Abstract
Background
We assessed practice patterns for monitoring mucosal inflammation after biologic initiation and the association between monitoring approaches and development of disease-related complications for Crohn’s disease (CD) and ulcerative colitis (UC).
Methods
This study used a Truven Health MarketScan (2007–2016) query for CD and UC patients initiating biologic therapy. Cumulative 24-month disease-related complications (corticosteroids, change of biologic, hospitalization, surgery) in patients undergoing proactive disease monitoring with lower endoscopy, fecal calprotectin, or cross-sectional radiographic enterography (computed tomography enterography or magnetic resonance enterography) within 6 months of biologic initiation vs no early monitoring after biologic initiation were compared. Cox proportional hazard ratios (HRs with 95% confidence intervals [CIs]) adjusted for propensity score were used.
Results
Within the first 24 months after biologic initiation, monitoring (proactive or reactive) was performed in 56.4% of CD patients and 67.8% of UC patients, with considerable geographic variability. Early (within 6 months) proactive monitoring was endoscopy-based (87.9%), performed in 11% of CD (n = 2195/19,899) and 12.8% of UC (n = 925/7247) patients. Compared with no early monitoring, early proactive monitoring was associated with a reduction in disease-related complications for CD (adjusted HR [aHR], 0.90; 95% CI, 0.84–0.96) and UC (aHR, 0.87; 95% CI, 0.78–0.97) and predominately driven by a reduction in corticosteroid use (CD: aHR, 0.83; 95% CI, 0.77–0.90; UC: aHR, 0.77; 95% CI, 0.69–0.87). Results were consistent across multiple sensitivity analyses.
Conclusions
Early proactive monitoring of mucosal inflammation in CD and UC within 6 months of biologic initiation was associated with reduction in disease-related complications over 24 months, primarily related to reduced steroid utilization. Wide variation exists in practice patterns for monitoring of mucosal inflammation after biologic initiation.
Keywords: IBD, biologic, value, monitoring
Achieving mucosal healing is associated with reductions in disease related complications, however, we have observed that a minority of biologic treated IBD patients undergo follow-up assessments of disease activity to confirm healing and this has direct impacts on disease-related complications.
Introduction
Inadequate control of intestinal inflammation in Crohn’s disease (CD) and ulcerative colitis (UC) can lead to disease-related complications including prolonged corticosteroid therapy, hospitalization, and penetrating complications requiring intestinal surgery. Although the advent of biologic therapies has improved our ability to control inflammation, the timing of intervention appears to influence longitudinal outcomes in CD. When early combined immunosuppressive strategies are paired with frequent clinical disease activity assessments and targeted adjustments in therapy, this strategy has been proven to reduce the risk of CD-related complications.1, 2 These findings form the basis for the treatment paradigm of proactive disease monitoring and “treat-to-target.”
The achievement of mucosal healing (MH) is associated with a reduction in long-term disease-related complications.3 In clinical practice, endoscopic assessment of mucosal disease activity with accompanying adjustments in therapy when persistent mucosal inflammation is observed is associated with increased probabilities for ultimately achieving MH.4, 5 Clinical trial data have now demonstrated that escalation of therapy based on clinical symptoms and biomarkers of mucosal inflammation results in improvements in the achievement of MH, as compared with symptom-based assessments alone.6 Taken together, these data support follow-up assessments of mucosal inflammation to optimize the achievement of MH, particularly in at-risk inflammatory bowel disease (IBD) populations initiating or adjusting biologic therapy.7
What is lacking, however, is an understanding of current practice patterns for monitoring of mucosal inflammation in the United States and associated outcomes with monitoring approaches. Using a large real-world patient population, we (1) evaluated current practice patterns for monitoring of mucosal inflammation in IBD patients newly initiating biologic therapy and (2) assessed whether early proactive monitoring of mucosal inflammation with endoscopy, fecal calprotectin (FC), and/or cross-sectional imaging after the initiation of biologic therapy was associated with longitudinal reductions in disease-related complications.
METHODS
Study Population
The study population was derived from the Truven Health MarketScan Commercial Database. This represents approximately 170 million covered lives and a mix of 350 commercial insurance plans that cover large employers, health plans, and government and public organizations. We identified all enrollees in the database from January 1, 2007, through December 31, 2016, who possessed a diagnosis of CD (555, K50) or UC (556, K51) based on the International Classification of Diseases (ICD), 9th and 10th revisions, codes in any diagnosis position. We used pharmaceutical claims data to identify the subset of IBD patients who initiated biologic therapy (new users of infliximab, adalimumab, certolizumab pegol, golimumab, natalizumab, vedolizumab, ustekinumab) without any history of use since start of enrollment. The combination of an ICD code for CD or UC with a claim for a biologic therapy has a high diagnostic accuracy for correctly identifying IBD patients using claims data.8, 9
We required a period of drug-free enrollment, and patients were excluded from the primary analysis if they had less than 6 months between the time of enrollment into the database and initiation of biologic therapy. Patients were also excluded if they had unclear follow-up (eg, insurance enrollment without recorded clinical encounters) or ≤6 months of follow-up data after initiation of biologic therapy. Patients with preexisting inflammatory arthropathy (ICD-9: 714; ICD-10: M05–M08), inflammatory spondylopathy (ICD-9: 720; ICD-10: M45–M49), and psoriasis (ICD-9: 696; ICD-10 L40) were also excluded.
