Abstract
Prematurity is the leading cause of perinatal morbidity and mortality worldwide. In most cases, preterm birth is preceded by spontaneous preterm labor, a syndrome that is associated with intra-amniotic inflammation, the most studied etiology. However, the remaining etiologies of preterm labor are poorly understood and, therefore, most preterm births are categorized as idiopathic. Herein, we provide evidence showing that the fetal immune system undergoes premature activation in women with preterm labor without intra-amniotic inflammation, providing a potential new mechanism of disease for some cases of idiopathic preterm birth. First, we showed that fetal T cells are a predominant leukocyte population in amniotic fluid during preterm gestations. Interestingly, only fetal CD4+ T cells were increased in amniotic fluid of women who underwent idiopathic preterm labor and birth. This increase in fetal CD4+ T cells was accompanied by elevated amniotic fluid concentrations of T-cell cytokines such as IL-2, IL-4, and IL-13, which are produced by these cells upon in vitro stimulation, but was not associated with the prototypical cytokine profile observed in women with intra-amniotic inflammation. Also, we found that cord blood T cells, mainly CD4+ T cells, obtained from women with idiopathic preterm labor and birth displayed enhanced ex vivo activation, which is similar to that observed in women with intra-amniotic inflammation. Finally, we showed that the intra-amniotic administration of activated neonatal CD4+ T cells induces preterm birth in mice. Collectively, these findings provide evidence suggesting that fetal T-cell activation is implicated in the pathogenesis of idiopathic preterm labor and birth.
Keywords: fetus, placenta, amniotic fluid, funisitis, chorioamnionitis, inflammation
Visual Abstract
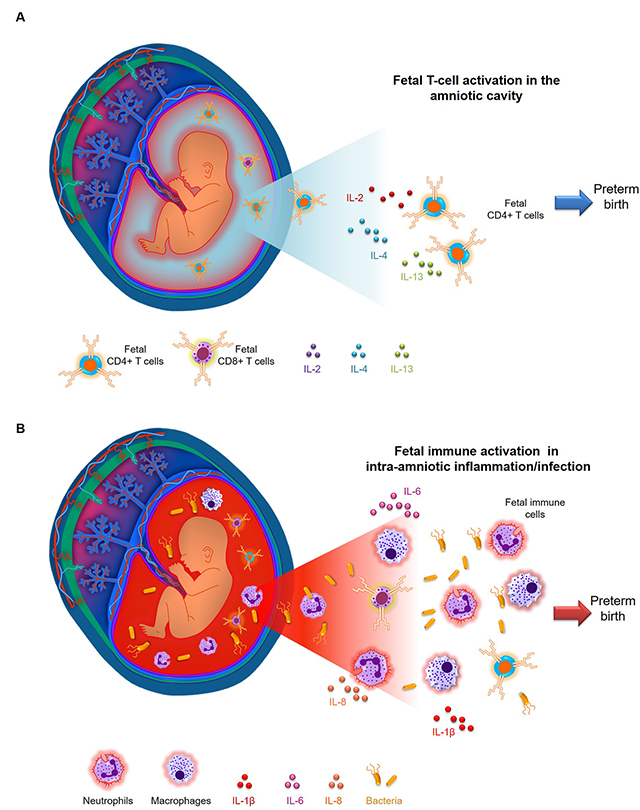
(A) Fetal T cells mediate a stereotypical inflammatory response in the amniotic cavity, partially mediated by IL-2, IL-4, and IL-13, which precedes preterm birth in a subset of women who underwent idiopathic preterm labor without intra-amniotic inflammation. This mechanism of disease is distinct from that observed in (B) women with preterm labor and intra-amniotic inflammation, in which maternal and fetal innate immune cells (neutrophils and macrophages) and T cells respond towards microbial products, involving the prototypical cytokines IL-6, IL-1β, and IL-8.
INTRODUCTION
Preterm birth remains one of the most common obstetrical syndromes today, and is a primary cause of perinatal morbidity and mortality around the world (1–4). Neonates born preterm have increased risk of both short- and long-term morbidities (5–8) that can persist throughout adulthood and seriously impact the quality of life. Two-thirds of all preterm births are preceded by spontaneous preterm labor (9), a syndrome of multiple pathological processes (10). Yet, among these etiologies, only intra-amniotic inflammation has been causally linked to preterm birth (11–20).
Intra-amniotic inflammation can result from microbial invasion of the amniotic cavity (i.e. intra-amniotic infection) (12, 13, 15, 21–32) or can be initiated by endogenous danger signals or alarmins found in amniotic fluid (33–37) (i.e. sterile intra-amniotic inflammation (38, 39)). The mechanisms implicated in intra-amniotic inflammation-associated preterm labor involve immune responses in the mother (40–42), the amniotic cavity (43–55), the fetus (53, 56–66), and at the maternal-fetal interface (67–79). Although intra-amniotic inflammation has been the main focus of study in the field of perinatal immunology, this etiology solely accounts for 37% of all preterm labor cases (38). The remaining etiologies of preterm labor (comprising approximately 60%) are poorly understood; therefore, most cases of preterm birth are categorized as idiopathic (9, 80).
Herein, we hypothesized that the fetus plays a central role in the mechanisms leading to idiopathic preterm labor and birth (i.e. preterm labor and birth in the absence of intra-amniotic inflammation). This hypothesis is based on a seminal study showing that fetal innate immune activation occurs in women with preterm labor in the presence and absence of infection in the amniotic cavity (56). Predictably, in the context of intra-amniotic infection-associated preterm labor, fetal immune activation has been associated with increased IL-6 and cortisol concentrations (57, 58). Furthermore, animal studies demonstrated that intra-amniotic inflammation induces activation and alteration in fetal T-cell populations prior to preterm birth (61, 63). More recently, it was reported that cord blood T cells from women with preterm labor are activated and respond towards maternal alloantigens, which suggested for the first time that the adaptive immune limb of the fetus can trigger preterm parturition and preterm birth (66). However, whether fetal T-cell activation occurs during idiopathic preterm labor is still unknown.
Given that fetal immune cells can be detected in the amniotic cavity even in the absence of inflammation (51, 81–84), we proposed the study of amniotic fluid immune cells as a window into the fetal immune system in utero. Using clinical and immunological tools, we aimed to investigate whether fetal T cells are implicated in the pathophysiology of idiopathic preterm labor which occurs in the absence of intra-amniotic inflammation.
MATERIALS AND METHODS
Study population and characteristics
This study included patients who underwent transabdominal amniocentesis due to clinical indications. The collection of samples was approved by the Institutional Review Boards of the Detroit Medical Center (Detroit, MI, USA), Wayne State University, and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS). Maternal and cord blood samples from these women were also collected in some cases. All participating women provided written informed consent prior to the collection of samples. Three separate cohorts of women were used in this study.
The first cohort included 21 women in preterm gestation (15–30 weeks of gestation) whose amniotic fluid was collected from November 2013 to January 2015 due to clinical indications and used for studies of the exploratory immunophenotyping, origin, and characterization of T cells, which was compared to that of cord blood and maternal blood samples. The demographic and clinical characteristics of these women are shown in Table I.
Table I.
Clinical and demographic characteristics of women whose amniotic fluid was used for exploratory experiments.
| Clinical characteristics | Values |
|---|---|
| Maternal age (years; median [IQR]) | 27 (23–35) |
| Body mass index (kg/m2; median [IQR]) | 25.9 (22.6–34.2) |
| Primiparity | 19.1% (4/21) |
| Race | |
| African-American | 85.7% (18/21) |
| Caucasian | 4.8% (1/21) |
| Asian | 4.8% (1/21) |
| Other | 4.8% (1/21) |
| Gestational age at amniocentesis (weeks; median [IQR]) | 21.6 (19.5–26.5) |
| IL-6 (ng/mL; median [IQR]) | 0.46 (0.15–0.91) |
| White blood cell count, cells/mm3 | 1 (0–4) |
| Amniotic fluid glucose, mg/dL | 28 (24.5–38) |
Available data are given as median (interquartile range, IQR) and percentage (n/N).
The second cohort included amniotic fluid samples collected from November 2013 to November 2016 of 43 women in preterm labor. These samples were used for confirmatory immunophenotyping and the determination of cytokine/chemokine concentrations in amniotic fluid. This cohort included the following groups: i) women with spontaneous preterm labor who delivered at term without intra-amniotic inflammation (PTL-TB, negative control); ii) women with spontaneous preterm labor who delivered preterm without intra-amniotic inflammation (PTL-PTB, study group); and iii) women with spontaneous preterm labor and birth with intra-amniotic inflammation (PTL-PTB-IAI, positive control). The demographic and clinical characteristics of these women are shown in Table II.
