Abstract
Elimination of hepatitis C virus (HCV) infection as a public health threat is a goal of the World Health Organization. Official treatment guidelines are comprehensive and may be most appropriate for experienced treaters of HCV; however, global elimination of HCV infection will require the engagement of frontline health care providers (HCPs) to increase the capacity to treat patients. Thus, a simplified treatment algorithm is needed to provide guidance to non-HCV specialists. To accomplish this, a panel of 13 HCV specialists held a consensus meeting in September 2018 to create an easy-to-use, effective, safe, and scalable algorithm for the management and treatment of HCV infection through discussion and collective decision-making. The algorithm begins with universal HCV screening and diagnosis by testing for HCV antibody with reflex to polymerase chain reaction to detect HCV RNA. The pretreatment evaluation uses platelet-based stratification to initially assess fibrosis, and the pan-genotypic regimens glecaprevir/pibrentasvir or sofosbuvir/velpatasvir are recommended for treatment. Unless clinically indicated, on-treatment monitoring is optional. Confirmation of cure (undetectable HCV RNA 12 weeks posttreatment) is followed by harm-reduction measures, as well as surveillance for hepatocellular carcinoma every 6 months in patients with advanced fibrosis/cirrhosis. This algorithm provides guidance for management of uncomplicated cases of HCV by frontline HCPs and indicates when referral to an HCV specialist is warranted. The algorithm was created to enable more HCPs to screen for and manage HCV infection, and thus contribute to its elimination.
Keywords: Hepatitis C, glecaprevir/pibrentasvir, sofosbuvir/velpatasvir, simplified treatment
Corresponding Author
Douglas T. Dieterich, MD
Icahn School of Medicine at Mount Sinai 1468 Madison Avenue Box 1123, Annenberg 5-04 New York, New York Phone: 212-659-8879 Fax: 212-659-8377 E-mail: douglas.dieterich@mountsinai.org
Committee Members
Douglas T. Dieterich, MD
Icahn School of Medicine at Mount Sinai New York, New York
Joseph Ahn, MD, MS
Oregon Health & Science University Portland, Oregon
Bruce Bacon, MD
Saint Louis University Liver Center St. Louis, Missouri
David Bernstein, MD
North Shore University Hospital Manhasset, New York
Marc Bourlière, MD
Hôpital Saint Joseph Marseilles, France
Steven Flamm, MD
Northwestern University Feinberg School of Medicine Chicago, Illinois
Paul Kwo, MD
Stanford University School of Medicine Palo Alto, California
Joseph K. Lim, MD
Yale University School of Medicine New Haven, Connecticut
Christian Ramers, MD, MPH
Family Health Centers of San Diego San Diego, California
Nancy Reau, MD
Rush University Medical Center Chicago, Illinois
Mark Sulkowski, MD
Johns Hopkins University School of Medicine Baltimore, Maryland
Norman Sussman, MD
Baylor College of Medicine Houston, Texas
Stefan Zeuzem, MD
Goethe University Hospital Frankfurt, Germany
Conflicts of Interest
DD has received research support from, and served in a consulting/advisory/speaking capacity for, AbbVie, Gilead, and Merck. JA has served in a consulting/advisory capacity for Gilead. BB has received research support from AbbVie, Bristol-Myers Squibb, Gilead, and Merck; served in a consulting/advisory capacity for AbbVie, Bristol-Myers Squibb, Gilead, Intercept, and Merck; was a speaker for AbbVie, Gilead, Merck, Intercept, and Salix/Valeant; and sat on the Data and Safety Monitoring Board for Gilead and Isis. DB has received research support from AbbVie and Gilead; and served in a consulting/advisory capacity for AbbVie, Gilead, and Merck. MB was a speaker and has served in a consulting/advisory capacity for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Intercept, Janssen, and Merck Sharp & Dohme. SF has received research support from AbbVie and Gilead; and served in a consulting/advisory capacity for AbbVie, Gilead, and Merck. PK has received research support from AbbVie, Bristol-Myers Squibb, and Gilead; served in a consulting/advisory capacity for AbbVie, Bristol-Myers Squibb, Gilead, Merck, and Quest; and was a non-CME speaker for Merck. JL has received research support from AbbVie, Bristol-Myers Squibb, and Gilead; and served in a consulting/advising capacity for Bristol-Myers Squibb and Gilead. CR has received research support from Gilead; served in a consulting/advisory capacity for AbbVie and Gilead; and was a speaker for AbbVie, Gilead, and Merck. NR has served in a consulting/advisory capacity for AbbVie, Gilead, and Merck. MS has received research support from NIH/NIDA (R01DA16065, K24DA034621), AbbVie, Allergan, Assembly Biosciences, Gilead, and Proteus Digital Health; served in a consulting/advisory capacity for AbbVie, Arbutus, Gilead, Merck, Janssen, and Trek; and sat on the Data and Safety Monitoring Board for Gilead. NS has served in a consulting/advisory capacity, and was a speaker, for AbbVie and Gilead. SZ has served in a consulting/advisory capacity, and was a speaker, for AbbVie, Gilead, Janssen, and Merck/Merck Sharp Dohme.
Declaration of Funding Interests
The meeting and manuscript were funded by Gilead Sciences. The funder procured logistical support for the meeting from The Kinetix Group. Writing support was provided by Laura Yee of Caudex, New York, and funded by Gilead Sciences. The funder’s role was limited to attendance at the meeting and review of the manuscript for medical accuracy. The opinions expressed in this manuscript reflect those of the panel of HCV specialists.
Acknowledgements
Tram Tran, MD, and Michael Mertens, PhD, both of Gilead Sciences, attended the consensus meeting. Dr Tran reviewed the manuscript for medical accuracy. Laura Yee of Caudex, New York, provided medical writing support for this article, funded by Gilead Sciences. Mark Sulkowski, MD, receives research support from the National Institutes of Health/National Institute for Drug Abuse (NIH/NIDA; grant numbers R01DA16065 and K24DA034621).