Disease Monitoring
Disease monitoring was defined as having undergone an assessment for mucosal disease activity with either a lower endoscopy (flexible sigmoidoscopy or colonoscopy) or FC, and for CD this also included computed tomography enterography (CTE) or magnetic resonance enterography (MRE). Early disease monitoring was defined as having undergone an assessment for mucosal disease activity within 6 months after starting index biologic initiation. Proactive monitoring was defined as having undergone an assessment of mucosal disease activity without corticosteroid use, emergency department (ED) visit, hospitalization, or intestinal surgery within 90 days before and up to 30 days after the monitoring test. Reactive monitoring was defined as having undergone an assessment of mucosal disease activity with corticosteroid use, hospitalization, or intestinal surgery within 90 days before and up to 30 days after the test. Early proactive monitoring was considered the intervention group, with no early disease monitoring being used as the reference. Early reactive monitoring was used as a control group.
Definitions of Disease-Related Complications
Potential disease-related complications included the initiation of corticosteroids, change of biologic agent, hospitalization, and/or intestinal surgery >30 days after disease monitoring. Change in biologic agent was used as a surrogate to represent disease worsening while on active therapy, which could represent primary nonresponse or secondary loss of response given the strong association between the presence or persistence of mucosal inflammation and the need to change biologic therapy for nonresponse or loss of response.10 ICD and CPT codes are provided in the Supplementary Methods.
Objectives
Our primary objectives were to evaluate current practice patterns for monitoring of mucosal inflammation in IBD patients newly initiating biologic therapy and to assess whether an association was present between monitoring approaches and development of disease-related complications after biologic initiation in CD and UC. Secondary objectives were to compare rates of disease-related complications between monitoring approaches (lower endoscopy, FC, or enterography) and varying timing of monitoring (months 0 to 6 and months 6 to 12, compared with months 12 and beyond).
We compared early proactive monitoring (intervention) with no early disease monitoring (reference). To ensure that findings from this comparison were associated with the proactive nature of monitoring, a second comparison was made between early reactive monitoring (control) and no early disease monitoring (reference). Our a priori hypothesis was that when monitoring was done before disease worsening, as defined by need for corticosteroids, ED visit, hospitalization, or surgery (proactive), it would be associated with a reduction in 24-month cumulative rates of disease-related complications compared with no monitoring, whereas when monitoring was done in response to disease worsening (reactive), it would be associated with either an increased risk of 24-month cumulative rates of disease-related complications or with no difference, compared with no monitoring. To compare rates of disease-related complications between monitoring approaches and varying timing of monitoring, we performed subgroup analyses for each monitoring approach (endoscopy, FC, enterography) stratified by timing (0–6, 6–12, >12) and the nature of the monitoring (proactive or reactive vs no monitoring).
Statistical Analyses
Descriptive statistics were used to describe practice patterns for monitoring of mucosal inflammation and secular trends and geographic variations in monitoring. Descriptive analyses were used to compare baseline patient characteristics between those who did and did not undergo early disease monitoring (ie, lower endoscopy, FC, or enterography within 6 months after initiation of biologic therapy). Categorical variables were compared using the χ2 statistic or Fisher exact test, where appropriate. Continuous variables were compared using analysis of variance.
Propensity score weighting with a 1-to-1 nearest-neighbor without-replacement matching scheme was used to statistically balance the groups (proactive, reactive, no monitoring) by taking into consideration baseline characteristics that could theoretically reflect disease severity and/or influence clinical practice patterns and relevant longitudinal clinical outcomes (Supplementary Methods). Cox proportional hazards models adjusted for propensity scores were used to evaluate the individual and composite risk of disease-related complications, including the need for corticosteroids, change of biologic agent, hospitalization, and intestinal surgery, when comparing groups. To reduce the risk of immortal time bias, the time of entry into the survival analysis was set at 6 months after initiation of biologic therapy for analyses. Censoring at 24 months was determined a priori to balance between having adequate time of follow-up and adequate remaining patients in the analyses. Post hoc analysis later revealed as few as 16 patients in 1 comparison group at 24 months.
Sensitivity analyses were performed by (1) varying the time frame for no event before monitoring for defining proactive vs reactive, (2) limiting the analysis to patients who did not experience a disease-related complication within the first 6 months of biologic initiation, and (3) limiting the analysis to patients with at least 12 months between the time of enrollment and initiation of biologic therapy, where lower endoscopy was used for monitoring of mucosal inflammation. We performed an additional sensitivity analysis excluding steroid events when the end of a prior steroid prescription was within 30 days of the survival start time to account for the potential that a new steroid prescription after reactive monitoring was simply a continuation of the previously prescribed steroid. Furthermore, we assessed the change in corticosteroid exposure among the proactive and reactive groups to account for variability in length of steroid prescriptions. This was calculated as the number of days on steroids divided by total time, with total time being calculated from entry to exit of survival analysis.
The Kaplan-Meier method was used to graph the longitudinal overall risk of disease-related complications. Statistical significance was defined as a 2-tailed α of <0.05. All statistical analyses were performed using SAS 9.4 (Cary, NC, USA) and Stata SE 14.2 (College Station, TX, USA).
Ethics
The study protocol was reviewed and deemed exempt by the Institutional Review Board of Stanford University. Use of primary data was performed in compliance with contractual agreements between the Stanford Center for Population Health Sciences and Truven Health Analytics, an IBM Company.
RESULTS
Study Cohort and Practice Patterns for Monitoring
A total of 55,950 IBD patients initiating biologic therapy were identified, of whom 27,146 (n = 19,899 CD, n = 7247 UC) were included in the current analyses (Supplementary Fig. 2). The biologics used for these 27,146 patients were adalimumab (n = 22,078), certolizumab (n = 2397), infliximab (n = 2006), golimumab (n = 430), vedolizumab (n = 119), ustekinumab (n = 85), and natalizumab (n = 31). Rates of early monitoring and baseline differences in patient characteristics across groups (early proactive, early reactive, and no early monitoring) were comparable in the included and excluded cohorts (Table 1; Supplementary Table 1).