Table II.
Clinical and demographic characteristics of women whose amniotic fluid was used for descriptive studies.
| Clinical characteristics | PTL TB (n=9) | PTL PTB (n=8) | PTL PTB IAI (n=26) | p-value |
|---|---|---|---|---|
| Maternal age (years; median [IQR])a | 25 (20–26) | 23.5 (23–24.8) | 26 (22–31.3) | 0.5 |
| Body mass index (kg/m2; median [IQR])a | 28.4 (21.8–30)c | 22.5 (20.5–25.2)c | 27.4 (24.8–32.7)d | 0.1 |
| Primiparityb | 22.2% (2/9) | 12.5% (1/8) | 7.7% (2/26) | 0.5 |
| Raceb | 0.3 | |||
| African-American | 88.9% (8/9) | 75% (6/8) | 80.8% (21/26) | |
| Caucasian | 0% (0/9) | 12.5% (1/8) | 15.4% (4/26) | |
| Asian | 0% (0/9) | 12.5% (1/8) | 0% (0/26) | |
| Other | 11.1% (1/9) | 0% (0/8) | 3.8% (1/26) | |
| Gestational age at Amniocentesis (weeks; median [IQR])a | 32.6 (31–33.7) | 30.6 (26.9–33.6) | 26.4 (22.8–30.7) | 0.009 |
| IL-6 (ng/mL; median [IQR])a | 0.3 (0.2–0.4) | 0.5 (0.3–0.7) | 65.3 (23.6–129.1) | <0.001 |
| White blood cell count, cells/mm3,a | 2 (0–4) | 2 (0–8)c | 10.5 (1.3–323.5) | 0.058 |
| Amniotic fluid glucose, mg/dLa | 37 (23–38) | 22.5 (16–39.5) | 10.5 (1.5–17.5) | 0.001 |
| Gestational age at delivery (weeks; median [IQR])a | 39 (38.7–39.6) | 30.8 (27.1–33.8) | 26.9 (23.3–30.9) | <0.001 |
| Cesarean sectionb | 0% (0/9) | 25% (2/8) | 19.2% (5/26) | 0.3 |
| Birthweight (grams)a | 2990 (2720–3525) | 1760 (970–2065) | 1030 (607.5–1641.3) | <0.001 |
| Maternal inflammatory response (moderate/severe acute chorioamnionitis)b | 37.5% (3/8)c | 12.5% (1/8) | 71.4% (15/21)e | 0.01 |
| Fetal inflammatory response (moderate/severe acute funisitis)b | 0% (0/8)c | 0% (0/8) | 14.3% (3/21)e | 0.3 |
Data are given as median (interquartile range, IQR) and percentage (n/N). PTL TB = preterm labor who delivered at term birth; PTL PTB = preterm labor and birth; PTL PTB IAI = preterm labor and birth with intra-amniotic inflammation.
Kruskal-Wallis test.
Fisher’s exact test.
One missing data.
Three missing data.
Five missing data
The third cohort included 20 women from whose neonates’ cord blood was obtained and biobanked from November 2015 to November 2016, and the inflammatory status (amniotic fluid white blood cell count and IL-6 concentrations) and microbiology of amniotic fluid was performed. These samples were used to assess the functional status of T cells. This cohort included the following groups: i) women who delivered at term with labor but without intra-amniotic inflammation (TB, negative control), ii) women with spontaneous preterm labor and birth without intra-amniotic inflammation (PTL-PTB, study group), and iii) women with spontaneous preterm labor and birth with intra-amniotic inflammation (PTL-PTB-IAI, positive control). The demographic and clinical characteristics of these women are shown in Table III.
Table III.
Clinical and demographic characteristics of women from whose neonates umbilical cord blood was obtained for in vitro studies.
| TB (n=6) | PTL PTB (n=9) | PTL PTB IAI (n=5) | p-value | |
|---|---|---|---|---|
| Maternal age (years; median [IQR])a | 23.5 (22.3–24.8) | 26 (23–27) | 24 (21–25) | 0.3 |
| Body mass index (kg/m2; median [IQR])a | 29.8 (23.3–34.9) | 21.7 (20–24.2) | 28.6 (23.3–32.5)c | 0.08 |
| Primiparityb | 50% (3/6) | 22.2% (2/9) | 40% (2/5) | 0.5 |
| Raceb | 0.7 | |||
| African-American | 83.3% (5/6) | 88.9% (8/9) | 80% (4/5) | |
| Caucasian | 16.7% (1/6) | 0% (0/9) | 20% (1/5) | |
| Other | 0% (0/6) | 11.1% (1/9) | 0% (0/5) | |
| Gestational age at Amniocentesis (weeks; median [IQR])a | 40.5 (40.3–40.6) | 33.3 (31.1–33.6) | 30.3 (26–32.6) | 0.05 |
| IL-6 (ng/mL; median [IQR])a | 2.1 (1.9–30.5) | 0.3 (0.2–0.5)c | 17.7 (16.8–67.1) | 0.002 |
| Gestational age at delivery (weeks; median [IQR])a | 40.5 (40.3–40.6) | 35.4 (33.7–36.3) | 31.9 (26.1–32.6) | 0.001 |
| Cesarean sectionb | 83.3% (5/6) | 44.4% (4/9) | 20% (1/5) | 0.1 |
| Birthweight (grams)a | 3342.5 (3070–3465) | 2585 (1860–2640) | 1955 (1040–2120) | 0.003 |
| Maternal inflammatory response (moderate/severe acute chorioamnionitis)b | 33.3% (2/6) | 33.3% (3/9) | 25% (1/4)c | 1 |
| Fetal inflammatory response (moderate/severe acute funisitis)b | 16.7% (1/6) | 0% (0/9) | 0% (0/4)c | 0.5 |
Data are given as median (interquartile range, IQR) and percentage (n/N). TB = term labor and birth; PTL PTB = preterm labor and birth; PTL PTB IAI = preterm labor and birth with intra-amniotic inflammation.
Kruskal-Wallis test.
Fisher’s exact test.
One missing data.
Labor was defined by the presence of regular uterine contractions at a frequency of at least two contractions every 10 min with cervical changes resulting in delivery. Preterm delivery was defined as delivery <37 wk of gestation. Our study groups included women who underwent preterm labor with intact membranes; thus, intra-amniotic inflammation was defined as an amniotic fluid IL-6 concentration ≥ 2.6 ng/mL (30).
Clinical determinations in amniotic fluid and placentas
Immediately after amniocentesis, samples of amniotic fluid were transported to the clinical laboratory in a capped sterile syringe, and the rest of the sample was used for this study. The clinical tests included the determination of amniotic fluid white blood cell count (43), Gram stain (85), and glucose concentration (86). IL-6 concentrations were determined as previously described (30). Acute inflammatory lesions of the placenta (maternal inflammatory response and fetal inflammatory response) were diagnosed according to established criteria, including staging and grading (87, 88).
Immunophenotyping by flow cytometry
Amniotic fluid samples (0.5–1 mL) were centrifuged at 300 x g for 5 min at room temperature. The resulting amniotic fluid cell pellet was re-suspended in 1 mL of 1X phosphate-buffered saline (PBS) (Life Technologies, Grand Island, NY, USA) and stained with the BD Horizon Fixable Viability Stain 510 dye (BD Biosciences, San Jose, CA, USA). Amniotic fluid cells were washed in 1X PBS and incubated with 20 μL of human FcR blocking reagent (Miltenyi Biotec, San Diego, CA, USA) in 80 μL of stain buffer (BD Biosciences) for 10 min at 4°C prior to incubation with extracellular antibodies.
Maternal peripheral blood samples were collected from healthy individuals by venipuncture into ethylene diamine tetra-acetic acid (EDTA)-containing tubes. Umbilical cord blood was collected at birth in EDTA-containing tubes by venipuncture of the umbilical vein. Mononuclear cells were isolated using the density gradient reagent Ficoll-Paque Plus (GE Healthcare Life Sciences, Piscataway, NJ, USA), according to the manufacturer’s instructions. For the samples with only extracellular staining, 50 μL of maternal or cord blood were used.