1. Introduction
Douglas T. Dieterich, Joseph Ahn, Bruce Bacon, David Bernstein, Marc Bourlière, Steven Flamm, Paul Kwo, Joseph K. Lim, Christian Ramers, Nancy Reau, Mark Sulkowski, Norman Sussman, Stefan Zeuzem
Pan-genotypic regimens with high cure rates and minimal toxicities have greatly simplified the treatment of hepatitis C virus (HCV) infection.1,2 Thus, the World Health Organization (WHO) has set an objective for elimination of HCV infection as a public health threat by 2030, defined as a 90% reduction in incidence and 65% reduction in mortality compared with 2015 values.3 Estimates from 2015 suggest that chronic HCV infection affects approximately 71 million individuals worldwide.4 In the United States, the recent rise in new HCV infections is associated with the increased use of injection drugs.5-7 Without a vaccine, expanding the availability of prevention strategies (harm-reduction services) and scaling up treatment form the basis of HCV elimination efforts. However, many persons with HCV infection have not been diagnosed, and current treatment rates are estimated at only 7% to 26% worldwide.3,8
Access to treatment is limited in part by lack of screening and the number of available specialists (eg, hepatologists, gastroenterologists, infectious disease specialists) who typically treat HCV infection. Screening and management must extend to frontline health care providers (HCPs), such as primary care physicians, nurse practitioners, and physician assistants; however, lack of experience in HCV may pose a barrier.9 The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD/IDSA), and the European Association for the Study of the Liver (EASL), provide professional guidelines for treating HCV infection,1,10 but non-HCV specialists may consider these guidelines impractical and overwhelming given their complexity and length. Therefore, simplified recommendations are needed to help HCPs manage and treat HCV infection.
In this article, we present a simplified algorithm to manage and treat uncomplicated cases of HCV infection based on expert opinion and consensus of leading HCV specialists. The algorithm targets HCPs (nontraditional HCV treaters) in countries conducive to a simplified treatment strategy for HCV, such as the United States and those in Europe, with the goal of treating patients safely, effectively, and efficiently to facilitate HCV elimination.
2. Methods
The authors participated in a consensus meeting in September 2018 to develop a simplified algorithm to help HCPs treat HCV infection. A committee of 13 members (authors) with expertise in evaluating and treating HCV was formed. The committee included physicians from a variety of clinical settings, including primary care US Federally Qualified Health Centers and infectious disease practices. Subcommittees developed the preliminary content for the various sections, which was presented to the whole committee for discussion at the meeting. Through collaborative decision-making, a consensus was reached on the treatment algorithm presented in this article.
3. Simplified Algorithm for HCV Management and Treatment
3.1 Screening and Diagnosis
Chronic HCV infection can be acquired and can persist without symptoms; many people with HCV remain unaware of their infection until they have advanced liver disease. Current guidelines in the United States and Europe recommend HCV screening in specific at-risk populations,1,10,11 but underreporting of risk factors limits this approach. In the United States, the AASLD/IDSA, the Centers for Disease Control and Prevention, and the US Preventive Services Task Force recommend a one-time screening for persons born from 1945 to 1965 regardless of risk,10-12 based on a higher prevalence of HCV among persons in this birth cohort in the United States.13 However, estimates of prevalence may not account for the rising incidence of HCV associated with increasing rates of injection drug use in youth and adults younger than 30 years old,6,7 leaving a substantial proportion of patients with newer HCV infections subject to HCV screening only on the basis of risk assessment. Furthermore, these patients are less likely to be engaged in the health care system, precluding HCV screening entirely.
To identify all cases of HCV and achieve elimination, outreach programs designed to link high-risk patients to medical care and a more comprehensive screening strategy are needed. WHO and EASL guidelines support general population testing in settings where the HCV antibody has at least a 2% to 5% seroprevalence.1,14 Recent modeling studies in the United States and France have demonstrated that universal screening is cost-effective compared with other age-based screening strategies, even in regions with low HCV prevalence (eg, >0.07%15 and 0.23%16). Thus, we recommend one-time universal screening for HCV infection in all adults ages 18 years or older.
Until universal screening is feasible, we recommend screening all persons with any risk factors (including birth cohort), as shown in Table 1. Consistent with AASLD/IDSA guidelines, we recommend routine screening for pregnant women, based on recent data suggesting a significant increase in HCV infection among women of reproductive age in the United States and the potential for mother-to-child transmission.17 Persons with abnormal liver enzyme levels, including those with mild elevations above normal, should also be tested for HCV infection.
Table 1.
Recommendations for One-Time HCV Screening
| Birth Cohort |
|
| Risk Exposures |
|
| Other |
|
Risk factors are based on AASLD/IDSA 2018 guidelines,10 except for “men who have sex with men,” which is based on WHO 2017 guidelines14 and the NIH guidelines for HIV55 and HBV infection.
AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; HBV, hepatitis C virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDSA, Infectious Diseases Society of America; NIH, National Institutes of Health; WHO, World Health Organization.
We additionally recommend annual screening for persons with ongoing risk of infection: persons who inject drugs, persons infected with the human immunodeficiency virus (HIV), men who have sex with men, persons on hemodialysis, and incarcerated persons. If possible, screening for HCV should be done concurrently with hepatitis B virus (HBV) and HIV screening, as they share the same primary routes of transmission and similar risk factors for infection. Patients who screen negative should be counseled on risk-reduction strategies, including pre-exposure prophylaxis (HIV); vaccination (HBV); and harm-reduction services (HIV, HBV, and HCV), such as opioid agonist therapy or syringe service programs.