Table 1.
Baseline Patient Characteristics
| Characteristic | Crohn’s Disease | Ulcerative Colitis | ||||||
|---|---|---|---|---|---|---|---|---|
| Early Proactive Monitoring | Early Reactive Monitoring | No Early Monitoring | P | Early Proactive Monitoring | Early Reactive Monitoring | No Early Monitoring | P | |
| (n = 2195) | (n = 1919) | (n = 15,785) | (n = 925) | (n = 925) | (n = 5397) | |||
| Age (SD), y | 38.5 (14.0) | 39.1 (13.8) | 36.9 (13.8) | <0.01 | 41.4 (13.4) | 42.0 (13.7) | 39.3 (14.1) | <0.01 |
| Sex, No. (%) | ||||||||
| Male | 7054 (44.7) | 979 (44.6) | 795 (41.4) | 0.02 | 2654 (49.2) | 470 (50.8) | 460 (49.7) | 0.65 |
| Female | 8728 (55.3) | 1216 (55.4) | 1124 (58.6) | 2742 (50.8) | 455 (49.2) | 465 (50.3) | ||
| Corticosteroid use, No. (%) | ||||||||
| None | 6443 (40.8) | 919 (41.9) | 566 (29.5) | <0.01 | 1543 (28.6) | 253 (27.4) | 148 (16.0) | <0.01 |
| Remote | 3933 (24.9) | 532 (24.2) | 568 (29.6) | 1696 (31.4) | 265 (28.6) | 302 (32.6) | ||
| Recent (≤90 d) | 5409 (34.3) | 744 (33.9) | 785 (40.9) | 2158 (40.0) | 407 (44.0) | 475 (51.4) | ||
| ED visits, No. (%)a | ||||||||
| None | 9967 (63.1) | 1391 (63.4) | 927 (48.3) | <0.01 | 3733 (69.2) | 642 (69.4) | 514 (55.6) | <0.01 |
| 1 to 2 | 5213 (33.0) | 702 (32.0) | 797 (41.5) | 1506 (27.9) | 260 (28.1) | 359 (38.8) | ||
| ≥ 3 | 605 (3.8) | 102 (4.6) | 195 (10.2) | 158 (2.9) | 23 (2.5) | 52 (5.6) | ||
| GI clinic visits, No. (%)a | ||||||||
| None | 5448 (34.5) | 667 (30.4) | 607 (31.6) | <0.01 | 1757 (32.6) | 297 (32.1) | 257 (27.8) | <0.01 |
| 1 to 2 | 5904 (37.4) | 834 (38.0) | 645 (33.6) | 1810 (33.5) | 281 (30.4) | 271 (29.3) | ||
| ≥ 3 | 4433 (28.1) | 694 (31.6) | 667 (34.8) | 1830 (33.9) | 347 (37.5) | 397 (42.9) | ||
| Hospitalizations, No. (%)a | ||||||||
| None | 11,699 (74.1) | 1551 (70.7) | 1151 (60.0) | <0.01 | 4273 (79.2) | 723 (78.2) | 620 (67.0) | <0.01 |
| 1 to 2 | 3587 (22.7) | 560 (25.5) | 620 (32.3) | 1018 (18.9) | 182 (19.7) | 264 (28.5) | ||
| ≥ 3 | 499 (3.2) | 84 (3.8) | 148 (7.7) | 106 (2.0) | 20 (2.2) | 41 (4.4) | ||
| Intestinal surgery, No. (%)a | 673 (4.3) | 150 (6.8) | 101 (5.3) | <0.01 | UTRb | UTRb | UTRb | 0.48 |
| 5-aminosalicylate use, No. (%) | 2275 (14.4) | 329 (15.0) | 246 (12.8) | 0.11 | 1660 (30.8) | 329 (35.6) | 297 (32.1) | 0.01 |
| Immunodulator use, No. (%) | 2286 (14.5) | 334 (15.2) | 247 (12.9) | 0.09 | 872 (16.2) | 165 (17.8) | 133 (14.4) | 0.13 |
| Assessment of disease activity in 6 mo before biologic initiation | ||||||||
| Lower endoscopy | 728 (33.2) | 828 (43.2) | 6439 (40.8) | <0.01 | 360 (38.9) | 484 (52.3) | 2588 (48.0) | <0.01 |
| Calprotectin | 51 (2.3) | 50 (2.6) | 286 (1.8) | 0.02 | 25 (2.7) | 48 (5.2) | 105 (2.0) | <0.01 |
| CTE/MRE | 542 (24.7) | 591 (30.8) | 3253 (20.6) | <0.01 | 109 (11.8) | 184 (19.9) | 626 (11.6) | <0.01 |
Abbreviations: GI, gastroenterology; UTR, unable to report.
aEncounters within 365 days before initiation of biologic therapy.
bContractual data use agreements prohibit reporting data where 1 or more cells are small.