For all samples, cells were then incubated with extracellular fluorochrome-conjugated anti-human monoclonal antibodies for 30 min at 4°C in the dark (Supplemental Table I). After extracellular staining, the cells were fixed. For intracellular staining, cells were fixed and permeabilized using the Foxp3 transcription factor fixation/permeabilization solution (Cat#00–5523-00; eBioscience by Thermo Fisher Scientific, Wilmington, DE, USA) prior to incubation with intracellular antibodies (Supplemental Table I). Stained cells were then washed and re-suspended in 0.5 mL of stain buffer and acquired using the BD LSR Fortessa flow cytometer and BD FACSDiva 6.0 software. The analysis was performed and the figures were generated using the FlowJo version 10 software (FlowJo, Ashland, OR, USA). The absolute number of cells was determined using CountBright absolute counting beads (Molecular Probes, Eugene, OR, USA).
DNA fingerprinting
Genomic DNA was isolated from amniotic fluid cell pellets using the QIAamp UCP DNA Micro kit (Cat#56204, Qiagen, Hilden, Germany), following the manufacturer’s instructions. Fetal or maternal genomic DNA from matching cases was isolated from frozen buffy coat samples of either umbilical cord or maternal blood using the DNeasy Blood and Tissue Kit (Qiagen), according to the manufacturer’s instructions. DNA concentrations and purity were assessed using the NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). DNA samples were submitted for DNA fingerprinting to Genetica DNA Laboratories, a LabCorp brand (Burlington, NC, USA). Briefly, analytical procedures for polymerase chain reaction and capillary electrophoresis were performed on a 3130xl genetic analyzer (Applied Biosystems, Foster City, CA, USA). The 13-core CODIS STR loci plus PENTA E and PENTA D, and the gender-determining locus, amelogenin, were analyzed using the commercially available PowerPlex 16HS amplification kit (Promega Corp, Madison, WI, USA) and GeneMapper ID v3.2.1 software (Applied Biosystems). Appropriate positive and negative controls were concurrently used throughout the analysis. The interpretation of the DNA profile of each sample was performed by geneticists from LabCorp and returned as a written report. The reported sensitivity for this method was between 2–5%, indicating that this technology is capable of detecting as low as 2–5% of the DNA in question among the total DNA mixture.
Amniotic fluid cytokine/chemokine concentrations
Amniotic fluid samples were assessed using the V-PLEX Pro-inflammatory Panel 1 kit (Meso Scale Discovery, Rockville, MD, USA) to measure amniotic fluid concentrations of IFNγ, TNFα, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, and IL-13, according to the manufacturer’s instructions. Plates were read using the SECTOR Imager 2400 (Meso Scale Discovery). Standard curves were generated and the assay values of the samples were interpolated from the curves. The detection limits of the assays were 0.37 pg/mL (IFNγ), 0.04 pg/mL (TNFα), 0.05 pg/mL (IL-1β), 0.09 pg/mL (IL-2), 0.02 pg/mL (IL-4), 0.06 pg/mL (IL-6), 0.07 pg/mL (IL-8), 0.04 pg/mL (IL-10), 0.11 pg/mL (IL-12p70), and 0.24 pg/mL (IL-13). Inter-assay and intra-assay coefficients of variation were less than 10.5%.
Amniotic fluid concentrations of CXCL10 (Cat#DIP100, R&D Systems, Minneapolis, MN, USA) and CXCL11 (Cat#K151UWK-1, Meso Scale Discovery) were determined using individual sensitive and specific immunoassays, according to the manufacturer’s instructions. Assays were read using the SpectraMax M5 (CXCL10; Molecular Devices, San Jose, CA, USA) or the SECTOR Imager 2400 (CXCL11). The concentrations of CXCL10 and CXCL11 were determined by interpolation from the standard curve. The detection limits of the assays were 1.7 pg/mL (CXCL10) and 1.5 pg/mL (CXCL11). The inter-assay and intra-assay coefficients of variation were less than 9.8% for CXCL10 and less than 16.8% for CXCL11.
Amniotic fluid T-cell cytokine expression upon stimulation
Amniotic fluid samples (5–10mL) from women in preterm gestations (n = 3) were centrifuged at 300 x g for 10 min at room temperature. The resulting amniotic fluid cell pellet was washed with 1mL of 1X PBS and re-suspended in supplemented RPMI [10% FBS and 1% penicillin/streptomycin antibiotic (Thermo Fisher Scientific)]. To evaluate IL-4 and IL-13 production, amniotic fluid cells were stimulated with immobilized anti-human CD3 antibody (clone OKT3, 10μg/mL, Cat#16–0037-85, eBioscience), soluble anti-human CD28 antibody (clone CD28.2, 2μg/mL, Cat#16–0289-85, eBioscience), recombinant human IL-2 (10ng/mL, Cat#554603, BD Biosciences) and recombinant human IL-4 (20ng/mL, Cat#554605, BD Biosciences) for 2 d. The cells were washed and subsequently cultured in supplemented RPMI containing IL-2 and IL-4 for another 2 d. Finally, the cells were collected and stimulated with Cell Stimulation Cocktail [containing phorbol 12-myristate 13-acetate (PMA), ionomycin, brefeldin A and monensin)] (Cat#00–4975-93, eBioscience) for 4 h. To evaluate IL-2 production, amniotic fluid cells were washed and treated with Cell Stimulation Cocktail for 4 h. Untreated cells were included as controls for each experiment.
After treatment, amniotic fluid cells were washed with 1X PBS and incubated with extracellular fluorochrome-conjugated anti-human monoclonal antibodies for 30 min at 4°C in the dark (Supplemental Table I). After extracellular staining, the cells were fixed, permeabilized, and incubated with specific fluorochrome-conjugated anti-human monoclonal antibodies against IL-2, IL-4 and IL-13 (Supplemental Table I). Stained cells were then washed and re-suspended in 0.5mL of stain buffer and acquired using the BD LSR Fortessa flow cytometer and BD FACSDiva 6.0 software. The analysis was performed and the figures were generated using FlowJo version 10 software.
Determination of cord blood T-cell functionality
Cord blood samples were obtained from neonates born to women who underwent spontaneous labor at term, spontaneous preterm labor, or spontaneous preterm labor with intra-amniotic inflammation (IL-6 >2.6 ng/mL) (Table III) and preserved in freezing media (90% fetal bovine serum and 10% DMSO) until use. Non-viable cells were depleted using the Dead Cell Removal kit (Cat#130–090-101, Miltenyi Biotec) in the cord blood samples, following the manufacturer’s instructions. The cells were washed, re-suspended in supplemented RPMI at 1×106 cells/mL, and then either treated with Cell Stimulation Cocktail or left untreated as a control. Cells were then incubated at 37°C and 5% CO2 for 5 hours. After incubation, cells were collected, washed in 1X PBS, and re-suspended in 10 μL of human FcR blocking reagent and 40 μL of stain buffer for 10 min at 4°C. The cells were then incubated with extracellular fluorochrome-conjugated anti-human monoclonal antibodies for 30 min at 4°C in the dark (Supplemental Table I). After washing with stain buffer, the cells were fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer Set prior to incubation with intracellular antibodies for 30 min at 4°C in the dark (Supplemental Table I). A portion of the cells were stained with fluorochrome-conjugated isotypes as a control. Stained cells were then washed, re-suspended in 0.5 mL of stain buffer, and acquired using the BD LSRFortessa Flow Cytometer and BD FACSDiva 6.0 software. The analysis was performed and the figures were generated using the FlowJo version 10 software, and the absolute number of cells was determined using CountBright absolute counting beads.