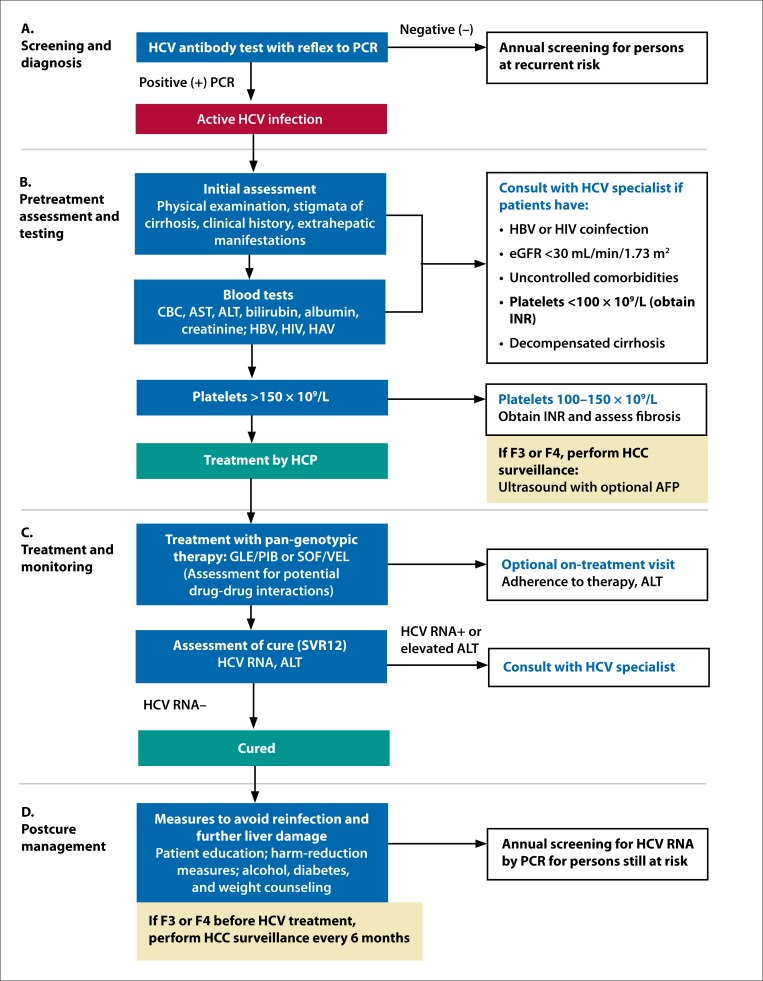
HCV screening is commonly performed through antibody testing, although HCV antibodies may not be detectable for 2 months or more after acute infection.18 A positive HCV antibody test indicates either current (active) or past HCV infection, or a false-positive test result.19 Therefore, to confirm a current infection, detection of HCV RNA by polymerase chain reaction (PCR) is required. We recommend “reflex testing,” in which a sample that tests positive for HCV antibody is automatically tested for the presence of HCV RNA by PCR (Figure 1A). If reflex testing is not available, then diagnosis requires a separate PCR test (second phlebotomy); however, this approach may lose many patients during follow-up.20,21
Figure 1.
A simplified treatment algorithm for HCV. AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBC, complete blood count; eGFR, estimated glomerular filtration rate; F, fibrosis score (METAVIR); GLE, glecaprevir; HAV, hepatitis A virus; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCP, health care provider; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INR, international normalized ratio; PCR, polymerase chain reaction; PIB, pibrentasvir; SOF, sofosbuvir; SVR12, sustained virologic response at 12 weeks; VEL, velpatasvir.
Screening and Diagnosis Recommendations
Screen all adults once using an HCV antibody test with reflex to HCV RNA by PCR
If universal screening is not feasible, screen all persons born from 1945 to 1965 and those with any identified risk factors (Table 1)
Continue annual screening for individuals with ongoing risk behaviors
3.2 Pretreatment Assessment and Testing
Studies have demonstrated that general practitioners can safely and effectively treat most uncomplicated cases of HCV, including patients with compensated cirrhosis.22,23 However, persons with more complicated HCV disease, such as those with decompensated cirrhosis or hepatocellular carcinoma (HCC), warrant care from a specialist, as they may require more complex management considerations and/or procedures such as esophagogastroduodenoscopy (EGD), paracentesis, or evaluation for liver transplantation. A pretreatment evaluation is necessary to assess the severity of fibrosis and other baseline factors that may impact HCV treatment, and to determine if a patient should be co-managed with specialist support (Figure 1B).
Initial Assessment During the initial assessment, the HCP should perform a physical examination and take a detailed patient history, which should include the risk of HCV acquisition, prior HCV therapies, presence of other liver diseases, and stigmata of cirrhosis. We recommend that HCPs assess for alcohol consumption, injection drug use, body mass index, diabetes, and the metabolic syndrome. Initial laboratory tests to assess HBV and HIV status, immunity to hepatitis A, and renal function are described below. HCPs should carefully assess all current medications, including over-the-counter drugs, vitamins, and herbal supplements, and those used on an as-needed basis. Women of childbearing age should be tested for pregnancy. HCPs should also consider extrahepatic manifestations associated with HCV, including vasculitis or renal disease related to cryoglobulinemia and porphyria cutanea tarda.24,25 Patients should be referred to an HCV specialist if they have been previously treated for HCV infection but not cured, have coinfection with HBV (positive for hepatitis B surface antigen [HBsAg]) or HIV, have severe renal impairment (renal replacement therapy or an estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2), or have uncontrolled comorbidities. Patients should be educated on modes of HCV transmission and counseled on measures to prevent reinfection. Drug and alcohol treatment, counseling, and harm-reduction services should be provided if appropriate.