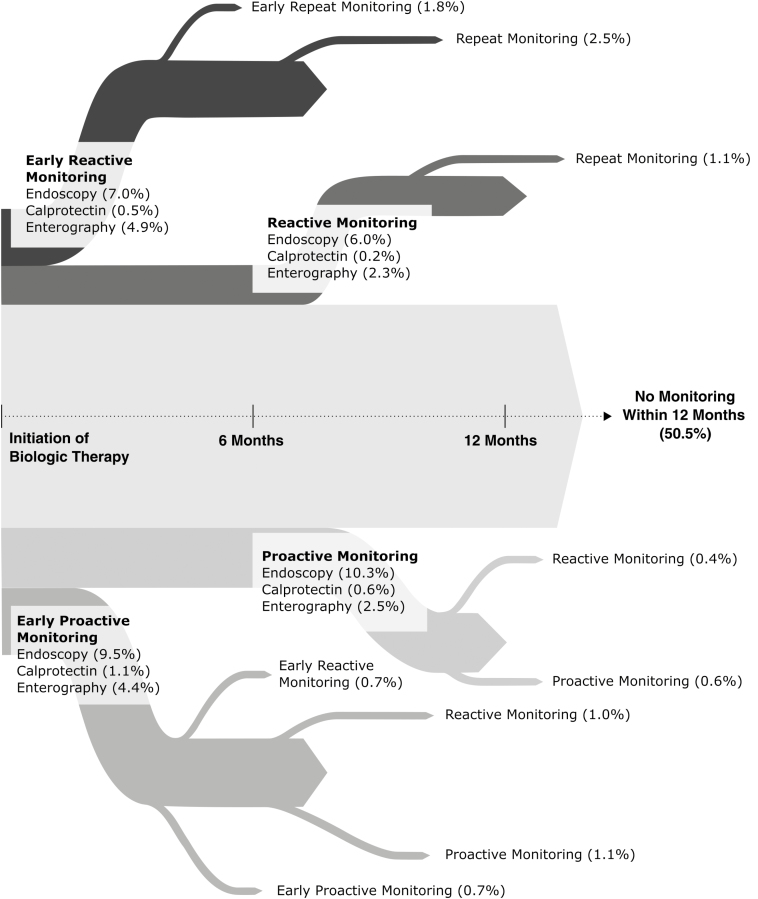
Within the 6 months before starting biologic therapy, an assessment of mucosal disease activity was performed in 64% of CD patients (n = 12,768/19,899) and 63% of UC patients (n = 4529/7247), with the no early monitoring group having undergone an assessment of mucosal disease in the 6 months before biologic initiation more often than the proactive or reactive monitoring group (Table 1). Within 12 months after biologic initiation, 49.5% of patients underwent some form of disease monitoring for the assessment of mucosal inflammation (Fig. 1). Within 24 months after biologic initiation, any form of monitoring for mucosal inflammation (endoscopy, enterography, or FC; proactive or reactive) was performed in only 56.4% of CD patients and 67.8% of UC patients. When monitoring was performed, it was predominately lower endoscopy (87.9%).
Figure 1.
Practice patterns for monitoring of mucosal inflammation after biologic initiation among IBD patients in the United States.
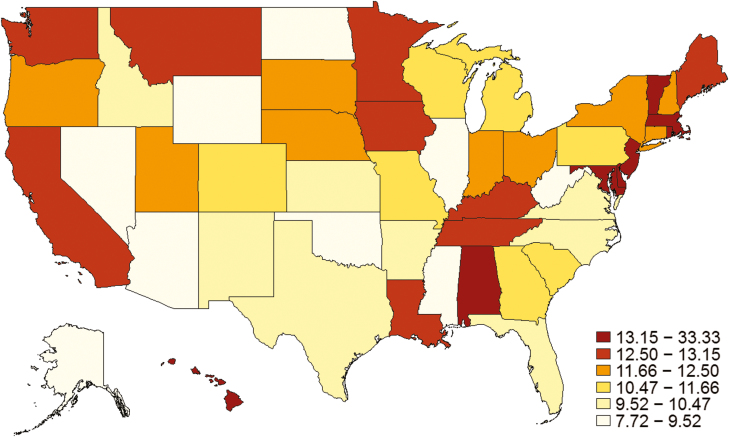
Within the first 6 months after biologic initiation specifically, monitoring of mucosal inflammation was done in 20.5% of CD patients (10.9% proactive, 9.6% reactive) and 25.6% of UC patients (12.8% proactive, 12.8% reactive). Crohn’s disease patients who had no early monitoring were more likely to have recent (within 90 days of biologic initiation) corticosteroid use (40.9% no early monitoring vs 33.9% reactive vs 34.3% proactive, P < 0.01), 3 or more ER visits in the preceding year (10.2% vs 4.6% vs 3.8%, P < 0.01), and more often had 3 or more hospitalizations in the preceding year (7.7% vs 3.8% vs 3.2%, P < 0.01), as compared with patients who underwent early reactive or early proactive monitoring. Ulcerative colitis patients who had no early monitoring were more likely to have recent corticosteroid use (51.4% vs 44% vs 40%, P < 0.01), 3 or more ER visits in the preceding year (5.6% vs 2.5% vs 2.9%, P < 0.01), and more often had 3 or more hospitalizations in the preceding year (4.4% vs 2.2% vs 2.0%, P < 0.01) (Table 1). Significant variation across the United States was observed for early proactive monitoring (P < 0.01) (Fig. 2).
Figure 2.
Geographic distribution of rates of early proactive monitoring practices for IBD. Significant variation across the United States was observed for early proactive monitoring (P < 0.01), and over time there has been a minimal increase of 0.2% per year in proactive monitoring after biologic initiation.