Mice
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred in the animal care facility at the C.S. Mott Center for Human Growth and Development, Wayne State University, Detroit, MI, USA and housed under a circadian cycle (light:dark = 12:12 h). Eight- to twelve-week-old females were mated with males of proven fertility. Female mice were examined daily between 8:00 and 9:00 AM for the presence of a vaginal plug, which indicated 0.5 d post coitum (dpc). Upon observation of vaginal plugs, female mice were removed from the mating cages and housed separately. A weight gain of ≥2g confirmed pregnancy at 12.5 dpc. All animal experiments were approved by the Institutional Animal Care and Use Committee at Wayne State University (Protocol No. A-18–03-0584). The authors adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Isolation and activation of neonatal T cells
Murine neonates of one week of age were sacrificed and the thymi were collected into sterile 1X PBS and thymocytes were isolated by gently dissociating, as previously reported (89). Thymocytes were then passed through a 30μm filter (Miltenyi Biotec), washed with sterile 1X PBS, and centrifuged at 500 x g for 5 min at 4°C. The cell pellet was resuspended in 2mL of sterile ACK lysis buffer (Cat#A10492; Thermo Fisher Scientific) followed by a 5 min incubation at 37°C and washed with sterile 1X PBS. Finally, the thymocytes were resuspended in 1mL of RPMI media (supplemented with 10% FBS and 1% penicillin/streptomycin antibiotic), and cells were counted using the Cellometer Auto 2000. Thymocytes were immediately stained for the cell sorting of non-activated CD4+ T cells (Day 0; see below) or placed at 2 × 106 cells/well in a six-well culture plate previously coated with anti-mouse CD3ε (clone 145–2C11, Cat#553058; BD Biosciences). After plating, the following stimulators were added to each well: anti-mouse CD28 (clone 37.51; Cat#553295, BD Biosciences) (1μg/mL), anti-mouse IL-4 (clone 11B11, Cat#16–7041-85; eBioscience) (10μg/mL), recombinant mouse IL-2 (Cat#575402; Biolegend) (10ng/mL), and recombinant mouse IL-12 (Cat#577002; Biolegend) (10ng/mL). Lastly, mercaptoethanol (Cat#21985023; Thermo Fisher Scientific) (2μL) was added to each well and thymocytes were cultured at 37°C and 5% CO2 for 4 d.
Determination of neonatal T-cell activation by flow cytometry
To confirm neonatal T-cell activation on Day 4 of culture, a portion of the neonatal thymocytes were collected and stimulated for 4 hours with 2μL/mL of Cell Stimulation Cocktail. Stimulated cells were then washed with 1X PBS and incubated with fluorochrome-conjugated anti-mouse monoclonal antibodies (Supplemental Table 1) for 30 min at 4°C in the dark. Following extracellular staining, cells were fixed and permeabilized using the Foxp3 Transcription Factor Staining Buffer Set (Thermo Fisher Scientific) and incubated with specific fluorochrome-conjugated anti-mouse monoclonal antibodies against IFNγ and TNFα (Supplemental Table 1) for 30 min at 4°C in the dark. Non-stimulated cells were stained as controls. The cells were acquired using the BD LSRFortessa flow cytometry and BD FACSDiva v6.0 software. The analysis and figures were performed using FlowJo v10 software.
Fluorescence-activated cell sorting of viable activated or non-activated neonatal T cells
After confirming neonatal T-cell activation (Day 4), thymocytes were collected from the culture plate and centrifuged at 500 x g for 5 min at room temperature. The cells were then resuspended in 100μL of sterile stain buffer and incubated with fluorochrome-conjugated anti-mouse monoclonal antibodies (Supplemental Table 1) for 30 min at 4°C in the dark. Non-activated thymocytes (Day 0) were also stained for cell sorting. The cells were then washed with supplemented RPMI media to remove excess antibody and resuspended in 4mL of RPMI. Viable CD3+CD8-CD4+ T cells were sorted using the BD FACSMelody cell sorter (BD Biosciences) and BD FACSChorus v1.3 software (BD Biosciences) under sterile conditions. Sorted cells were counted, centrifuged at 300 x g for 5 min, and resuspended at 1 × 104 cells/25μL in sterile 1X PBS for ultrasound-guided intra-amniotic injection.
Ultrasound-guided intra-amniotic injection of activated or non-activated neonatal CD4+ T cells
Pregnant C57BL/6 dams were anesthetized on 16.5 dpc by inhalation of 2–3% isoflurane (Aerrane, Baxter Healthcare Corporation, Deerfield, IL, USA) and 1–2 L/min of oxygen in an induction chamber. Anesthesia was maintained with a mixture of 1.5–2% isoflurane and 1.5–2 L/min of oxygen. Dams were positioned on a heating pad and stabilized with adhesive tape. Fur removal from the abdomen was achieved by applying Nair cream (Church & Dwight Co., Inc., Ewing, NJ, USA) to this area. Body temperature was maintained in the range of 37 ± 1°C and detected with a rectal probe (VisualSonics, Inc., Toronto, ON, Canada), and respiratory and heart rates were monitored by electrodes embedded in the heating pad. An ultrasound probe from the Vevo 2100 Imaging System (VisualSonics, Inc.) was fixed and mobilized with a mechanical holder, and the transducer was slowly moved toward the abdomen. Ultrasound-guided intra-amniotic injection of 1 × 104 non-activated (Day 0; n = 3) or activated (Day 4; n = 8) neonatal T cells in 25μL of sterile 1X PBS was performed in each amniotic sac using a 30G needle (BD PrecisionGlide Needle, Becton Dickinson, Franklin Lakes, NJ, USA). The amount of neonatal T cells injected in each amniotic sac was based on preliminary experiments showing that pregnant dams require higher doses of inflammatory stimuli to deliver preterm. Vehicle controls were injected with 25μL of sterile 1X PBS in each amniotic sac (n = 9). The syringe was stabilized by a mechanical holder (VisualSonics, Inc.). Following the ultrasound, mice were placed under a heat lamp for recovery (defined as when the mouse resumes normal activity, such as walking and responding), which typically occurred 10–20 min after removal from anesthesia. Dams were monitored via video camera (Sony Corporation, Tokyo, Japan) until delivery to obtain the gestational age and rate of preterm birth, which was defined as delivery occurring before 18.5 dpc, and its rate was represented by the percentage of dams delivering preterm among the total number of mice injected.
Statistical analysis
Statistical analyses were conducted using SPSS software version 19.0 (IBM Corporation, Armonk, NY, USA). For patient demographics, the Kruskall-Wallis test was performed for continuous variables and the Fisher’s exact test for nominal variables. t-SNE plots were generated using the FlowJo v10 software. The Mann-Whitney U-test was performed when comparing non-normally distributed data between study groups. The log2 fold changes in cytokine concentrations were also calculated after data transformation. The Wilcoxon signed rank paired test was used when comparing the same sample with and without stimulation. Kaplan-Meier survival curves were used to plot and compare the murine gestational age data (Mantel–Cox test). A p-value ≤ 0.05 was considered statistically significant.
RESULTS
Characterization and origin of amniotic fluid immune cells
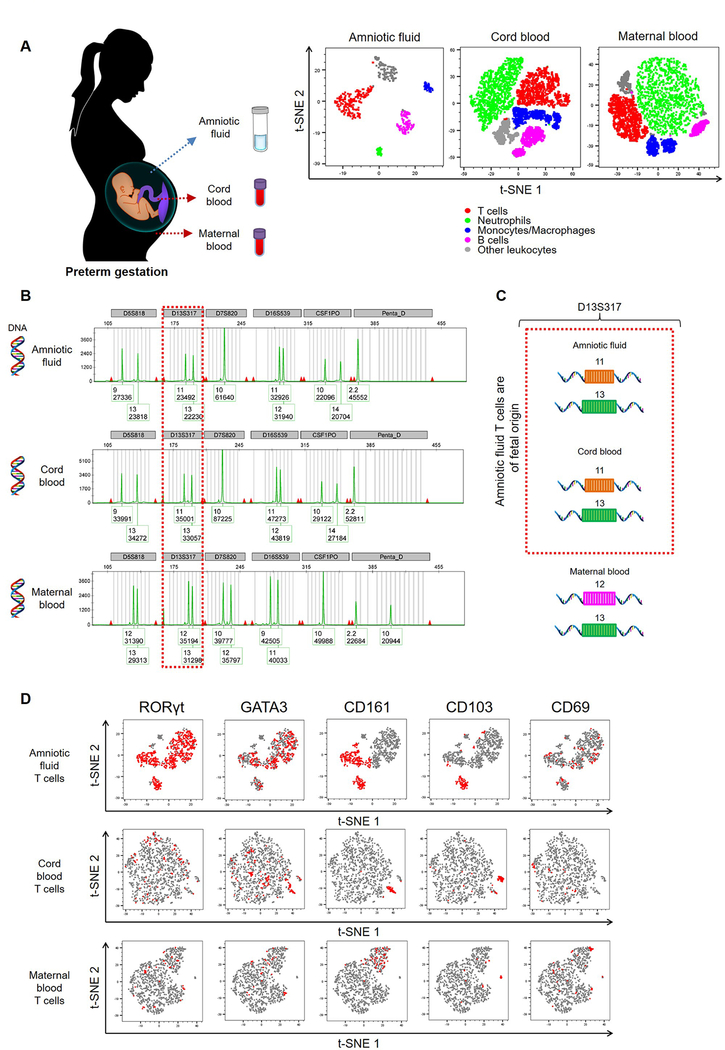
Amniotic fluid contains both innate (83, 84, 90–93) and adaptive (84) immune cells throughout pregnancy. First, we showed that in preterm gestations the amniotic fluid immune cell profile is distinct from that seen in the fetus (umbilical cord blood) or mother (peripheral blood), and that T cells are the dominant physiological population in this compartment (Fig. 1A). However, amniotic fluid T cells have been poorly investigated. Therefore, we collected amniotic fluid from women in preterm gestations and performed DNA fingerprinting to determine the origin of these T cells (Fig. 1B and Fig. S1). In each case, DNA fingerprinting revealed that amniotic fluid T cells were of fetal origin (Fig. 1B&C). Amniotic fluid T cells and innate lymphoid cells, another immune cell population present in preterm gestation, have been proposed to originate from the fetal intestine, based on their expression of transcription factors such as RAR-related orphan receptor-γt (RORγt) (83, 84, 94) and GATA-binding protein-3 (GATA3) (94) as well as the surface markers CD161 and CD103 (83, 84). Our exploratory immunophenotyping revealed that fetal T cells in amniotic fluid highly expressed RORγt and GATA3, as well as moderate levels of CD161, CD103, and the activation marker CD69 (Fig. 1D). The expression of these markers was low on T cells from the fetal or maternal circulations, further indicating that fetal T cells in amniotic fluid are a unique subset (Fig. 1D). We also determined the expression of the mucosal effector molecules IL-17 and IL-22 by amniotic fluid T cells, which were low and no differences were found when compared to cord blood or maternal blood (data not shown).
