Abstract
Background
Children with very early onset inflammatory bowel diseases (VEO-IBD) often have a refractory and severe disease course. A significant number of described VEO-IBD-causing monogenic disorders can be attributed to defects in immune-related genes. The diagnosis of the underlying primary immunodeficiency (PID) often has critical implications for the treatment of patients with IBD-like phenotypes.
Methods
To identify the molecular etiology in 5 patients from 3 unrelated kindred with IBD-like symptoms, we conducted whole exome sequencing. Immune workup confirmed an underlying PID.
Results
Whole exome sequencing revealed 3 novel CARMIL2 loss-of-function mutations in our patients. Immunophenotyping of peripheral blood mononuclear cells showed reduction of regulatory and effector memory T cells and impaired B cell class switching. The T cell proliferation and activation assays confirmed defective responses to CD28 costimulation, consistent with CARMIL2 deficiency.
Conclusion
Our study highlights that human CARMIL2 deficiency can manifest with IBD-like symptoms. This example illustrates that early diagnosis of underlying PID is crucial for the treatment and prognosis of children with VEO-IBD.
Keywords: immunodeficiency, very early onset inflammatory bowel diseases, CARMIL2
Patients with inherited CARMIL2 deficiency can present with pediatric inflammatory bowel disease. Early diagnosis of the underlying primary immunodeficiency has critical implications for the clinical management of affected children.
INTRODUCTION
Children with very early onset inflammatory bowel diseases (VEO-IBD) often show a severe—and sometimes life-threatening—course refractory to conventional treatment. The diagnosis and management of VEO-IBD is challenging, because patients show a broad phenotypic spectrum and cannot be categorized based on routine clinical and histological assessment. Advances in next-generation sequencing have facilitated characterization of monogenic disorders altering intestinal homeostasis through multiple mechanisms including epithelial barrier dysfunctions, T and B lymphocyte abnormalities, neutropenia and defects of phagocyte bacterial killing, hyper- and autoinflammatory disorders, and defects in intestinal innervation.1 Deciphering the molecular pathomechanisms of VEO-IBD is critical for the medical management and prognosis as it can provide rational arguments for or against unconventional biological treatment options, surgery, or hematopoietic stem cell transplantation (HSCT). Moreover, the knowledge of the molecular mechanisms of the disease is the basis for the development of innovative, personalized treatment strategies.
Primary immunodeficiencies (PID) are a heterogeneous group of disorders that can manifest with variable phenotypes.2 In addition to increased susceptibility to infections, immunodeficient patients are predisposed to allergies, inflammation, autoimmunity, lymphoproliferation, and malignancy.3 PID can also manifest with gastrointestinal symptoms and mimic IBD-like phenotypes. Notably, the majority of published monogenic disorders in patients with VEO-IBD are caused by defects in PID-related genes, as evidenced by IL-10R,4 XIAP,5 and NADPH oxidase6 deficiencies.
The cytosolic capping protein, Arp2/3 and myosin-I linker protein 2 (CARMIL2), also known as RLTPR, has been reported to regulate cytoskeletal organization, endocytosis, and cell migration by controlling actin polymerization dynamics.7 Using a mouse N-ethyl-N-nitrosourea–mutagenesis screen, Liang et al have demonstrated that CARMIL2 is essential for costimulation of T cells via CD28 and development of regulatory T cells (Tregs).8 The critical importance of CARMIL2 for human immune homeostasis has been recently evidenced by the identification of children with germline loss-of-function mutations in CARMIL2. The patients manifested with variable immune-related phenotypes in early childhood characterized by bacterial, viral, and fungal infections (eg, invasive mycobacterial diseases, mucocutaneous candidiasis, warts, molluscum contagiosum, dermatitis, and esophagitis), cutaneous and pulmonary allergy, and/or EBV-positive disseminated smooth muscle tumors.9–12 Reduced numbers of Tregs and impaired differentiation towards effector CD4+ T cells have been observed.
In this study, we report 5 patients from 3 unrelated kindred with novel loss-of-function mutations in CARMIL2 and IBD-like phenotypes. Our study reinforces the knowledge of immunological pathomechanisms and expands the clinical spectrum of CARMIL2 deficiency, highlighting the fact that it should be considered as a molecular cause in children with VEO-IBD.