Crohn’s Disease
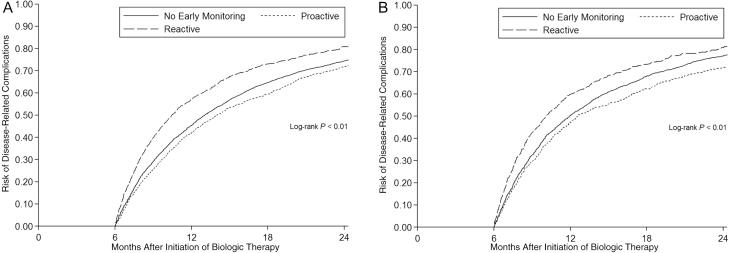
Crohn’s disease patients undergoing early proactive monitoring within 6 months of biologic initiation had a lower overall risk of disease-related complications vs those not undergoing any early monitoring (adjusted hazard ratio [aHR], 0.90; 95% confidence interval [CI], 0.84–0.96; P < 0.01) (Table 2, Fig. 3A). The associated reduction in risk from early proactive monitoring (vs no early monitoring) was mainly due to a reduction in need for corticosteroids (aHR, 0.83; 95% CI, 0.77–0.90; P < 0.01), and the early proactive monitoring group had a 3.1% (95% CI, 2.5%–3.6%; P < 0.01) reduction in corticosteroid exposure over time as compared with the no early monitoring group. Conversely, undergoing early reactive monitoring within 6 months of biologic initiation was associated with an increased risk for development of disease-related complications as compared with no early monitoring (aHR, 1.22; 95% CI, 1.14–1.31; P < 0.01) and as compared with early proactive monitoring (aHR, 1.45; 95% CI, 1.36–1.55; P < 0.01) (Tables 2 and 3). The early reactive monitoring group had a 1% (95% CI, 0.4–1.6; P < 0.01) increase in corticosteroid exposure over time as compared with the no early monitoring group.
Table 2.
Risk of Disease-Related Complications Comparing Patients With Early Proactive, Reactive, and No Monitoring in Entire Cohort
| No Early Monitoring | Early Proactive Monitoring vs No Early Monitoring |
Early Reactive Monitoring vs No Early Monitoring |
Early Reactive Monitoring vs Early Proactive Monitoring | ||||
|---|---|---|---|---|---|---|---|
| IR (95% CI), per 1000 Person-Years | aHR (95% CI) | P | aHR (95% CI) | P | aHR (95% CI) | P | |
| Crohn’s disease | |||||||
| Corticosteroid use | 1.62 (1.56–1.68) | 0.83 (0.77–0.90) | <0.01 | 1.13 (1.05–1.22) | <0.01 | 1.40 (1.30–1.50) | <0.01 |
| Change biologic | 0.95 (0.91–0.99) | 1.01 (0.94–1.09) | 0.81 | 1.02 (0.94–1.10) | 0.61 | 1.03 (0.96–1.10) | 0.40 |
| Hospitalization | 0.67 (0.64–0.70) | 0.95 (0.86–1.05) | 0.35 | 1.59 (1.45–1.74) | <0.01 | 1.83 (1.68–1.99) | <0.01 |
| Surgery | 0.15 (0.13–0.16) | 0.99 (0.81–1.21) | 0.94 | 1.51 (1.26–1.80) | <0.01 | 1.72 (1.46–2.04) | <0.01 |
| Overall | 2.86 (2.77–2.97) | 0.90 (0.84–0.96) | <0.01 | 1.22 (1.14–1.31) | <0.01 | 1.45 (1.36–1.55) | <0.01 |
| Ulcerative colitis | |||||||
| Corticosteroid use | 1.95 (1.85–2.06) | 0.77 (0.69–0.87) | <0.01 | 0.93 (0.84–1.04) | 0.22 | 1.20 (1.06–1.35) | <0.01 |
| Change biologic | 0.87 (0.81–0.92) | 0.94 (0.83–1.06) | 0.33 | 0.77 (0.67–0.88) | <0.01 | 0.83 (0.73–0.94) | <0.01 |
| Hospitalization | 0.54 (0.49–0.59) | 1.10 (0.94–1.31) | 0.24 | 2.12 (1.85–2.44) | <0.01 | 2.19 (1.89–2.54) | <0.01 |
| Surgery | 0.09 (0.06–0.13) | 1.53 (1.00–2.34) | 0.05 | 3.73 (2.68–5.20) | <0.01 | 3.08 (2.14–4.43) | <0.01 |
| Overall | 4.14 (3.81–4.50) | 0.87 (0.78–0.97) | 0.01 | 1.19 (1.07–1.31) | <0.01 | 1.36 (1.23–1.51) | <0.01 |
Comparisons are made between the early proactive group and no early monitoring group and the early reactive monitoring group and no early monitoring group.
Abbreviation: IR, incidence rate.
Figure 3.
Kaplan-Meier survival curves of disease-related complications comparing those who underwent endoscopy or not within 6 months after initiation of biologic therapy. A, Crohn’s disease. B, Ulcerative colitis.
Table 3.