Figure 1. Characterization and origin of amniotic fluid T cells.
(A) (Left) Amniotic fluid samples were obtained from women between 15–30 weeks of gestation for exploratory studies. Maternal peripheral blood and umbilical cord blood samples were collected from each case for comparison. (Right) Representative t-SNE plots showing the relative distribution of immune cell populations in amniotic fluid, umbilical cord blood, and maternal peripheral blood (n = 3 – 14 each). (B) DNA fingerprinting of amniotic fluid cells, the fetus (umbilical cord blood), and the mother (peripheral blood) is shown in electropherograms. Each electropherogram contains 6 genetic sites: D5S818, D13S317, D7S820, D16S539, CSF1PO, and Penta_D. Each genetic site has STR alleles, which are represented by peaks. The numbers below each STR allele (peak) are the number of repeats and the signal strength for each STR allele. The DNA fingerprinting of the amniotic fluid cells is identical to the DNA fingerprinting of the fetus. (C) A representative comparison between the D13S317 STR alleles expressed by amniotic fluid cells, the fetus, and the mother is shown. (D) Representative t-SNE plots showing the expression patterns of RORγt, GATA3, CD161, CD103, and CD69 on T cells (CD45+CD14-CD15-CD3+ cells) from amniotic fluid, cord blood, or maternal peripheral blood. Red represents the expression of the indicated marker, and grey indicates no expression. Demographic and clinical characteristics of the study population are shown in Table I.
Together, these exploratory studies indicate that fetal T cells are the predominant immune cell population in preterm gestations and display a phenotype that is distinct from that observed in the fetal and maternal systemic circulations.
Are fetal T cells in amniotic fluid associated with preterm labor and birth?
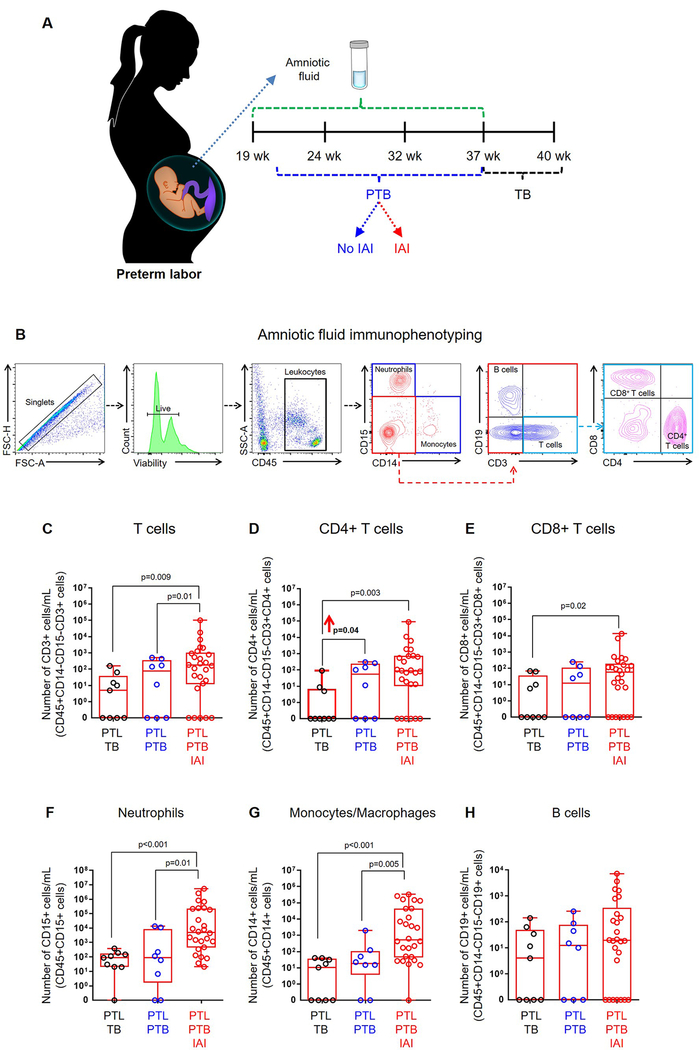
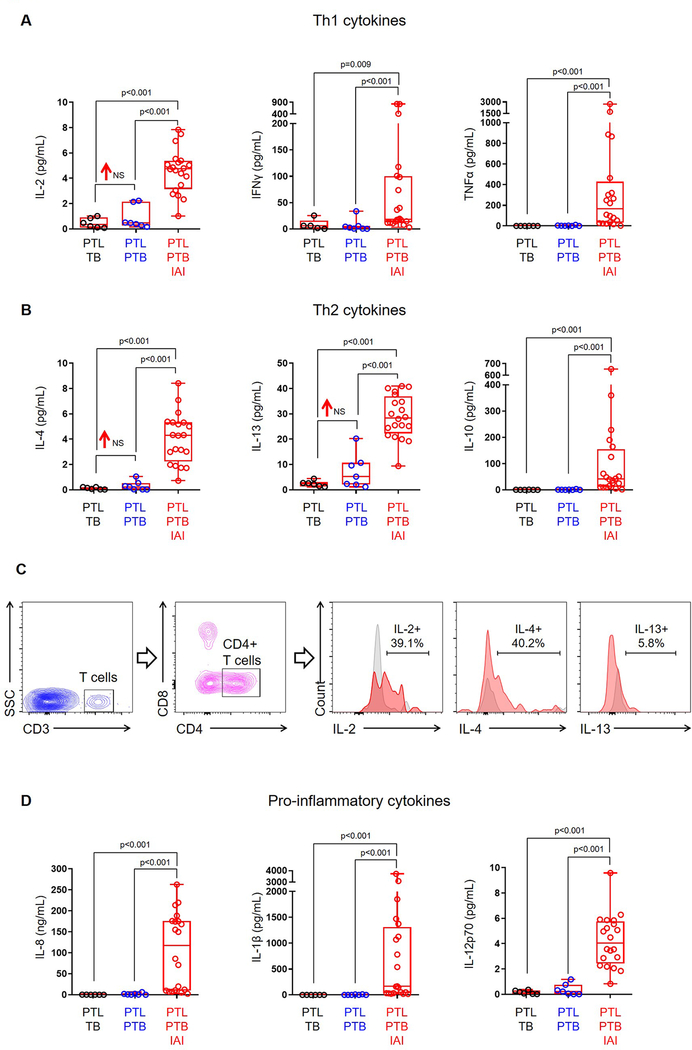
Given that amniotic fluid contains a large population of fetal T cells during preterm gestations, we next investigated whether this subset is associated with idiopathic preterm labor and birth. To determine this, we undertook a comprehensive three-year study in which amniotic fluid was obtained from women with preterm labor who delivered preterm without intra-amniotic inflammation (study group), and performed immunophenotyping of amniotic fluid immune cells to determine the absolute numbers of each subset compared to women with preterm labor who delivered at term (negative control) and those with preterm labor who delivered preterm with intra-amniotic inflammation (positive control) (Fig. 2A&B). Interestingly, we found that the number of CD4+ T cells in amniotic fluid was increased in our study group compared to negative controls (Fig. 2D). As previously reported (66, 84), the numbers of amniotic fluid neutrophils, monocytes/macrophages, and T cells were highest in positive controls (Fig. 2C–G). The number of B cells in amniotic fluid was unaltered when compared among the study group and controls (Fig. 2H). Since fetal CD4+ T cells were increased in our study group, we next investigated whether such an increase was associated with T-cell cytokines in amniotic fluid. The increased number of fetal CD4+ T cells was accompanied by a modest elevation in amniotic fluid concentrations of IL-2 (1.85 log2 fold change), IL-4 (1.59 log2 fold change), and IL-13 (2.18 log2 fold change) in our study group compared to negative controls (Fig. 3A&B). To confirm that amniotic fluid fetal T cells were capable of releasing these cytokines in vivo (i.e. preterm labor), we stimulated preterm gestation-derived amniotic fluid T cells and observed that these cells can express IL-2, IL-4, and IL-13 ex vivo (Fig. 3C). These data suggest that fetal T cells undergo premature activation prior to idiopathic preterm birth.