MATERIAL AND METHODS
DNA Sequencing
Genomic DNA was isolated from patients’ and family members’ peripheral blood using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) following manufacturer’s instructions. For whole exome sequencing analysis, exomes were enriched by employing Agilent Exome enrichment or Ampliseq exome panel, and sequencing was conducted on Illumina platforms. To detect rare sequence variants following Mendelian inheritance patterns, we used bioinformatic algorithms as described previously.13, 14 Polymerase chain reaction (PCR) amplification of CARMIL2 sequence variants was performed using specific primers.
Cell Culture and Stimulation
Peripheral blood mononuclear cells (PBMC) were isolated from patients, first-degree relatives, and healthy donors by density gradient centrifugation using Ficoll-Hypaque (Pharmacia, New Jersey, USA). PBMC were maintained in Roswell Park Memorial Institute 1640 supplemented with 2 mM of glutamine, 100 U/mL of penicillin, 100 µg/mL of streptomycin, and 10% FCS (Thermo Fisher Scientific, USA) in a 5% CO2 incubator at 37°C. For immunoblot analysis, T lymphoblasts were generated from PBMC by stimulation with 5 ng/mL PMA (phorbol 12-myristate 13-acetate), 1 µM of ionomycin (Sigma Aldrich, St. Louis, MO, USA), and 100 U/ml IL-2 (Novartis, Basel, Schweiz) for 2 days followed by expansion for 8 days in complete medium supplemented with 100 U/mL of IL-2. For T cell activation and proliferation experiments, isolated PBMC were labeled with 2.5 µM of carboxyfluorescein diacetate succinimidyl ester (CFSE, Thermo Fisher Scientific, Waltham, MA, USA) and stimulated with anti-CD3-coupled beads (Biotin-anti-CD3, OKT3, Thermo Fisher Scientific; coupled with anti-Biotin MACSiBeads, Miltenyi Biotec, Bergisch Gladbach, Germany) at a ratio of 5:1 in the absence or presence of 1 µg/mL of soluble anti-CD28 (Bio-anti-CD28.2, Thermo Fisher Scientific) or with 0.5 ng/mL of phorbol 12-myristate 13-acetate (PMA) and 1 µM of ionomycin (Sigma-Aldrich) in round-bottom 96-well plates at a density of 105 cells per well.
Immunoblotting
Whole cell lysates were prepared from T lymphoblasts and separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-CARMIL2 (HPA041402, 1:1000, Sigma Aldrich), anti-Actin (sc-8432, 1:1000, Santa Cruz, Dallas, USA), anti-rabbit HRP-conjugated secondary antibody (#7074, 1:3000, Cell Signaling, Danvers, USA) and goat anti-mouse IgG-HRP (sc-2005, 1:10,000, Santa Cruz, USA).
Immunophenotyping
For immunophenotyping of PBMC, blood samples were washed with PBS, stained with fluorochrome-labeled antibodies in BD Brillant stain buffer (BD Bioscience, Franklin Lakes, New Jersey, USA), and lysed with 1× BD FACS Lysing Solution, as previously reported.13 Treg cells were surface stained with APC-H7-anti-CD3 (SK7, 1:50), APC-anti-CD4 (SK3, 1:100), PE-anti-CD25 (M-A251, 1:50) (all BD Biosciences), and FITC-anti-CD127 (eBioRDR5, 1:50, Thermo Fisher Scientific). Intracellular staining of FOXP3 was done with PerCP-Cy5.5-anti-FOXP3 (PCH101, 1:25) using the FOXP3 staining kit (Thermo Fisher Scientific).
Samples were acquired on a CantoII or LSRFortessa Flow Cytometer (BD Biosciences). Data analysis was performed with FlowJo software (TreeStar, Ashland, USA).
T Cell Assays
T cell assays were conducted as previously reported.10 Activation of CD4+ and CD8+ T cells was measured by labelling PBMC with APC-H7-anti-CD3 (SK7, 1:50), APC-anti-CD4, PacB-anti-CD8 (SK1, 1:200), PE-anti-CD25 (M-A251, 1:50) (all BD), and APC-Cy7-CD69 (FN50, 1:50, Biolegend) 2 days after stimulation. Proliferative responses of CD4+ and CD8+ T cells was measured in CFSE dilution assays by labelling PBMC with APC-H7-anti-CD3 (SK7, 1:50), APC-anti-CD4, PacB-anti-CD8 (SK1, 1:200), PE-anti-CD25 (M-A251, 1:50) (all BD), and 7-aminoactinomycin D (7-AAD, 0.5 µg/mL, BD) 5 days after stimulation. Samples were analyzed using a CantoII flow cytometer (BD) and FlowJo software (TreeStar).