Risk of Disease-Related Complications Comparing Patients With Early Proactive, Reactive, and No Monitoring in Subcohort With 12 Months of Data Between Enrollment and Biologic Initiation and Only Endoscopy-Based Monitoring Used for Assessment of Mucosal Inflammation
| No Early Endoscopy | Early Proactive Endoscopy vs No Early Endoscopy | Early Reactive Endoscopy vs No Early Endoscopy | Early Reactive Endoscopy vs Proactive Endoscopy | ||||
|---|---|---|---|---|---|---|---|
| IR (95% CI), per 100 Person-Years | aHR (95% CI) | P | aHR (95% CI) | P | aHR (95% CI) | aHR (95% CI) | |
| Crohn’s disease | |||||||
| Corticosteroid use | 58.4 (56.2–60.8) | 0.82 (0.75–0.89) | <0.01 | 1.10 (1.01–1.21) | 0.03 | 1.35 (1.20–1.51) | <0.01 |
| Changed biologic | 34.3 (32.9–35.9) | 0.97 (0.89–1.06) | 0.52 | 0.99 (0.90–1.10) | 0.88 | 1.02 (0.90–1.15) | 0.73 |
| Hospitalization | 23.6 (22.3–25.0) | 0.85 (0.75–0.95) | <0.01 | 1.38 (1.23–1.54) | <0.01 | 1.63 (1.40–1.89) | <0.01 |
| Surgery | 5.3 (4.7–5.9) | 0.73 (0.57–0.93) | 0.01 | 1.08 (0.86–1.36) | 0.49 | 1.48 (1.09–2.02) | 0.01 |
| Overall | 103.3 (99.4–107.4) | 0.87 (0.80–0.95) | <0.01 | 1.13 (1.04–1.23) | <0.01 | 1.29 (1.16–1.44) | <0.01 |
| Ulcerative colitis | |||||||
| Corticosteroid use | 72.0 (67.9–76.4) | 0.76 (0.67–0.86) | <0.01 | 0.88 (0.78–1.00) | 0.04 | 1.16 (1.00–1.35) | 0.05 |
| Changed biologic | 31.4 (29.3–33.7) | 0.96 (0.83–1.10) | 0.53 | 0.76 (0.65–0.88) | <0.01 | 0.79 (0.66–0.94) | <0.01 |
| Hospitalization | 19.0 (17.2–20.9) | 1.09 (0.91–1.32) | 0.97 | 2.17 (1.86–2.53) | <0.01 | 1.98 (1.62–2.42) | <0.01 |
| Surgery | 1.9 (1.4–2.5) | 1.79 (1.12–2.87) | 0.02 | 4.20 (2.89–6.11) | <0.01 | 2.34 (1.50–3.67) | <0.01 |
| Overall | 118.1 (111.5–125.1) | 0.86 (0.76–0.96) | <0.01 | 1.13 (1.01–1.26) | 0.04 | 1.32 (1.14–1.51) | <0.01 |
Abbreviation: IR, incidence rate.
These associations were consistent when using a 60- or 180-day window of no events before monitoring (Supplementary Tables 2 and 3) and when excluding steroid events where the end of the prior steroid prescription was within days of the survival start (aHR for composite disease-related complications, 0.89; 95% CI, 0.83–0.95; P < 0.01). The strengths of associations were stronger when limiting the analysis to the subcohort with at least 12 months of data between enrollment and biologic initiation (12-month drug-free period), and only endoscopy-based monitoring was used for follow-up assessment of mucosal inflammation (Table 3). Notably, when limiting the analysis to this subcohort with a more stringent inclusion criterion, we observed an association between early proactive monitoring and reductions in risk for surgery (aHR, 0.70; 95% CI, 0.52–0.94; P = 0.02) and hospitalization (aHR, 0.87; 95% CI, 0.75–1.01; P = 0.06) separately. When compared with CD patients who underwent proactive endoscopic monitoring >12 months after biologic initiation, proactive endoscopy within the first 6 months or months 6–12 after biologic initiation were both associated with reductions in disease-related complications (aHR, 0.84 and 0.77) (Supplementary Tables 4–6).
Ulcerative Colitis
Ulcerative colitis patients undergoing early proactive monitoring within 6 months of biologic initiation had a lower overall risk of disease-related complications vs those not undergoing any early monitoring (aHR, 0.87; 95% CI, 0.78–0.97; P = 0.01) (Table 2, Fig. 3B). The associated reduction in risk from early proactive monitoring (vs no early monitoring) was primarily related to a reduction in need for corticosteroids (aHR, 0.77; 95% CI, 0.69–0.87; P < 0.01), and the early proactive monitoring group had a 4.4% (95% CI, 3.3%–5.4%; P < 0.01) reduction in corticosteroid exposure over time as compared with the no early monitoring group. Notably, early proactive monitoring was associated with an increased risk of surgery vs those not undergoing any early monitoring (aHR, 1.53; 95% CI, 1.00–2.34; P = 0.05). This risk was substantially lower as compared with the risk of surgery associated with early reactive monitoring vs those not undergoing any early monitoring (aHR, 3.73; 95% CI, 2.68–5.20; P < 0.01), and early reactive monitoring was associated with an increased risk for surgery when directly compared with early proactive monitoring (aHR, 3.08; 95% CI, 2.14–4.43; P < 0.01). Furthermore, UC patients undergoing early reactive monitoring were less likely to change biologic agents as compared with the no early monitoring group (aHR, 0.77; 95% CI, 0.67–0.88; P < 0.01) and the proactive monitoring group (aHR, 0.83; 95% CI, 0.73–0.94; P < 0.01), and they had an increased risk of hospitalization as compared with the no early monitoring group (aHR, 2.12; 95% CI, 1.85–2.44; P < 0.01) and the proactive monitoring group (aHR, 2.19; 95% CI, 1.89–2.54) (Table 2). These associations were consistent across sensitivity analyses (Table 3; Supplementary Tables 2 and 3), and there remained an association for reduction in disease-related complications with early proactive monitoring when excluding steroid events where the end of the prior steroid prescription was within days of the survival start (aHR, 0.89; 95% CI, 0.80–0.99; P = 0.01). When compared with UC patients who underwent proactive endoscopic monitoring >12 months after biologic initiation, proactive endoscopy within the first 6 months or months 6–12 after biologic initiation were both associated with reductions in disease-related complications (aHR, 0.78 and 0.81) (Supplementary Tables 4 and 6).
Secular Trends in Monitoring and Outcomes Stratified by Biologics
During the observed study period, there was a minimal increase of 0.2% per year in proactive monitoring (endoscopy, FC, or CTE/MRE) after biologic initiation. We did not observe any difference in associations between proactive monitoring and reductions in steroid utilization when stratified by calendar year (Supplementary Table 7). Results were similar when excluding patients treated with ustekinumab or vedolizumab, given the low utilization of these drugs during study period, and when stratified by infliximab or adalimumab therapy only (Supplementary Tables 8 and 9).