Figure 2. Amniotic fluid T cells are increased in preterm labor and birth.
(A) Amniotic fluid samples were obtained from women with preterm labor who delivered at term (PTL-TB) (negative control), women with preterm labor who delivered preterm without intra-amniotic inflammation (PTL-PTB) (study group), and women with preterm labor who delivered preterm with intra-amniotic inflammation (PTL-PTB-IAI) (positive control). (B) Flow cytometry gating strategy for immunophenotyping of immune cells. Immune cells were initially gated within the viability gate and CD45+ gate followed by lineage gating for neutrophils (CD45+CD15+ cells), monocytes/macrophages (CD45+CD14+ cells), T cells (CD45+CD3+ cells) that were subsequently gated for CD4+ T cells (CD45+CD3+CD4+ cells) and CD8+ T cells (CD45+CD3+CD8+ cells), and B cells (CD45+CD19+ cells). The numbers of (C) total T cells, (D) CD4+ T cells, (E) CD8+ T cells, (F) neutrophils, (G) monocytes/macrophages, and (H) B cells in amniotic fluid. Data are shown as box-and-whisker plots where midlines indicate medians, boxes indicate interquartile ranges, and whiskers indicate minimum and maximum ranges. Demographic and clinical characteristics of the study population are shown in Table II.
Figure 3. Concentrations of amniotic fluid T-cell cytokines.
Concentrations of (A) T helper 1 cytokines (IL-2, IFNγ, TNFα) and (B) T helper 2 cytokines (IL-4, IL-13, IL-10) in amniotic fluid from women who underwent spontaneous preterm labor and delivered at term (PTL-TB) (negative control), those who delivered preterm without intra-amniotic inflammation (PTL-PTB) (study group), and those who delivered preterm with intra-amniotic inflammation (PTL-PTB-IAI) (positive control). (C) Representative flow cytometry gating strategy showing the expression of IL-2, IL-4 and IL-13 by CD3+CD4+ preterm gestation-derived amniotic fluid T cells. Concentrations of (D) pro-inflammatory cytokines (IL-8, IL-1β, IL-12p70) in amniotic fluid. Data are shown as box-and-whisker plots where midlines indicate medians, boxes indicate interquartile ranges, and whiskers indicate minimum and maximum ranges. NS = no significance. Demographic and clinical characteristics of the study population are shown in Table II.
Next, we investigated whether the increase in fetal CD4+ T cells was associated with a rise in amniotic fluid concentrations of the T cell-specific chemokines CXCL10 (95) and CXCL11 (96). No differences were observed between the study group and the negative controls (Fig. S2), suggesting that CXCL10 and CXCL11 are not actively chemoattracting fetal T cells in the amniotic cavity during the process of idiopathic preterm labor that leads to premature birth. As previously reported, the amniotic fluid concentration of CXCL10 was increased in cases with intra-amniotic inflammation (97, 98). Of interest, the pro-inflammatory cytokines that are typically implicated in inflammation-associated preterm labor (e.g. IL-8 (99), IL-1β (33, 100), and IL-12p70 (101)) were increased in our positive controls, but not in our study group (Fig. 3D), indicating that the process of idiopathic preterm labor associated with fetal T-cell activation involves a distinct inflammatory milieu from that induced by intra-amniotic inflammation.
Fetal T-cell activation in idiopathic preterm labor and birth
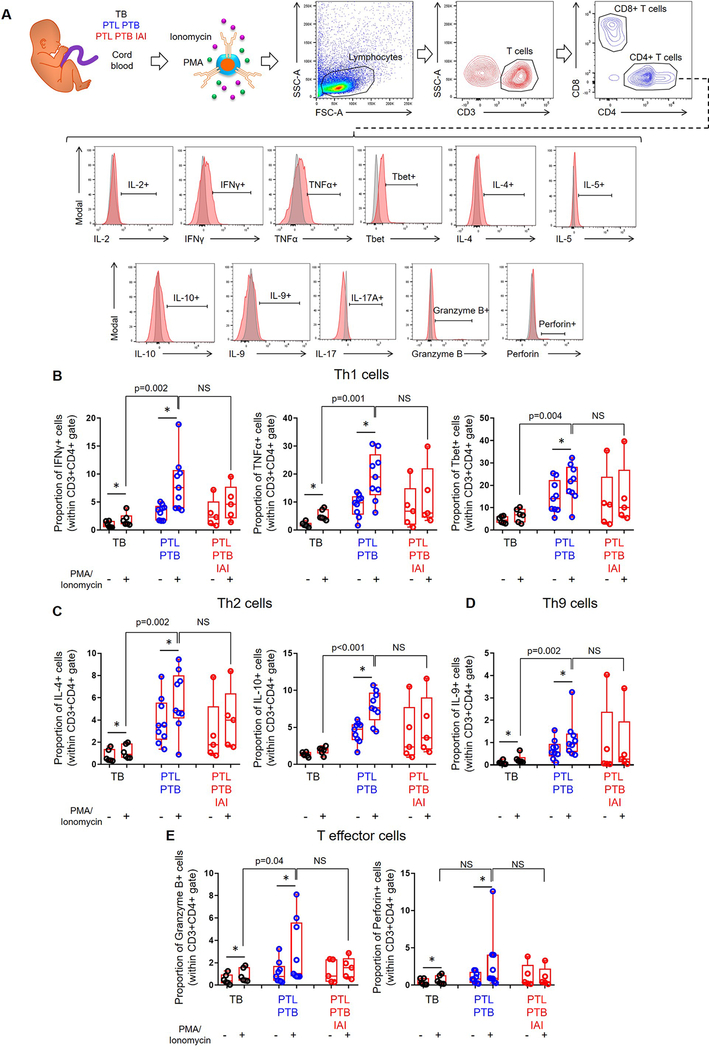
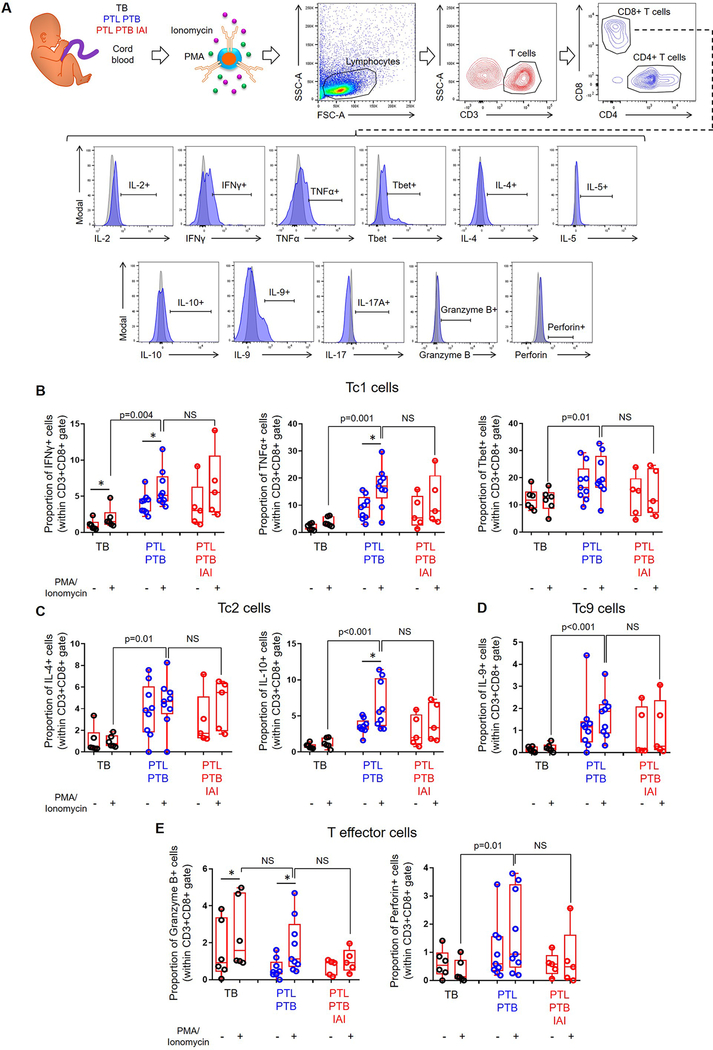
Amniotic fluid contains low numbers of T cells in physiological conditions (Fig. 1A&2C). Moreover, the amount of amniotic fluid that can be obtained for research purposes is limited. Therefore, as a surrogate for fetal T cells obtained from amniotic fluid, we utilized cord blood T cells from women with available data for the inflammatory status and microbiology of amniotic fluid. Hence, we tested the functionality of fetal T cells from neonates born to women with idiopathic preterm labor who delivered preterm without intra-amniotic inflammation (study group), those born to women with term labor and birth (negative control), and those born to women with preterm labor who delivered preterm with intra-amniotic inflammation (positive control) (Fig. 4A&5A). Immunophenotyping was used to determine the expression of IL-2, IFNγ, TNFα, Tbet, IL-4, IL-5, IL-10, IL-9, IL-17A, granzyme B, and perforin by CD4+ or CD8+ T cells (Fig. 4A&5A). Significant differences among PMA-stimulated groups are reported, since we sought to study fetal T-cell function under inflammatory conditions. Overall, fetal CD4+ T cells from our study group and from negative controls responded to stimulation, which did not occur in CD4+ T cells from positive controls (Fig. 4B–E). Specifically, fetal CD4+ T cells from women with preterm labor and birth displayed higher expression of Th1 (IFNγ, TNFα, and Tbet), Th2 (IL-4 and IL-10), Th9 (IL-9), and effector (granzyme B) mediators compared to those from term controls (Fig. 4B–E). Similarly, fetal CD8+ T cells from the study and negative control groups were responsive, although this was milder than that of CD4+ T cells (Fig. 5B–E vs. Fig4B–E). Fetal CD8+ T cells from women with preterm labor and birth displayed higher expression of Tc1 (IFNγ, TNFα, and Tbet), Tc2 (IL-4 and IL-10), Tc9 (IL-9), and effector (perforin) mediators compared to those from term controls (Fig. 