Blood Tests At a minimum, we recommend that initial blood tests include a complete blood count (CBC) and a metabolic panel that includes aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, albumin, and creatinine. Tests should also be ordered for HBsAg, hepatitis B surface antibody (anti-HBs), hepatitis B core antibody (anti-HBc total), HIV antibody, hepatitis A immunity (anti-HAV), and eGFR if not already completed during prior testing. In general, genotyping of HCV is not required for treatment with the pan-genotypic regimens recommended below but may be necessary if these regimens are not available for first-line treatment. We specifically do not recommend routine toxicology testing for illicit drug or alcohol use as a requirement for HCV treatment.
Fibrosis Assessment The primary purpose of fibrosis assessment is to detect advanced fibrosis (consistent with a METAVIR score of F3 [bridging fibrosis]) or cirrhosis (F4) to identify patients who may require procedures such as EGD, and those who will require posttreatment HCC surveillance. Fibrosis staging is not required for persons with acute HCV infection (presenting within 6 months of exposure), but is recommended for all persons with chronic HCV infection. Thrombocytopenia is closely linked to chronic liver disease26; therefore, we propose that the platelet count from a CBC can serve as an initial indication of whether advanced fibrosis may be present. If the platelet count is greater than 150 × 109/L, then the risk of advanced fibrosis/cirrhosis is low; no additional routine tests are necessary prior to HCV treatment unless there is other evidence of cirrhosis. If the platelet count is less than 100 × 109/L, the likelihood of advanced fibrosis/cirrhosis is high, and we recommend obtaining an international normalized ratio (INR) and consulting a liver specialist. For patients with platelet levels from 100–150 × 109/L, we recommend obtaining an INR and using additional noninvasive methods of fibrosis assessment. These include calculation of the AST-to-platelet ratio index (APRI) or the fibrosis index based on 4 factors (FIB-4) score; evaluation using serum-based fibrosis markers (FibroTest™/FibroSure®, FibroMeter™); and liver stiffness measurement using elastography (FibroScan®) or abdominal ultrasound with acoustic radiation force impulse imaging.27-33 These tests require a score equivalent to F3 or higher to diagnose advanced fibrosis (Table 2). Cross-sectional imaging methods (computed tomography scan and magnetic resonance imaging) may provide another modality to detect cirrhosis. Due to the high cost, risk, and potential for sampling error, liver biopsy is not required for fibrosis assessment or treatment of HCV. If any of these methods confirm advanced fibrosis or cirrhosis, the patient should undergo a baseline screening for HCC. HCC screening consists of an ultrasound examination with optional assessment of serum α-fetoprotein (AFP) concentration. If the ultrasound reveals no lesions, the patient may proceed to treatment for HCV, with continued HCC surveillance every 6 months in those with advanced fibrosis/cirrhosis.1,10 Patients with an abnormal ultrasound result and/or an elevated AFP concentration trending upwards should be referred to an HCV specialist. Additionally, all patients with advanced fibrosis should be educated on medication avoidance and proper diet.
Table 2.
Cutoffs for Noninvasive Methods of Fibrosis Equivalent to Advanced Fibrosis (F3) or Cirrhosis (F4)
| Cutoff for F3 or Higher | Sensitivity | Specificity | PPV | NPV | Reference | |
|---|---|---|---|---|---|---|
| APRI | 1.0 1.5 |
61% 50% |
64% 87% |
40% NR |
81% NR |
27 |
| FIB-4 | 1.45 3.25 |
70% 22% |
74% 97% |
42% 65% |
90% 82% |
28 |
| FibroTest™/FibroSure® | 0.58 | NR | NR | NR | NR | 56 |
| FibroMeter® | 0.786 | NR | NR | NR | NR | 57 |
| FibroScan® | 9.6 kPa | 72% | 80% | 62% | 89% | 31 |
Higher scores indicate more severe advanced fibrosis. Cutoffs are provided as approximate guidelines; a combination of methods can be used to confirm F3 or higher. APRI and FIB-4 scores are calculated from data derived from the CBC (platelet count), chemistry profile (AST, ALT), and patient age (FIB-4 only).
ALT, alanine aminotransferase; APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; CBC, complete blood count; F, fibrosis score (METAVIR); FIB-4, fibrosis index based on 4 factors; NPV, negative predictive value; NR, not reported; PPV, positive predictive value.
If cirrhosis is confirmed, HCPs should refer patients for EGD to assess for complications associated with portal hypertension, including esophageal varices. HCPs should calculate the Child-Pugh score and determine whether cirrhosis is compensated (no signs of liver failure) or decompensated (eg, ascites, variceal hemorrhage, hepatic encephalopathy, jaundice).34 Patients with decompensated cirrhosis should be referred to an HCV specialist.
Pretreatment Recommendations
Perform a physical examination and obtain a patient history, including HCV treatment history, stigmata of cirrhosis, extrahepatic manifestations, and all current medications
Obtain blood tests, including CBC, AST, ALT, total bilirubin, albumin, and creatinine; test for HBsAg, anti-HBs, anti-HBc total, HIV antibody, anti-HAV, and eGFR
Estimate fibrosis stage using platelet count and, if appropriate, detect advanced fibrosis/cirrhosis using APRI, FIB-4, serum-based biomarkers, elastography and/or imaging
If advanced fibrosis/cirrhosis (F3 or F4) is detected, screen for HCC by ultrasound with optional AFP testing
Consult with a specialist before managing a patient who has been previously treated for HCV or has active HBV or HIV coinfection, severe renal impairment, uncontrolled comorbidities, platelet count less than 100 × 109/L, or decompensated cirrhosis
3.3 Treatment
HCV treatment is recommended for all persons with active infection to prevent development of complications from cirrhosis, decompensation, HCC, and extrahepatic manifestations. Additionally, treatment is recommended to prevent transmission of HCV infection to others, including children born to mothers with HCV infection. The use of pan-genotypic therapy simplifies treatment by removing the need for HCV genotype testing. Genotype-specific regimens have no advantage over pan-genotypic therapies, with the exception of the approval of ledipasvir/sofosbuvir (SOF) for the treatment of adolescents ≥12 years of age.35,36 Studies of pan-genotypic therapies in children ages ≥3 years and adolescents are ongoing.37,38 Therefore, children and adolescents should be referred to HCV specialists until more safety and efficacy data are available.