RNA Scope Duplex in Situ Hybridization
To assess FOXP3 expression in intestinal tissue of CARMIL2-deficient patients, we performed in situ hybridization with the RNAscope 2.5 Duplex Detection Kit (Chromogenic; Advanced Cell Diagnostics) according to manufacturer’s protocol. Formalin-fixed and paraffin-embedded intestinal samples from CARMIL2-deficient patients were compared with subjects without IBD (control) or with active Crohn’s disease (undefined VEO-IBD). Samples were cut into 10 µm-thick sections and stained per the manufacturer’s protocol with reagents and probes from Advanced Cell Diagnostics. Following deparaffinization and pretreatment (hydrogen peroxide, target retrieval), slides were hybridized with probes specific for CD4 (C1 channel, blue) and FOXP3 (C2 channel, red). After amplification and signal detection, the slides were counterstained with hematoxylin and evaluated under 40× magnification using an Olympus BX41 microscope.
Data Availability
The identified CARMIL2 sequence variants will be submitted to the ClinVar database (www.ncbi.nlm.nih.gov/clinvar/; 08 May 2019, date last accessed) upon publication. Exome sequencing data will not be published to protect privacy of affected families.
Statistical Analysis
Data were analyzed with the software environment R (version 3.5.1) for statistical computing and graphics. Significances between 2 groups were calculated with the 2-sided unequal variances Welch t test. All P values <0.05 were considered significant.
Ethical Considerations
Patients were originally identified by the National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russia (P1 and P2), the Bai Jerbai Wadia Children Hospital, Mumbai, India (P3), and the Erciyes University, Kayseri, Turkey (P4 and P5). The affected children were referred for genetic analysis to the University Hospital, Ludwig Maximilian University (LMU) of Munich. Clinical information and biospecimens from patients, unaffected family members, and healthy donors were obtained upon written consent. The study protocol was approved by the Institutional Review Board at the LMU and the investigations were conducted in accordance with the current ethical and legal framework.
RESULTS
Five patients from 3 unrelated families with IBD-like symptoms presenting during early childhood were referred for genetic analysis due to suspected underlying primary immunodeficiency.
All patients presented with pancolitis associated with failure to thrive, abdominal pain, and mucous, non-bloody, or bloody diarrhea. Perianal disease was observed in P4 and P5. Histopathological examination of colon biopsies from patients P1, P2, and P3 revealed unspecific inflammatory and regenerative changes (Fig. 1A). While the morphological picture in P1 was suggestive of an infectious etiology, P2 and P3 showed morphological features reminiscent of ulcerative colitis. Disease was refractory despite anti-inflammatory or immunosuppressive medication (eg, sulfasalazine, mesalazine, steroids, azathioprine, infliximab). Patient 1 required a colectomy at 9 years of age, and P4 and P5 underwent surgery due to duodenal stenosis at the age of 6 and 4 years, respectively. Notably, all patients suffered from recurrent infections (eg, pneumonia, otitis media) and oral aphthous lesions. Furthermore, P1 and P2 showed dermatitis, and P4 suffered from psoriasis. Patient 5 died due to septic complications at the age of 4 years. Demographics and clinical details of individual patients are described in Table 1.
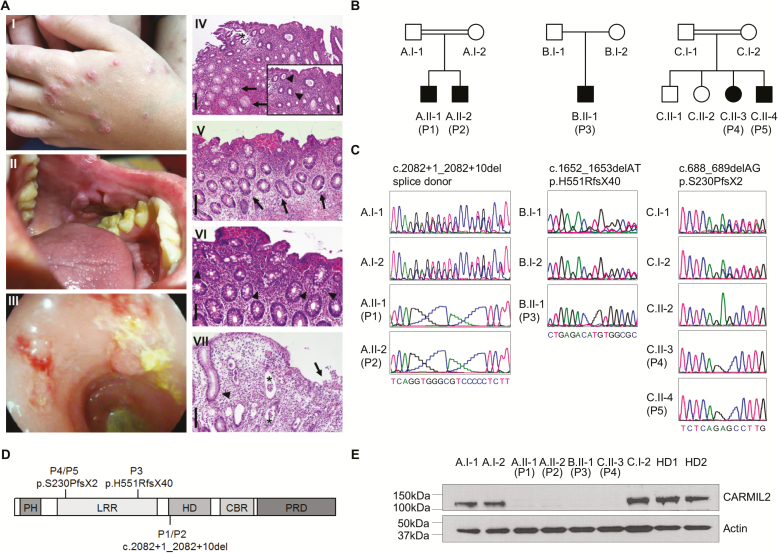
FIGURE 1.