5B–E). Alike fetal CD4+ T cells, fetal CD8+ T cells from positive controls did not display significantly higher expression of cytokines/effector mediators after stimulation (Fig. 5B–E). Importantly, fetal T cells from our study group displayed significantly greater activation (based on the increased expression of cytokines and effector mediators) than those from negative controls, even reaching similar levels to fetal T cells that had been activated by microorganisms invading the amniotic cavity (positive controls) (Fig. 4B–E&5B–E). The expression of IL-2, IL-5, and IL-17A by fetal CD4+ or CD8+ T cells did not vary among the study groups (Fig. S3A–F). Together, these findings show that idiopathic preterm labor that leads to premature birth is accompanied by fetal T-cell activation, which upon stimulation can be similar to that observed in cases with intra-amniotic infection.
Figure 4. Functional status of cord blood CD4+ T cells.
(A) Experimental design and flow cytometry gating strategy used to determine the expression of cytokines and effector molecules on CD4+ T cells from neonates born to mothers who delivered with spontaneous labor at term (TB) (negative control), those who underwent spontaneous preterm labor and birth without intra-amniotic inflammation (PTL-PTB) (study group), or those who underwent spontaneous preterm labor and birth with intra-amniotic inflammation (PTL-PTB-IAI) (positive control). Isolated T cells were either treated with PMA/ionomycin or left untreated. Red histograms indicate the expression of each T-cell marker, and grey histograms indicate isotype controls. (B) Proportions of CD4+ T cells expressing the type 1 cytokines IFNγ and TNFα and the transcription factor Tbet. (C) Proportions of CD4+ T cells expressing the type 2 cytokines IL-4 and IL-10. (D) Proportion of CD4+ T cells expressing the cytokine IL-9. (E) Proportion of CD4+ T cells expressing the effector molecules granzyme B and perforin. Data are shown as box-and-whisker plots where midlines indicate medians, boxes indicate interquartile ranges, and whiskers indicate minimum and maximum ranges. Asterisks indicate p-values ≤0.05 for paired comparisons between the same samples with and without stimulation. P-values are shown for comparisons between stimulated fetal T cells from each group. NS = no significance. Demographic and clinical characteristics of the study population are shown in Table III.
Figure 5. Functional status of cord blood CD8+ T cells.
(A) Experimental design and flow cytometry gating strategy used to determine the expression of cytokines and effector molecules on CD8+ T cells from neonates born to mothers who delivered with spontaneous labor at term (TB) (negative control), those who underwent spontaneous preterm labor and birth without intra-amniotic inflammation (PTL-PTB) (study group), or those who underwent spontaneous preterm labor and birth with intra-amniotic inflammation (PTL-PTB-IAI) (positive control). Isolated T cells were either treated with PMA/ionomycin or left untreated. Blue histograms indicate the expression of each T-cell marker, and grey histograms indicate isotype controls. (B) Proportions of CD8+ T cells expressing the type 1 cytokines IFNγ and TNFα, and the transcription factor Tbet. (C) Proportions of CD8+ T cells expressing the type 2 cytokines IL-4 and IL-10. (D) Proportion of CD8+ T cells expressing the cytokine IL-9. (E) Proportion of CD8+ T cells expressing the effector molecules granzyme B and perforin. Data are shown as box-and-whisker plots where midlines indicate medians, boxes indicate interquartile ranges, and whiskers indicate minimum and maximum ranges. Asterisks indicate p-values ≤0.05 for paired comparisons between the same samples with and without stimulation. P-values are shown for comparisons between stimulated fetal T cells from each group. NS = no significance. Demographic and clinical characteristics of the study population are shown in Table III.
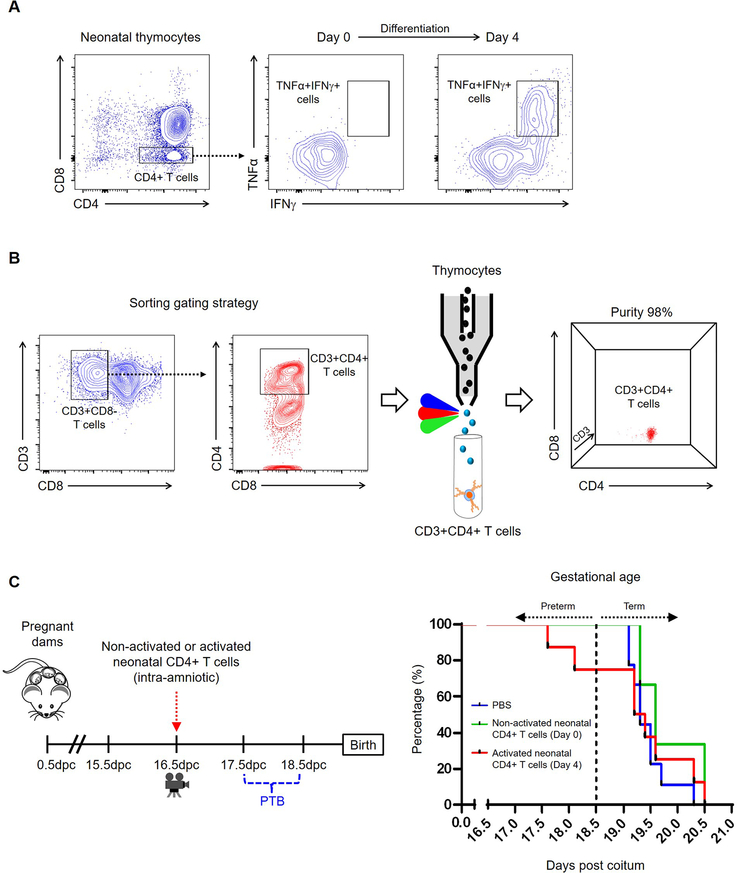
Activated neonatal T cells induce preterm birth in mice
Our above findings suggested that idiopathic preterm labor and birth is associated with fetal T-cell activation. Therefore, we lastly investigated whether the intra-amniotic injection of activated neonatal CD4+ T cells could induce preterm birth. Murine neonates are comparable to human fetuses due to differences in the developmental timelines (102); hence, we isolated thymocytes from one-week-old neonates for in vitro activation. Neonatal thymocytes were activated in vitro, as indicated by the expression of TNFα and IFNγ (Day 4), or were used immediately after isolation (Day 0) (Fig. 6A). Activated or non-activated neonatal T cells were then sorted to obtain viable CD4+ T cells (Fig. 6B). Importantly, the ultrasound-guided intra-amniotic injection of in vitro activated neonatal CD4+ T cells induced 25% of preterm birth, whereas dams injected with non-activated neonatal CD4+ T cells or vehicle control delivered term neonates. Consequently, dams injected with activated neonatal CD4+ T cells delivered sooner than those injected with non-activated neonatal CD4+ T cells or vehicle control (Fig. 6C). These observations provide further evidence that activated T cells alone can trigger the premature process of labor in a subset of preterm births.