Discussion
In this large real-world cohort of >27,000 patients with IBD initiating biologic therapy, we assessed practice patterns for monitoring of mucosal inflammation and have made several key observations that should be informative for providers, payers, and stakeholders alike. First, approximately 40% of the US IBD population initiating biologic therapy had no assessment of mucosal disease activity within the 6 months before initiating biologic therapy, and nearly half had no assessment of mucosal disease activity within the 12 months after biologic initiation, with significant variation across the United States in the performing early proactive monitoring group. Second, for CD, the use of early proactive monitoring is associated with a lower risk of disease-related complications, namely a reduction in corticosteroid use and risk of surgery. For UC, early proactive monitoring was associated with a reduction in corticosteroid use, but the impact of this strategy on hospitalization or surgery was less clear. Third, the benefits of proactive monitoring with endoscopy appear to be similar when done in the first 6 months vs between 6 and 12 months after biologic initiation. Taken together, early proactive monitoring has a potential beneficial role for offsetting risks of disease-related complications in IBD, particularly for CD, and efforts should focus on broader adoption of this strategy.
The premise of treat-to-target strategies imploring objective assessments of mucosal inflammation is that symptoms are not adequately representative of mucosal inflammation and more objective measurements are needed to accurately guide treatment adjustments. This is most notable in CD, where a substantial disconnect exists between symptomatic activity and mucosal activity.11 In UC, normalization of rectal bleeding and stool frequency appear to be adequate surrogates for mucosal inflammation; however, some degree of discrepancy remains between symptomatic activity and mucosal activity, particularly among UC patients with persistent increased stool frequency.12, 13 In a single-center retrospective cohort study, endoscopic assessment of mucosal disease activity with accompanying adjustments in therapy when persistent mucosal inflammation was observed was associated with increased probabilities for ultimately achieving MH in CD,5 and to a lesser extent in UC.4 In our routine practice cohort of >27,000 IBD patients across the United States, we observed an association between early proactive monitoring of mucosal inflammation (predominately with endoscopy) and reductions in disease-related complications, which appeared to be stronger for CD than UC. Notably, we observed a reduction in risk for corticosteroid use and surgery in CD patients undergoing proactive monitoring, which is broadly aligned with the results of the REACT trial.1 For UC, we observed a reduction in corticosteroid use when using a proactive monitoring strategy; however, the impact on other health outcomes of importance was less clear. There appeared to be an increased risk for surgery with proactive monitoring as compared with no monitoring; however, this increase was half the increase seen when UC patients underwent reactive monitoring. Furthermore, patients in the reactive monitoring group were less likely to change biologics and more likely to be hospitalized, suggesting that disease severity or natural disease history may have influenced or attenuated the observations made for proactive monitoring. A dedicated clinical trial is needed to determine if proactive monitoring of any kind (symptom, biomarker, and/or endoscopy) reduces disease-related complications in UC, akin to observations in CD.
The CALM trial recently demonstrated that interim biomarker-based adjustments lead to higher endoscopic mucosal healing rates in patients with newly diagnosed CD,6 further supporting this rationale for objective measurements of mucosal inflammation to guide treatment decisions for CD. Surprisingly, a substantial proportion of CD patients starting biologic therapy in the United States have no baseline assessment or follow-up assessment of mucosal inflammation (endoscopy, enterography, or FC), suggesting that symptom-based monitoring is still widely used in CD. It is unclear if this overall lack of objective monitoring is a result of variations in provider or patient preferences, burden (for endoscopy or stool collection), reimbursement (particularly for FC), health care infrastructure, and/or uncertainty regarding the value of these strategies. This could nonetheless serve as a quality metric of high-quality health care delivery for CD; similar to adenoma detection rates, which now have national benchmarking standards, follow-up assessments of mucosal disease activity in high-risk CD patients requiring initiation of biologic therapy should be considered for incorporation into societal guidelines for quality metrics.14 Whether follow-up assessments of mucosal disease activity in UC should be considered a quality metric is unclear, given that symptoms are better correlated with disease activity in UC than CD, the lack of association between proactive monitoring and reductions in hospitalization or surgery in our data, and the lack of a randomized trial demonstrating benefit for proactive monitoring in UC.
Our study has several strengths, including the large cohort and routine practice nature of our analyses, propensity matching to account for variations in study populations and drivers of practice patterns, assessment of disease-related complications as composite and individual outcomes, consistency of findings across subgroups and sensitivity analyses, and setting the survival analyses at 6 months after biologic initiation to address the risk of immortal time bias.