The pan-genotypic therapies glecaprevir/pibrentasvir (GLE/PIB), SOF/daclatasvir (SOF/DAC) and SOF/velpatasvir (SOF/VEL) are currently approved in the United States and the European Union for the initial treatment of HCV39; SOF/VEL/voxilaprevir (VOX) is approved for direct-acting antiviral (DAA)-naive patients only in the European Union.40 We recommend SOF/VEL or GLE/PIB (Figure 1C) and, in regions of the world where it is accessible, SOF/DAC.41 As the use of SOF/DAC is not generally feasible in the United States and European Union due to issues related to access and cost, our algorithm focuses on GLE/PIB and SOF/VEL. From our perspective, GLE/PIB and SOF/VEL have generally comparable efficacy and safety profiles with 2 exceptions. SOF/VEL can be used in patients with Child-Pugh B and C cirrhosis (with ribavirin), whereas GLE/PIB is not recommended in Child-Pugh B and contraindicated in Child-Pugh C cirrhosis. SOF/VEL is restricted to patients with an eGFR ≥30 mL/min/1.73 m2, whereas GLE/PIB has no restrictions for renal disease.42,43 In registration trials, GLE/PIB and SOF/VEL achieved overall cure rates ≥95%,44-48 defined as sustained virologic response at 12 weeks (SVR12; undetectable HCV RNA 12 weeks after treatment). Minimal side effects occur with these therapies, and most are mild.42,43 Common side effects include headache, fatigue, and nausea with both GLE/PIB and SOF/VEL, as well as asthenia and insomnia with SOF/VEL. Laboratory abnormalities such as elevated lipase and creatine kinase with SOF/VEL,42 and ALT and bilirubin levels with GLE/PIB, are rare.46-48
Details of treatment duration and dosage of GLE/PIB and SOF/VEL are shown in Table 3. SOF/VEL is given as an oral tablet once daily with or without food for 12 weeks in patients without cirrhosis or with compensated cirrhosis.42 As initial treatment for patients without cirrhosis, GLE/PIB is given as 3 oral tablets once per day with food for 8 weeks; for patients with compensated cirrhosis, treatment duration is currently 12 weeks,43 although recent data support treatment for 8 weeks.49 Those with decompensated cirrhosis can be treated by an HCV specialist using a regimen containing SOF/VEL plus ribavirin. In general, patients treated with DAAs who are not cured should be evaluated for re-treatment by an HCV specialist, with SOF/VEL/VOX as the preferred regimen1,10,50; for patients who are not cured with GLE/PIB, re-treatment with SOF plus GLE/PIB and ribavirin may also be an option.1
Table 3.
Comparison of Recommended Pan-Genotypic Regimens
| SOF/VELa | GLE/PIBb | |
|---|---|---|
|
Treatment duration,
wk No cirrhosis Compensated cirrhosis Decompensated cirrhosis |
12 12c 12d |
8 12 Not indicated |
| Dosage | 1 tablet (400 mg SOF + 100 mg VEL) daily with or without food | 3 tablets (100 mg GLE + 40 mg PIB per tablet) once daily with food |
| Common side effects | Headache, fatigue, nausea, asthenia, insomnia | Headache, fatigue, nausea |
| Key drug-drug interactionse | Amiodarone, anticonvulsants, proton pump inhibitors (high dose), rifampicin, efavirenz, St John’s wort, statins | Dabigatran, anticonvulsants, rifampicin, ethinyl estradiol–containing contraceptives, St John’s wort, atazanavir, darunavir, ritonavir, efavirenz, statins, cyclosporine |
| Common drugs without interactionse | ARBs, methadone, buprenorphine, calcium channel blockers, lamotrigine, omeprazole, progestin-only contraceptives | |
ARBs, angiotensin II receptor blockers; GLE, glecaprevir; HCV, hepatitis C virus; NS, nonstructural protein; PIB, pibrentasvir; SOF, sofosbuvir; VEL, velpatasvir; wk, week.
aTreatment duration of SOF/VEL for treatment-experienced patients is 12 weeks.
bGLE/PIB is also indicated for patients with HCV genotype 1 infection with no cirrhosis or compensated cirrhosis (Child-Pugh A) who have been treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor, but not both. Duration of treatment for patients with prior NS5A inhibitor experience or NS3/4A protease inhibitor experience is 16 weeks or 12 weeks, respectively.
cPrescribing information in the European Union, but not the United States, states that the addition of ribavirin may be considered for patients with HCV genotype 3 and compensated cirrhosis.
dFor patients with decompensated cirrhosis, SOF/VEL is indicated in combination with ribavirin.
eHealth care providers should consult prescribing information, their local pharmacist, and/or online tools (eg, HEP Drug Interactions, http://www.hepdruginteractions.org) to confirm interaction or lack of interaction for specific drugs within a class, as exceptions may exist.
Drug-drug interactions may impact the treatment choice between GLE/PIB and SOF/VEL; common drugs with or without interactions are shown in Table 3. HCPs should pay attention to anticonvulsants, statins, and over-the-counter herbal medicines. Amiodarone should not be administered with SOF/VEL due to the risk of bradycardia, but is acceptable with GLE/PIB. Drugs that should not be administered with GLE/PIB, but are acceptable with SOF/VEL, include ethinyl estradiol and dabigatran. Of note, medications used to treat opiate-use disorder, including methadone, buprenorphine, and buprenorphine/naloxone, do not have significant interactions with SOF/VEL or GLE/PIB. We recommend that HCPs work with their local pharmacists and refer to online tools (eg, HEP Drug Interactions, http://www.hep-druginteractions.org) to identify potential drug-drug interactions and determine if additional monitoring or dosing modifications should be made.