Identification of patients with IBD-like phenotypes and novel loss-of-function mutations in CARMIL2. A, The index patient P2 presented with nodular dermatitis (I), oral aphthous lesions (II), and pancolitis with edematous colonic mucosa with loss of vascularity, ulcerations, fresh bleeding, and pseudopolypoid lesions (III). Histopathological analysis of colonic biopsies from P1, P2, and P3 confirmed active colitis: P1 displayed a moderately active colitis with granulocytic accentuation in the upper third of the mucosa and the surface epithelial layer (inlay; arrowheads) and mucoid metaplasia (arrows) (IV). The asterisk indicates an artificial defect. P2 showed a mildly chronic active colitis with increased epithelial regeneration (arrows, V) and marked increase of eosinophilic granulocytes (arrowheads, VI). P3 displayed a moderately chronic and highly active colitis with mucosal edema and ulceration (arrow), the formation of numerous crypt abscesses (astericks) and regenerative crypt hyperplasia (arrowhead) (VII). Scale bars indicate 200 µm in (IV, V, VII) and 100 µm in (VI, inlay of IV), respectively. B, Pedigrees of 5 patients from 3 unrelated kindred (A–C) with IBD-like symptoms (P1–P5). C, DNA Sanger sequencing confirmed biallelic CARMIL2 mutations. D, Schematic representation of CARMIL2 and localization of the identified mutations. Abbreviations: PH, pleckstrin homology domain; LRR, leucine-rich repeat; HD, homodimerization domain; CBR, capping protein binding region; PRD, proline-rich region. E, Immunoblotting of CARMIL2 protein expression in T lymphoblasts derived from healthy donors (HD), parents, and patients. Actin was used as loading control. Data are representative for 3 experiments.
TABLE 1.
Demographic, Genetic, and Clinical Data of CARMIL2-Deficient Patients
| P1 | P2 | P3 | P4 | P5 | |
|---|---|---|---|---|---|
| Demographics and Genetics | |||||
| Mutation | c.2082 + 1_2082 + 10 delGTGGGCGTCC | c.2082 + 1_2082 + 10 delGTGGGCGTCC | c.1652_1653delAT, p.His551ArgfsTer40 | c.688_689delAG p.Ser230ProfsTer2 | c.688_689delAG p.Ser230ProfsTer2 |
| Consanguinity | yes | yes | not reported | yes | yes |
| Ethnicity/country | Arab/Russia | Arab/Russia | South Asian/India | Turkish/Turkey | Turkish/Turkey |
| Sex | male | male | male | female | male |
| Age of onset/ diagnosis | 6 mo/3 yrs | 7 mo/9 yrs | 5 yrs/9 yrs | 6 yrs/14 yrs | 4.5 yrs/post mortem |
| Clinical presentation | |||||
| Gastrointestinal manifestations | |||||
| Colitis | pancolitis | pancolitis | pancolitis | unknown | pancolitis |
| Growth failure | + | + | + | + | + |
| Abdominal pain | + | + | + | + | + |
| Diarrhea | non-bloody, bloody | non-bloody, bloody | mucous, non-bloody | mucous, non-bloody | mucous, bloody |
| Oral lesions | aphthous lesions | aphthous lesions | aphthous lesions | aphthous lesions | aphthous lesions |
| Perianal disease | - | - | - | + | + |
| Others | gastritis, duodenitis | duodenitis, jejunitis | esophageal webs | duodenal stenosis | Candida esophagitis, ileoileal invagination, duodenal stenosis |
| Extraintestinal manifestations | |||||
| Recurrent infections | pneumonia (eg CMV), Salmonellosis | sinusitis, pneumonia, oral candidiasis | Candidiasis (oral, penile) | sinusitis, upper and lower respiratory tract infections | sinusitis, upper and lower respiratory tract infections, Candida infections (urine tract, skin), sepsis |
| Skin | dermatitis (>12 mo) | dermatitis (2 yrs) | — | severe generalized skin and nail psoriasis | — |
| Others | chorioretinitis | asthma (4 yrs) | hypothyroidism (TPO-Ab neg, 8 yrs) | psoriatic arthritis | — |
| Treatment | |||||
| Nutrition | elimination diet | elimination diet | exclusive enteral nutrition | — | parenteral (p) |
| Medication | antibiotics (p), azathioprine (n), infliximab (n), mesalazine (n), steroids (p) | abatacept (p), azathioprine (p), infliximab (p), mesalazine (n), steroids (p) | azathioprine (p), steroids (p), sulfasalazine (p) | topical steroids | azathioprine (p), mesalazine (p), metronidazole (p), steroids (p) |
| Surgery | colectomy (9 yrs) | — | — | duodenal stenosis (6 yrs 5 mo) | duodenal stenosis (4 yrs 2 mo) |
| Outcome | |||||
| alive | alive | alive | alive | dead (pulmonary infection, sepsis, bilateral pneumothorax, respiratory distress at the age of 4 yrs 9 mo) |
Abbreviations: g, good response; mo, months; n, no response; p, partial response; yrs, years.