Although our study adds to and expands the growing body of literature for treat-to-target monitoring in IBD, it is not without limitations. First, the data source was an administrative data set that did not include more granular data, and the absence of these data precluded deeper propensity score matching for important confounders. This is of potential importance when considering that the observed association for proactive monitoring with reduced disease-related complications was largely driven by steroid use. The performance of proactive endoscopy as defined in our study may therefore simply represent a surrogate for identifying providers who more closely adhere to standard of care and therefore are less likely to prescribe steroids. The groups were observed to be well balanced for measurable confounders related to health care utilization after propensity score matching, which could serve as surrogates for quality of care, but we cannot exclude the potential unleashing of an unmeasurable confounder after propensity score matching that drove this association. Nonetheless, it does still validate the finding of its potential value as a quality metric of high-value care in IBD. Second, the basis for defining “proactive” monitoring was the absence of an event before the test. We were unable to clarify whether the intent to perform monitoring was truly proactive or if it was performed due to the development of mild to moderate symptoms that did not necessitate corticosteroids, change in biologic therapy, hospitalization, or surgery—or if it was performed routinely for some other indication such as routine surveillance. On the other hand, if a significant number of patients with active disease were misclassified as having proactive monitoring, we would have expected a bias toward the null hypothesis; that is, proactive monitoring would not have been associated with a reduction in disease-related complications. Instead, we observed the opposite, and we could detect contrasting effects between those who underwent proactive and reactive monitoring. Third, the follow-up time was limited to 24 months due to constraints in the time span of available data. The effects of early proactive monitoring are therefore unclear beyond 2 years after initiation of biologic therapy; however, it was reassuring to observe that even within this 2-year span we observed an associated reduction in surgery risk for CD with early proactive monitoring, similar to that observed in the 2-year symptom-based treat-to-target monitoring trial REACT.1 Fourth, as this data set is not an inception cohort and patients could have entered the data set with a prior diagnosis of IBD of unclear duration, it is not possible to accurately calculate or capture disease duration, and this was not accounted for in analyses. Therefore, we cannot comment on how these analyses are impacted by disease duration. Finally, despite the large study population, the low utilization of FC limits our ability to comment on biomarker-based monitoring strategies. It is unclear why FC utilization was so low, and further work is required to understand the barriers to its implementation and/or the identification of alternative blood-based biomarkers for monitoring of mucosal inflammation.
In conclusion, in this large real-world study, we observed that a substantial proportion of IBD patients initiating biologic therapy have no assessment of mucosal inflammation within 6 months before biologic initiation or up to 24 months after initiation. Furthermore, performing early proactive monitoring within 6 months of biologic initiation was associated with a reduction in disease-related complications as compared with no early monitoring, primarily driven by a reduction in corticosteroid use, and this impact was most notable for CD. There was considerable variability across the United States in performing any form of proactive monitoring after biologic initiation. This wide variability has direct implications for population-level outcomes, and consideration will be needed regarding whether documented follow-up assessments of mucosal disease activity after biologic initiation should be considered as quality metrics of high-quality health care delivery for at-risk IBD patients who are escalated to biologic therapy.
Supplementary Material
Supported by: Access to the Truven Health MarketScan Commercial Database was provided by the Stanford Center for Population Health Sciences (PHS) Data Core. The PHS Data Core is supported by a National Institutes of Health (NIH) National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and internal Stanford funding.
Conflicts of interest: Dr. Singh is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K23DK117058, the American College of Gastroenterology Junior Faculty Development Award, and the Crohn’s and Colitis Foundation Career Development Award (#404614). He has received research grants from Pfizer and AbbVie and consulting fees from AbbVie, Takeda, Pfizer, and AMAG Pharmaceuticals. Dr. Jairath has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena Pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, and Celltrion; speaker’s fees from Takeda, Janssen, Shire, Ferring, Abbvie, and Pfizer. Dr. Sandborn reports research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, and Celgene/Receptos; consulting fees from Abbvie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Conatus, Cosmo, Escalier Biosciences, Ferring, Genentech, Gilead, Janssen, Lilly, Miraca Life Sciences, Nivalis Therapeutics, Novartis Nutrition Science Partners, Oppilan Pharma, Otsuka, Paul Hastings, Pfizer, Precision IBD, Progenity, Prometheus Laboratories, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust or HART), Salix, Shire, Seres Therapeutics, Sigmoid Biotechnologies, Takeda, Tigenix, Tillotts Pharma, UCB Pharma, and Vivelix; and stock options from Ritter Pharmaceuticals, Oppilan Pharma, Escalier Biosciences, Precision IBD, and Progenity. Dr. Dulai has received grant support from Takeda, Pfizer, Janssen, Prometheus, Polymedco, and ALPCO; has served as a consultant for Takeda, Janssen, and Abbvie; and has received speaker honoraria from Takeda.
Author contributions: B.N.L. participated in the study concept and design, data analysis, data interpretation, drafting of the manuscript, critical revision, and final approval of the manuscript. S.S., W.J.S., and P.S.D. participated in the study concept and design, data analysis, data interpretation, critical revision, and final approval of the manuscript. P.S.D. provided study supervision.
References
- 1. Khanna R, Bressler B, Levesque BG, et al. ; REACT Study Investigators Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386:1825–1834. [DOI] [PubMed] [Google Scholar]
- 2. Dulai PS, Siegel CA, Colombel JF, et al. Systematic review: monotherapy with antitumour necrosis factor α agents versus combination therapy with an immunosuppressive for IBD. Gut. 2014;63:1843–1853. [DOI] [PubMed] [Google Scholar]
- 3. Dulai PS, Levesque BG, Feagan BG, et al. Assessment of mucosal healing in inflammatory bowel disease: review. Gastrointest Endosc. 2015;82:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouguen G, Levesque BG, Pola S, et al. Feasibility of endoscopic assessment and treating to target to achieve mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2014;20:231–239. [DOI] [PubMed] [Google Scholar]
- 5. Bouguen G, Levesque BG, Pola S, et al. Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:978–985. [DOI] [PubMed] [Google Scholar]
- 6. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 7. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 8. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. [DOI] [PubMed] [Google Scholar]
- 9. Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685–698. [DOI] [PubMed] [Google Scholar]
- 11. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- 12. Narula N, Alshahrani AA, Yuan Y, et al. Patient-reported outcomes and endoscopic appearance of ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17:411–418.e3. [DOI] [PubMed] [Google Scholar]
- 13. Colombel JF, Keir ME, Scherl A, et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut. 2017;66:2063–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dulai PS, Singh S, Ohno-Machado L, et al. Population health management for inflammatory bowel disease. Gastroenterology. 2018;154:37–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.