Treatment Recommendations
Treat HCV infection with one of the pan-genotypic regimens GLE/PIB or SOF/VEL (or SOF/DAC, if available)
Before initiating treatment, assess for potential drug-drug interactions
3.4 Monitoring
Treatment with GLE/PIB or SOF/VEL requires minimal monitoring due to the high efficacy and favorable safety profile of these therapies (Figure 1C). AASLD/IDSA monitoring guidelines include laboratory tests after 4 weeks of therapy, more frequent assessments for drug-related adverse events, and HCV RNA testing 4 weeks after therapy initiation and 12 weeks after therapy completion (confirmation of cure).10 However, detectable HCV RNA at 4 weeks does not warrant treatment discontinuation, and side effects are generally mild; in clinical studies, only 0.2% and 0.1% of patients who received SOF/VEL or GLE/PIB, respectively, discontinued treatment due to adverse events.42,43 Therefore, routine on-treatment assessments are not recommended and should be performed only as clinically indicated.
At the discretion of the HCP, an optional on-treatment visit 4 to 6 weeks after treatment initiation may occur. If this visit is indicated, an ALT assay may be obtained; HCPs should refer to AASLD/IDSA guidelines if ALT levels are elevated above baseline. As an alternative to a clinic visit, HCPs can consider contacting the patient by telephone to inquire about adverse effects. Although few side effects are expected, patients should be directed to consult their HCP if they experience any unexpected or severe symptoms. Importantly, HCPs should encourage adherence to treatment by contacting patients by telephone or electronically.
Reports of HBV reactivation in patients who have received DAAs as HCV treatment—primarily in those with positive HBsAg, if status was known—have resulted in warnings on the GLE/PIB and SOF/VEL labels about the possible risk of HBV reactivation.42,43,51 All persons with active HBV infection (HBsAg positive) should be managed by or in collaboration with an HCV specialist. However, persons with evidence of prior HBV infection (anti-HBc total positive, HBsAg negative) with or without anti-HBs can be treated safely by non-HCV specialists. A recent systematic review and meta-analysis of 17 studies involving more than 1600 patients found that HBV reactivation occurred in 1.4% of patients with prior HBV infection who had taken DAAs for HCV52; thus, the risk of HBV reactivation is low. Based on these findings, we recommend that patients with prior HBV infection be educated on the signs and symptoms of HBV reactivation, but routine monitoring is unnecessary.
At least 12 weeks after treatment completion, HCPs should confirm cure (SVR12) by testing for HCV RNA by PCR. In cases where treatment with GLE/PIB or SOF/VEL does not result in a cure, patients should be referred to an HCV specialist. In addition, ALT should be measured to assess liver inflammation. Persons who have been cured but have persistently elevated ALT levels (>19 U/L for women and >30 U/L for men per AASLD guidelines53) should also consult a specialist.
Monitoring Recommendations
Advise patients, particularly those with prior HBV infection, to contact their HCP if they experience unexpected or severe symptoms
At least 12 weeks after treatment completion, confirm cure by assessing HCV RNA by PCR; refer patients with detectable HCV RNA to a specialist
At least 12 weeks after treatment completion, obtain the ALT level; if ALT remains abnormal on repeated measure, refer the patient to a specialist
3.5 Postcure Management
After a patient has been cured of HCV, follow-up measures are required to reduce the risk of reinfection and liver damage (Figure 1D). HCPs should inform patients that past HCV infection does not provide protective immunity against reinfection. Patients should be aware that the HCV antibody will persist, and a positive antibody test result does not indicate current infection; diagnosis of a recurrent HCV infection requires testing for HCV RNA by PCR.
Appropriate prevention measures must be taken to avoid reinfection with HCV. We recommend patients be offered harm-reduction resources, such as opioid agonist therapy and syringe service programs, which may reduce the risk of acquiring HCV by 50% and 76%, respectively.54 Persons at risk for HCV infection through sexual transmission should be educated on the use of condoms and other safe-sex practices. HCV testing and treatment should be offered to close personal contacts of patients to reduce the risk of reinfection. For patients with continued risk, we recommend surveillance for HCV RNA at least annually. We recommend offering counseling to patients with alcohol use issues, obesity, or diabetes to prevent additional liver damage due to steatohepatitis.
Patients with advanced fibrosis/cirrhosis (F3 or F4) prior to HCV treatment should undergo continued surveillance for HCC every 6 months. As with the pretreatment evaluation, an ultrasound should be performed with optional serum AFP testing.
Postcure Recommendations
Inform patients who are cured that they are susceptible to reinfection
Provide patients with appropriate HCV harm-reduction resources to minimize the possibility of reinfection
Continue HCC surveillance every 6 months in patients who had advanced fibrosis/cirrhosis before HCV treatment
4. Conclusions
The goal of simplifying HCV therapy is to enable expansion of treatment on a scale that will meaningfully contribute to HCV elimination. Frontline HCPs have the opportunity to reduce the spread of HCV by diagnosing and treating patients with HCV, if up-to-date resources, support mechanisms, and practical clinical recommendations are provided. Telehealth programs, such as Project ECHO, that use videoconferencing and case-based learning have already demonstrated that nonspecialists can successfully treat HCV with outcomes comparable to those seen in specialty centers.22 Likewise, open-source educational resources (www.hepatitisc.uw.edu) are available for HCPs to improve their knowledge and skills. By harnessing the expertise of a panel of HCV specialists, we have developed a streamlined algorithm to assist HCPs in managing and treating HCV. Together, these resources may facilitate diagnoses and lead to greater treatment access in the primary care setting, thus contributing to elimination of HCV infection.