To decipher the molecular etiology in our 5 patients, we conducted whole exome sequencing as part of a larger study by the VEO-IBD Consortium. Our genetic screen unraveled novel biallelic mutations in CARMIL2 that have not been reported with a homozygous genotype in the gnomAD database15 and were predicted to be deleterious (frameshift, splice acceptor, splice donor). Sanger sequencing showed segregation of the mutations with the disease phenotype in available first-degree relatives (Fig. 1B–D). Immunoblotting revealed abrogated CARMIL2 protein expression in P1, P2, P3, and P4, confirming that the 3 newly identified mutations have deleterious effects (Fig. 1E).
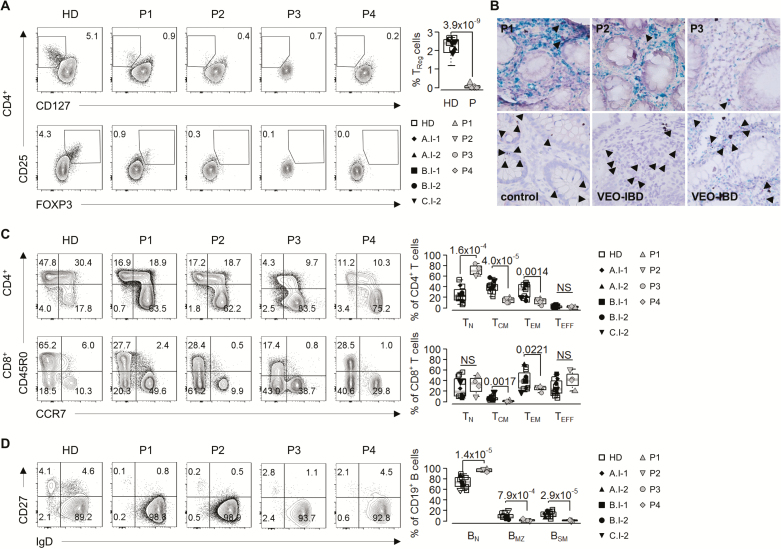
Immune evaluation revealed normal numbers of CD3+ T, CD19+ B, and CD16+CD56+ NK cells. Detailed immunophenotyping of peripheral blood mononuclear cells (PBMC) from P1, P2, P3, and P4 showed substantial reduction of peripheral blood CD127lowCD25+ or FOXP3+CD25+ Tregs (Fig. 2A). Correspondingly, RNAscope analysis revealed a reduced frequency of CD4+FOXP3+ cells in available intestinal biopsies from P1, P2, and P3 as compared with individuals without colonic inflammation (control) or unrelated patients with undefined VEO-IBD (Fig. 2B). In addition to Treg defects, our patients exhibited significant reduced levels of CD45R0+CCR7+ central memory (TCM) and CD45R0+CCR7-effector memory (TEM) CD4+ helper and CD8+ cytotoxic T cells (Fig. 2C). Moreover, our patients had increased CD19+ naïve (BN) but reduced IgD+CD27+ marginal zone (BMZ) and IgD-CD27+ switched memory (BSM) B cells (Fig. 2D), indicating defective B cell maturation.
FIGURE 2.
