Google Scholar]
- [17].Stoop D, Ermini B, Polyzos NP, et al. Reproductive potential of a metaphase II oocyte retrieved after ovarian stimulation: an analysis of 23 354 ICSI cycles. Hum Reprod 2012;27:2030–5. [DOI] [PubMed] [Google Scholar]
- [18].Eppsteiner EE, Sparks AET, Liu DW, et al. Change in oocyte yield in repeated in vitro fertilization cycles: effect of ovarian reserve. Fertil Steril 2014;101:399–402. [DOI] [PubMed] [Google Scholar]
- [19].Kroener L, Ambartsumyan G, Yee B. Impact of lead follicle size in clomiphene citrate-only minimal stimulation in-vitro fertilization (IVF) cycles. Fertil Steril 2014;102:e66–7. [Google Scholar]
- [20].Legro RS, Brzyski RG, Diamond MP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. New Engl J Med 2014;371:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Polyzos NP, Devos M, Humaidan P, et al. Corifollitropin alfa followed by rFSH in a GnRH antagonist protocol for poor ovarian responder patients: an observational pilot study. Fertil Steril 2013;99:422–6. [DOI] [PubMed] [Google Scholar]
- [22].König TE, Houwen LEEVD, Lambalk CB. Reply: Comment on ‘Recombinant LH supplementation to a standard GnRH antagonist protocol in women of 35 years or older undergoing IVF/ICSI: a randomized controlled multicentre study’. Hum Reprod 2014;29:637–8. [DOI] [PubMed] [Google Scholar]
- [23].Papanikolaou EG, Pados G, Grimbizis G, et al. GnRH-agonist versus GnRH-antagonist IVF cycles: is the reproductive outcome affected by the incidence of progesterone elevation on the day of HCG triggering? A randomized prospective study. Hum Reprod 2012;27:1822–8. [DOI] [PubMed] [Google Scholar]
- [24].Lan VT, Linh NK, Tuong HM, et al. Anti-Müllerian hormone versus antral follicle count for defining the starting dose of FSH. Reprod. Biomed Online 2013;27:390–9. [DOI] [PubMed] [Google Scholar]
- [25].Yates AP, Roberts SA, Nardo LG. Anti-Müllerian hormone-tailored stimulation protocols improve outcomes whilst reducing adverse effects and costs of IVF. Hum Reprod 2012;27:2353–62. [DOI] [PubMed] [Google Scholar]
- [26].Oppenheimer A, Bartmann A, Genro V, et al. Influence of antral follicle count (AFC) on IVF-ET outcome is modulated by age. Fertil Steril 2015;104:e62–162. [Google Scholar]
- [27].Broekmans FJ, Verweij PJM, Eijkemans MJC, et al. Prognostic models for high and low ovarian responses in controlled ovarian stimulation using a GnRH antagonist protocol. Hum Reprod 2014;29:1688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nelson S, Arce JC. Individual IVF clinic performance of anti-müllerian hormone and antral follicle count as biomarkers of ovarian response to controlled ovarian stimulation in multicentre trials. Fertil Steril 2014;102:e147–147. [Google Scholar]
- [29].Nelson SM, La Marca A. The journey from the old to the new AMHassay: how to avoid getting lost in the values. Reprod Biomed Online 2011;23:411–20. [DOI] [PubMed] [Google Scholar]
- [30].La Marca A, Broekmans FJ, Volpe A, et al. Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod 2009;24:2264–75. [DOI] [PubMed] [Google Scholar]
- [31].Hsu A, Arny M, Knee AB, et al. Antral follicle count in clinical practice: analyzing clinical relevance. Fertil Steril 2011;95:474–9. [DOI] [PubMed] [Google Scholar]
- [32].Wiser A, Shalom-Paz E, Sokal-Arnon T, et al. Age-related normogram for antral follicles count in women with polycystic ovary syndrome. Reprod Biomed Online 2013;27:414–8. [DOI] [PubMed] [Google Scholar]
- [33].Jayaprakasan K, Chan YY, Islam R, et al. Prediction of in vitro fertilization outcome at different antral follicle count thresholds in a prospective cohort of 1,012 women. Fertil Steril 2012;98:657–63. [DOI] [PubMed] [Google Scholar]
- [34].Ji J, Liu Y, Tong XH, et al. The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. Hum Reprod 2013;28:2728–34. [DOI] [PubMed] [Google Scholar]
- [35].Chen ZJ, Shi Y, Sun Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. New Engl J Med 2016;375:523–33. [DOI] [PubMed] [Google Scholar]
- [36].Wei D, Shi Y, Jing L, et al. Effect of pretreatment with oral contraceptives and progestins on IVF outcomes in women with polycystic ovary syndrome. Hum Reprod 2017;32:354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang N, Hao CF, Zhuang LL, et al. Prediction of IVF/ICSI outcome based on the follicular output rate. Reprod Biomed Online 2013;27:147–53. [DOI] [PubMed] [Google Scholar]
- [38].Norman RJ, Dewailly D, Legro RS, et al. Polycystic ovary syndrome. Med J Australia 2017;370:685–97. [DOI] [PubMed] [Google Scholar]
- [39].Mellembakken JR, Berga SL, Kilen M, et al. Sustained fertility from 22 to 41 years of age in women with polycystic ovarian syndrome. Hum Reprod 2011;26:2499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Loendersloot LL, van Wely M, Repping S, et al. Individualized decision-making in IVF: calculating the chances of pregnancy. Hum Reprod 2013;28:2972–80. [DOI] [PubMed] [Google Scholar]
- [41].Choi B, Bosch E, Lannon BM, et al. Personalized prediction of first-cycle in vitro fertilization success. Fertil Steril 2013;99:1905–11. [DOI] [PubMed] [Google Scholar]
- [42].Polyzos NP, Sunkara SK. Sub-optimal responders following controlled ovarian stimulation: an overlooked group? Hum Reprod 2015;30:2005–8. [DOI] [PubMed] [Google Scholar]
- [43].Drakopoulos P, Blockeel C, Stoop D, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod 2016;22:497–526. [DOI] [PubMed] [Google Scholar]
- [44].Benharoush A, Farhi J, Zahalka Y, et al. Correlations between antral follicle count and ultrasonographic ovarian parameters and clinical variables and outcomes in IVF cycles. Gynecol Endocrinol 2012;28:432–5. [DOI] [PubMed] [Google Scholar]