Postcure Recommendations
Inform patients who are cured that they are susceptible to reinfection
Provide patients with appropriate HCV harm-reduction resources to minimize the possibility of reinfection
Continue HCC surveillance every 6 months in patients who had advanced fibrosis/cirrhosis before HCV treatment
References
- 1. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018 J Hepatol 20186902461–511. [DOI] [PubMed] [Google Scholar]
- 2.Kapadia SN, Marks KM. Hepatitis C management simplification from test to cure: a framework for primary care providers. Clin Ther. 2018;40(08):1234–1245. doi: 10.1016/j.clinthera.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization Global Hepatitis Report, 2017. Geneva, Switzerland: World Health Organization; 2017https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=5649373E4ADD028FA 00B4421E3C7B687?sequence=1 Accessed April 15, 2019 [Google Scholar]
- 4.Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(03):161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Statistics & Surveillance: Surveillance for Viral Hepatitis—United States, 2016. https://www.cdc.gov/hepatitis/statistics/2016surveillance/index.htm#tabs-6-11 Accessed November 7, 2018.
- 6.Suryaprasad AG, White JZ, Xu F et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59(10):1411–1419. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- 7.Zibbell JE, Iqbal K, Patel RC et al. Centers for Disease Control and Prevention (CDC). Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
- 8.Vutien P, Jin M, Le MH et al. Regional differences in treatment rates for patients with chronic hepatitis C infection: systematic review and meta-analysis. PLoS One. 2017;12(09):e0183851. doi: 10.1371/journal.pone.0183851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGowan CE, Fried MW. Barriers to hepatitis C treatment. Liver Int. 2012;32(suppl 1):151–156. doi: 10.1111/j.1478-3231.2011.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org Accessed September 12, 2018.
- 11.Centers for Disease Control and Prevention. Testing recommendations for hepatitis C virus infection. https://www.cdc.gov/hepatitis/hcv/guidelinesc.htm Accessed September 12, 2018.
- 12.Moyer VA. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(05):349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 13.Smith BD, Morgan RL, Beckett GA et al. Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1–32. RR-4. [PubMed] [Google Scholar]
- 14.World Health Organization. WHO Guidelines on Hepatitis B and C Testing: February 2017. Geneva, Switzerland: World Health Organization; 2017. https://apps.who.int/iris/bitstream/handle/10665/254621/9789241549981-eng.pdf?sequence=1 Accessed April 15, 2019.
- 15.Eckman MH, Ward JW, Sherman KE. Cost effectiveness of universal screening for HCV infection in the era of direct-acting, pangenotypic treatment regimens. Clin Gastroenterol Hepatol. 2019;17(05):930–939.e9. doi: 10.1016/j.cgh.2018.08.080. [DOI] [PubMed] [Google Scholar]
- 16.Deuffic-Burban S, Huneau A, Verleene A et al. Assessing the cost-effectiveness of hepatitis C screening strategies in France. J Hepatol. 2018;69(04):785–792. doi: 10.1016/j.jhep.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C virus infection among reproductive-aged women and children in the United States, 2006 to 2014. Ann Intern Med. 2017;166(11):775–782. doi: 10.7326/M16-2350. [DOI] [PubMed] [Google Scholar]
- 18.Busch MP. Insights into the epidemiology, natural history and pathogenesis of hepatitis C virus infection from studies of infected donors and blood product recipients. Transfus Clin Biol. 2001;8(03):200–206. doi: 10.1016/s1246-7820(01)00125-2. [DOI] [PubMed] [Google Scholar]
- 19.Pawlotsky JM. Use and interpretation of virological tests for hepatitis C. Hepatology. 2002;36(05) suppl 1:S65–S73. doi: 10.1053/jhep.2002.36815. [DOI] [PubMed] [Google Scholar]
- 20.McGibbon E, Bornschlegel K, Balter S. Half a diagnosis: gap in confirming infection among hepatitis C antibody-positive patients. Am J Med. 2013;126(08):718–722. doi: 10.1016/j.amjmed.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Spradling PR, Tong X, Rupp LB et al. Chronic Hepatitis Cohort Study (CHeCS) Investigators. Trends in HCV RNA testing among HCV antibody-positive persons in care, 2003-2010. Clin Infect Dis. 2014;59(07):976–981. doi: 10.1093/cid/ciu509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arora S, Thornton K, Murata G et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199–2207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brew IF, Butt C, Wright N. Can antiviral treatment for hepatitis C be safely and effectively delivered in primary care?: a narrative systematic review of the evidence base. Br J Gen Pract. 2013;63(617):e842–e851. doi: 10.3399/bjgp13X675421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cacoub P, Gragnani L, Comarmond C, Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis. 2014;46(suppl)(05):S165–S173. doi: 10.1016/j.dld.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Dedania B, Wu GY. Dermatologic extrahepatic manifestations of hepatitis C. J Clin Transl Hepatol. 2015;3(02):127–133. doi: 10.14218/JCTH.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afdhal N, McHutchison J, Brown R et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48(06):1000–1007. doi: 10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Lin ZH, Xin YN, Dong QJ et al. Performance of the aspartate aminotransferaseto-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(03):726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 28.Sterling RK, Lissen E, Clumeck N et al. APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(06):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 29.Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102(11):2589–2600. doi: 10.1111/j.1572-0241.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 30.Calès P, Boursier J, Ducancelle A et al. ANRS HC EP 23 Fibrostar Study. Improved fibrosis staging by elastometry and blood test in chronic hepatitis C. Liver Int. 2014;34(06):907–917. doi: 10.1111/liv.12327. [DOI] [PubMed] [Google Scholar]
- 31.Afdhal NH, Bacon BR, Patel K et al. Accuracy of fibroscan, compared with histology, in analysis of liver fibrosis in patients with hepatitis B or C: a United States multicenter study. Clin Gastroenterol Hepatol. 2015;13(04):772–779. doi: 10.1016/j.cgh.2014.12.014. e771-e773. [DOI] [PubMed] [Google Scholar]
- 32.Lim JK, Flamm SL, Singh S, Falck-Ytter YT. Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute Guideline on the Role of Elastography in the Evaluation of Liver Fibrosis. Gastroenterology. 2017;152(06):1536–1543. doi: 10.1053/j.gastro.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Hu X, Qiu L, Liu D, Qian L. Acoustic Radiation Force Impulse (ARFI) elastography for non-invasive evaluation of hepatic fibrosis in chronic hepatitis B and C patients: a systematic review and meta-analysis. Med Ultrason. 2017;19(01):23–31. doi: 10.11152/mu-942. [DOI] [PubMed] [Google Scholar]
- 34.D’Amico G, Morabito A, D’Amico M et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68(03):563–576. doi: 10.1016/j.jhep.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 35. HARVONI® (ledipasvir and sofosbuvir) [prescribing information]. Foster City, CA: Gilead Sciences Inc; November 2017.