Impaired regulatory and effector T cell development and defective B cell maturation in patients with CARMIL2 deficiency. A, Representative contour plots of CD4+CD25+CD127low and CD4+CD25+FOXP3+ Treg cell percentages in PBMC from healthy donors (HD) and patients (P1, P2, P3, P4) (left panel). Graphical representation of CD4+CD25+CD127lowFOXP3+ Treg cell percentages (right panel) of 3 independent experiments with individual data points from five HD, parents, and patients. B, Representative RNAscope duplex in situ hybridization of CD4 (green, blue) and FOXP3 (red, arrowheads) in formalin-fixed paraffin-embedded colonic sections from CARMIL2-deficient patients (P1, P2, and P3), patients without IBD (control), and unrelated patients with undefined VEO-IBD. Images are 40X. C, Contour plots of CD45R0-CCR7+ naive (TN), CD45R0+CCR7+ central memory (TCM), CD45R0+CCR7-effector memory (TEM), and CD45R0-CCR7-effector (TE) CD4+ and CD8+ T cells from healthy donors (HD) and patients (P1, P2, P3, P4) (left panel). Graphs indicate CD4+ and CD8+ T cell subtypes for 6 different HD, parents and patients (right panel). D, Contour plots of IgD+CD27-naïve (BN), IgD+CD27+ marginal zone (BMZ), and IgDCD27+ switched memory (BSM) CD19+ B cells from healthy donors (HD) and patients (left panel). Graphs show CD19+ B cell subtypes for 6 different HD, parents, and patients (right panel). HD (white square), A.I-1 (black rhomb), A.I-2 (black up-pointing triangle), B.I-1 (black square), B.I-2 (black circle), C.I-2 (black down-pointing triangle), P1 (gray up-pointing triangle), P2 (gray down-pointing triangle), P3 (gray circle), and P4 (gray rhomb).
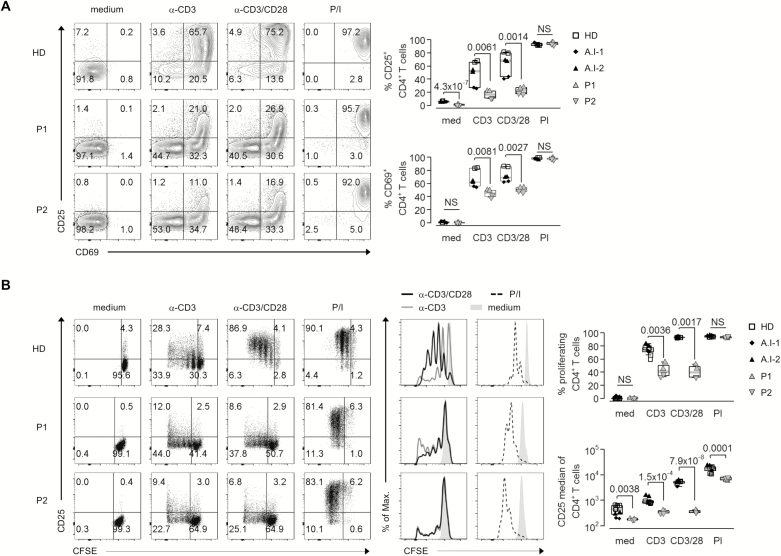
To confirm CD28-dependent functional defects of T cell activation and proliferation in CARMIL2-deficient patients, we stimulated patient PBMC with anti-CD3-coupled beads alone or in combination with soluble anti-CD28. Activation of T cells was determined by flow cytometric analysis of CD25 and CD69 upregulation 2 days after stimulation, and proliferative response was measured by CFSE dilution 5 days upon stimulation. Patient CD4+ and CD8+ T cells showed abrogated induction of CD25 and CD69 after anti-CD3 ± anti-CD28 stimulation as compared with T cells from healthy donors (HD) and parents (Fig. 3A and Supplementary Fig. S1A). Correspondingly, we observed a defective T cell proliferation in response to anti-CD3 ± anti-CD28 (Fig. 3B and Supplementary Fig. S1B). The response of patient T cells upon PMA/ionomycin stimulation, bypassing proximal TCR-mediated signaling, was comparable with HD.
FIGURE 3.
Defective proliferation and activation of CARMIL2-deficient T cells upon CD28 costimulation. A, Flow cytometric analysis of CD4+ T cell activation in PBMC from HD, P1, and P2 based on CD25 and CD69 surface expression without (medium) and 2d after stimulation with anti-CD3 coupled beads (α-CD3), anti-CD3 coupled beads plus soluble anti-CD28 (αCD3/CD28), or PMA/ionomycin (P/I) (left panel). B, Dot plots and overlay histogram plots of CFSE dilution and CD25 expression for CD4+ T cells in PBMC from HD, P1, and P2 without (medium) and after stimulation for 5d with anti-CD3 coupled beads (α-CD3), anti-CD3 coupled beads plus soluble anti-CD28 (αCD3/CD28), or PMA/ionomycin (P/I) (left panel). Graphs showing activated CD4+ T cells (A) and proliferated CD4+ T cells and median of CD25 expression (B) in HD (white square), A.I-1 (black rhomb), A.I-2 (black up-pointing triangle), P1 (gray up-pointing triangle), and P2 (gray down-pointing triangle), n = 2 (right panels).