- 36.European Medicines Agency. Harvoni summary of product characteristics. https://www.ema.europa.eu/documents/product-information/harvoni-epar-product-information_en.pdf Accessed October 31, 2018.
- 37.ClinicalTrials.gov. Sofosbuvir/velpatasvir in adolescents and children with chronic HCV infection. https://clinicaltrials.gov/ct2/show/NCT03022981.Identifier:NCT03022981 Accessed September 20, 2018.
- 38.ClinicalTrials.gov. A study to evaluate the pharmacokinetics, safety, and efficacy of glecaprevir/pibrentasvir in pediatric subjects with genotypes 1-6 chronic hepatitis C virus (HCV) infection (DORA) https://clinicaltrials.gov/ct2/show/NCT03067129.Identifier:NCT03067129 Accessed September 20, 2018.
- 39.World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed With Chronic Hepatitis C Virus Infection: July 2018. Geneva, Switzerland: World Health Organization; 2018. https://apps.who.int/iris/bitstream/han dle/10665/273174/9789241550345-eng.pdf?ua=1 Accessed April 15, 2019. [PubMed]
- 40.European Medicines Agency. Vosevi summary of product characteristics. https://www.ema.europa.eu/documents/product-information/vosevi-epar-product-information_en.pdf Accessed September 27, 2018. Accessed April 15, 2019.
- 41.Belperio PS, Shahoumian TA, Loomis TP, Mole LA, Backus LI. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J Hepatol. 2019;70(01):15–23. doi: 10.1016/j.jhep.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Foster City, CA: Gilead Sciences Inc; 2017. EPCLUSA® (sofosbuvir and velpatasvir) [prescribing information] November. [Google Scholar]
- 43.North Chicago, IL: AbbVie Inc; 2018. MAVYRETTM (glecaprevir and pibrentasvir) [prescribing information] August. [Google Scholar]
- 44.Feld JJ, Jacobson IM, Hézode C et al. ASTRAL-1 Investigators. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 45.Foster GR, Afdhal N, Roberts SK et al. ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 46.Zeuzem S, Foster GR, Wang S et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378(04):354–369. doi: 10.1056/NEJMoa1702417. [DOI] [PubMed] [Google Scholar]
- 47.Asselah T, Kowdley KV, Zadeikis N et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. 2018;16(03):417–426. doi: 10.1016/j.cgh.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 48.Forns X, Lee SS, Valdes J et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017;17(10):1062–1068. doi: 10.1016/S1473-3099(17)30496-6. [DOI] [PubMed] [Google Scholar]
- 49.Brown RS, Hezode C, Wang S et al. Preliminary efficacy and safety of 8-week glecaprevir/pibrentasvir in patients with HCV genotype 1–6 infection and compensated cirrhosis: the EXPEDITION-8 study [AASLD abstract LB-7] Hepatology. 2018;68(S1) [Google Scholar]
- 50.Bourlière M, Gordon SC, Flamm SL et al. POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2134–2146. doi: 10.1056/NEJMoa1613512. [DOI] [PubMed] [Google Scholar]
- 51.US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. https://www.fda.gov/Drugs/DrugSafety/ucm522932.htm Accessed October 16, 2018.
- 52.Mücke MM, Backus LI, Mücke VT et al. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3(03):172–180. doi: 10.1016/S2468-1253(18)30002-5. [DOI] [PubMed] [Google Scholar]
- 53.Kasarala G, Tillmann HL. Standard liver tests. Clin Liver Dis (Hoboken) 2016;8(01):13–18. doi: 10.1002/cld.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Platt L, Minozzi S, Reed J et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9:CD012021. doi: 10.1002/14651858.CD012021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Institutes of Health. AIDS Info: Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-opportunistic-infection/362/appendix-a--preventing-exposure Accessed September 27, 2018.
- 56.BioPredictive. Cut-off tables: FibroTest. https://www.biopredictive.com/services/cutoff-tables/ Accessed October 16, 2018.
- 57.Association Française pour l’Etude du Foie (AFEF). Recommandations AFEF pour l’élimination de l’infection par le virus de l’hépatite C, en France. https://afef.asso.fr/wp-content/uploads/2018/06/VF-INTERACTIF-RECO-VHCAFEF-v2103.pdf Accessed November 1, 2018.
Biography

Footnotes
Disclaimer
This supplement is supported by Gilead Sciences. Support of this supplement does not imply the supporter’s agreement with the views expressed herein. Every effort has been made to ensure that drug usage and other information are presented accurately; however, the ultimate responsibility rests with the prescribing physician. Gastro-Hep Communications, Inc., the supporter, and the participants shall not be held responsible for errors or for any consequences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorsements are made for any drug or compound at present under clinical investigation.