Taken together, immune workup showed that our patients with an IBD-like phenotype and CARMIL2 deficiency present with dual T and B dysfunctions characterized by reduction of Tregs, impaired effector T and B cell maturation, and defective T cell responses to CD28 costimulation.
DISCUSSION
The nonredundant role of human CARMIL2 has been recently highlighted by the characterization of germline loss-of-function mutations in patients with primary immunodeficiency.9–12 The reported cases showed a broad spectrum of immune-related phenotypes. Our study expands the clinical spectrum and demonstrates that CARMIL2 deficiency can also present with IBD-like phenotypes. This information is critical for the clinical management of identified VEO-IBD patients.
IBD is a complex, multifactorial disorder, and the exact pathogenesis still remains unclear. Both autoimmune and immune-mediated phenomena have been described. Whereas immune phenomena include abnormalities of humoral and cell-mediated immunity, the presence of autoantibodies against intestinal epithelial cells, immune cells, and/or microbial flora as well as activationof autoreactive immune cells can be detected in autoimmunity.16 Tregs play a critical role in maintaining immune homeostasis and tolerance,17 and Treg deficiencies have been shown to result in severe multi-organ autoimmunity and intestinal inflammation.18–20 Previously reported CARMIL2-deficient patients show a profound reduction of FOXP3+ Tregs, but no evidence of organ-specific or life-threatening autoimmune phenotypes in contrast to patients with inherited defects of Treg numbers and/or function caused by FOXP318, 20 (immunodysregulation polyendocrinopathy enteropathy X-linked syndrome), LRBA,21, 22 or CTLA423, 24 deficiencies. The absence of severe autoimmune phenotypes in CARMIL2 deficiency might be partially explained by defective memory T cell differentiation and CD28-mediated effector T cell activation. Correspondingly, CD28-deficient mice were protected from autoimmunity in experimental models.25–27 Our newly identified CARMIL2-deficient patients also did not present with severe autoimmune phenotypes, and thus, it is tempting to speculate that the IBD symptoms are rather secondary caused by immune dysregulation or triggered by infections. Further studies are required to (1) define genotype-phenotype correlations, (2) investigate functions of CARMIL2 in other immune and epithelial cell types, and (3) assess the underlying molecular pathomechanisms of intestinal inflammation in patients with loss-of-function of CARMIL2.
The identification of life-threatening CARMIL2 deficiency has critical implications for the clinical care of patients with VEO-IBD. Even though no targeted treatment for CARMIL2 deficiency has yet been established, the knowledge of an underlying immunodeficiency can provide rational arguments for preventing surgery (eg colectomy) and using unconventional treatments. Allogeneic HSCT has been used to treat a broad range of PID and shown to be effective in patients suffering from VEO-IBD on the basis of an underlying PID, as exemplified by IL-10R deficiency.28 Even though it cannot be ruled out that CARMIL2 has a critical role in epithelial cells, mouse and human studies suggest that the inflammation is caused by immune cell dysfunction, and thus HSCT might be considered as treatment option in our patients. Long-term follow-up studies are required to determine efficacy, risk, and prognosis of HSCT in CARMIL2-deficient patients.
Taken together, we show that CARMIL2 deficiency can manifest with IBD-like symptoms. Thus, our study highlights the critical role of CARMIL2-mediated immunity in regulating intestinal homeostasis, providing another critical example that PID can present with phenotypic characteristics of VEO-IBD and should be considered as molecular causes. Early diagnosis of CARMIL2 deficiency in VEO-IBD patients is critical to prevent fatal complications.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients and families for participating in the study and the interdisciplinary medical teams. Moreover, they thank Dr. Liza Konnikova for providing protocols for RNAscope analysis and Dr. Veysel Gök for providing clinical information on P4 and P5. Scott B. Snapper is supported by the NIH Grants P30 DK034854, and AI50950, The Leona M. and Harry B. Helmsley Charitable Trust, and the Wolpow Family Chair in IBD Treatment and Research. The authors declare no competing interests.
Supported by The Leona M. and Harry B. Helmsley Charitable Trust, the Collaborative Research Consortium SFB1054 project A05 (DFG), PID-NET (BMBF), the Wilhelm Sander-Foundation, German Centre for Infection Research (DZIF), and the Care-for-Rare Foundation. Daniel Kotlarz has been a scholar of the Daimler und Benz Stiftung, Reinhard-Frank Stiftung, and Else-Kröner-Fresenius-Stiftung.
REFERENCES
- 1. Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut. 2013;62:1795–1805. [DOI] [PubMed] [Google Scholar]
- 2. Bousfiha A, Jeddane L, Picard C, et al. . The 2017 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2018;38:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fischer A. Human primary immunodeficiency diseases: a perspective. Nat Immunol. 2004;5:23–30. [DOI] [PubMed] [Google Scholar]
- 4. Glocker EO, Kotlarz D, Boztug K, et al. . Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pachlopnik Schmid J, Canioni D, Moshous D, et al. . Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency). Blood. 2011;117:1522–1529. [DOI] [PubMed] [Google Scholar]
- 6. Dhillon SS, Fattouh R, Elkadri A, et al. . Variants in nicotinamide adenine dinucleotide phosphate oxidase complex components determine susceptibility to very early onset inflammatory bowel disease. Gastroenterology. 2014;147:680–689.e2. [DOI] [PubMed] [Google Scholar]
- 7. Edwards M, Zwolak A, Schafer DA, et al. . Capping protein regulators fine-tune actin assembly dynamics. Nat Rev Mol Cell Biol. 2014;15:677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang Y, Cucchetti M, Roncagalli R, et al. . The lymphoid lineage-specific actin-uncapping protein Rltpr is essential for costimulation via CD28 and the development of regulatory T cells. Nat Immunol. 2013;14:858–866. [DOI] [PubMed] [Google Scholar]
- 9. Alazami AM, Al-Helale M, Alhissi S, et al. . Novel CARMIL2 mutations in patients with variable clinical dermatitis, infections, and combined immunodeficiency. Front Immunol. 2018;9:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schober T, Magg T, Laschinger M, et al. . A human immunodeficiency syndrome caused by mutations in CARMIL2. Nat Commun. 2017;8:14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorte HS, Osnes LT, Fevang B, et al. . A potential founder variant in CARMIL2/RLTPR in three Norwegian families with warts, molluscum contagiosum, and T-cell dysfunction. Mol Genet Genomic Med. 2016;4:604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Ma CS, Ling Y, et al. . Dual T cell- and B cell-intrinsic deficiency in humans with biallelic RLTPR mutations. J Exp Med. 2016;213:2413–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lehle AS, Farin HF, Marquardt B, et al. . Intestinal inflammation and dysregulated immunity in patients with inherited caspase-8 deficiency. Gastroenterology. 2019;156:275–278. [DOI] [PubMed] [Google Scholar]
- 14. Kotlarz D, Marquardt B, Barøy T, et al. . Human TGF-β1 deficiency causes severe inflammatory bowel disease and encephalopathy. Nat Genet. 2018;50:344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lek M, Karczewski KJ, Minikel EV, et al. ; Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wen Z, Fiocchi C. Inflammatory bowel disease: autoimmune or immune-mediated pathogenesis? Clin Dev Immunol. 2004;11:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. [DOI] [PubMed] [Google Scholar]
- 18. Bennett CL, Christie J, Ramsdell F, et al. . The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. [DOI] [PubMed] [Google Scholar]
- 19. Brunkow ME, Jeffery EW, Hjerrild KA, et al. . Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. [DOI] [PubMed] [Google Scholar]
- 20. Wildin RS, Ramsdell F, Peake J, et al. . X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. [DOI] [PubMed] [Google Scholar]
- 21. Alangari A, Alsultan A, Adly N, et al. . LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J Allergy Clin Immunol. 2012;130:481–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez-Herrera G, Tampella G, Pan-Hammarström Q, et al. . Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90:986–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuehn HS, Ouyang W, Lo B, et al. . Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schubert D, Bode C, Kenefeck R, et al. . Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang TT, Jabs C, Sobel RA, et al. . Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J Exp Med. 1999;190:733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi FD, He B, Li H, et al. . Differential requirements for CD28 and CD40 ligand in the induction of experimental autoimmune myasthenia gravis. Eur J Immunol. 1998;28:3587–3593. [DOI] [PubMed] [Google Scholar]
- 27. Tada Y, Nagasawa K, Ho A, et al. . CD28-deficient mice are highly resistant to collagen-induced arthritis. J Immunol. 1999;162:203–208. [PubMed] [Google Scholar]
- 28. Kotlarz D, Beier R, Murugan D, et al. . Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143:347–355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The identified CARMIL2 sequence variants will be submitted to the ClinVar database (www.ncbi.nlm.nih.gov/clinvar/; 08 May 2019, date last accessed) upon publication. Exome sequencing data will not be published to protect privacy of affected families.