Figure 6. Ultrasound-guided intra-amniotic injection of activated and non-activated neonatal T cells.
(A) Representative flow cytometry plots showing the expression of TNFα and IFNγ by neonatal thymocytes after collection (Day 0) and after four days of in vitro activation (Day 4). (B) Representative gating used to sort neonatal CD3+CD4+ T cells. Purity was above 95% for all experiments. (C) Experimental design for the ultrasound-guided intra-amniotic injection of non-activated neonatal CD3+CD4+ T cells (n = 3), in vitro activated neonatal CD3+CD4+ T cells (n = 8), or PBS (vehicle control; n = 9) on 16.5 days post coitum (dpc). Kaplan-Meier survival curve showing the gestational ages at delivery for dams injected with PBS, non-activated CD3+CD4+ T cells, or activated CD3+CD4+ T cells. Preterm delivery was defined as birth before 18.5 dpc, whereas term delivery was considered as birth after 18.5 dpc.
DISCUSSION
To our knowledge, herein we report for the first time that the fetal immune system undergoes activation in women with preterm labor and birth in the absence of intra-amniotic inflammation, providing a potential new mechanism of disease for a subset of idiopathic preterm birth. Specifically, we first reported that fetal T cells are the predominant immune cell population in amniotic fluid during preterm gestations. Interestingly, only fetal CD4+ T cells were increased in amniotic fluid of women who underwent idiopathic preterm labor and birth. This increase in fetal CD4+ T cells was accompanied by mildly elevated amniotic fluid concentrations of T-cell cytokines such as IL-2, IL-4, and IL-13, which are expressed by these cells upon in vitro stimulation, but was not associated with the prototypical cytokine profile observed in cases with intra-amniotic inflammation. We also found that cord blood T cells, mainly CD4+ T cells, obtained from women with idiopathic preterm labor and birth displayed enhanced ex vivo activation, which is similar to that observed in women with intra-amniotic inflammation. Finally, we showed that the intra-amniotic administration of in vitro activated neonatal CD4+ T cells induces preterm birth in a subset of dams. These data suggest that, in a subset of women with idiopathic preterm birth, activated fetal T cells can trigger the premature process of labor.
The concept that amniotic fluid immune cells are of fetal origin emerged upon the observation that polymorphonuclear leukocytes obtained from amniotic fluid of women with preterm labor and clinical evidence of infection who delivered a male neonate carried X and Y chromosomes (81). A follow-up study in Rhesus macaques showed that amniotic fluid leukocytes in preterm gestations could be of either maternal or fetal origin: maternal and fetal leukocytes were observed under physiological conditions, whereas after the induction of intra-amniotic inflammation by infusion of IL-1β the fetal contribution predominated (82). More recently, using DNA fingerprinting, we demonstrated that, in cases with intra-amniotic inflammation/infection, fetal neutrophils invade the amniotic cavity of women in preterm gestations, whereas maternal neutrophils are predominantly present at term (51). These studies indicate that, in preterm gestations with intra-amniotic infection (81) or inflammation (82), amniotic fluid leukocytes are of fetal origin; yet, in cases at term, these cells can also be of maternal origin (51). In the absence of intra-amniotic infection or inflammation, amniotic fluid leukocytes (mostly lymphocytes (83, 84)) could be either of maternal or fetal origin (82); however, the origin of amniotic fluid leukocytes in preterm gestations requires further investigation since it is likely that gestational age and labor influence the origin of these immune cells. The present investigation provides conclusive evidence that amniotic fluid leukocytes in preterm gestations, primarily T cells, are exclusively of fetal origin in the absence of intra-amniotic inflammation. A proposed source for fetal immune cells in the amniotic cavity is the fetal organs, such as the gut and lungs, which are continuously exposed to amniotic fluid (83). Consistently, amniotic fluid T cells (84) and innate lymphoid cells (83) express a surface marker phenotype resembling that displayed by mucosal immune cells in the fetal gut (83, 103) and lungs (83). Moreover, fetuses with gastroschisis, a congenital defect of the abdominal wall that results in exposure and inflammation of the intestines, have increased T-cell infiltration in the gut mucosa (94) that may result in augmented numbers of such cells in amniotic fluid. Hence, T cells found in amniotic fluid during preterm gestations are of fetal origin and display a unique mucosal phenotype.
A major finding of our investigation was that fetal CD4+ T cells are increased in amniotic fluid of women with idiopathic preterm labor who delivered preterm. This rise in fetal CD4+ T cells coincided with mildly elevated amniotic fluid concentrations of the T cell-related cytokines IL-2, IL-4, and IL-13, and enhanced cord blood T-cell ex vivo activation. Notably, we found that amniotic fluid fetal T cells are capable of producing IL-2, IL-4, and IL-13 upon in vitro stimulation, indicating that these responses could occur in the context of preterm labor. These data suggest that fetal T-cell activation precedes preterm birth in the absence of intra-amniotic inflammation, which complements prior evidence showing that the fetal innate immune system is activated during preterm parturition independent of infection/inflammation (56), and that the fetus can signal the onset of labor (104–106). Thus, both the innate and adaptive immune system of the fetus are activated during the pathological process of idiopathic preterm labor that leads to preterm birth.
How do amniotic fluid fetal T cells trigger preterm parturition in the absence of intra-amniotic inflammation/infection? Answering this question would require the isolation of sufficient amniotic fluid fetal T cells to test their specificity and functionality ex vivo, which is extremely difficult when working with amniotic fluid leukocytes from women in preterm labor, from which we typically obtain few milliliters of amniotic fluid (10 – 100 cells per milliliter before sorting). The most tempting explanation for the induction of preterm labor by fetal T cells is that such cells are responding to maternal alloantigens. This concept is based on previous observations showing that central memory CD4+ T cells are increased in the cord blood of neonates born to women with preterm labor (66, 107) and display an activated phenotype (66, 108). Such fetal T cells possess the following properties: 1) proliferate in the presence of maternal antigens, but do not proliferate in response to third-party antigens; 2) release IFNγ and TNFα upon ex vivo stimulation; and 3) stimulate myometrial contractility ex vivo (66). Together with the abovementioned studies, our findings provide a potential mechanism whereby fetal T cells can lead to idiopathic preterm labor and birth in the absence of the clinical condition of intra-amniotic inflammation. Nonetheless, additional investigation, including HLA typing of the mother and neonate, will be required to investigate whether idiopathic preterm labor occurs as a result of the activation of allogeneic fetal T cells.
Interestingly, cord blood T cells from neonates born to women with idiopathic preterm labor and birth exhibited enhanced ex vivo cytokine responses. However, such a subset of preterm birth occurred in the absence of a prototypical cytokine response in the amniotic cavity (IL-6 (30), IL-8 (99), IL-1β (33, 100), and IL-12p70 (101)), which is observed in cases with inflammation-associated preterm labor and birth. These findings led us to propose that, in a subset of women with idiopathic preterm labor and birth, enhanced fetal T-cell cytokine responses in the amniotic cavity occurred in the absence of the canonical pro-inflammatory cytokine response, which is mainly initiated by neutrophils and monocytes invading this compartment in the context of infection or sterile inflammation (49, 54, 109). In line with this proposal, we demonstrated herein that the intra-amniotic administration of activated neonatal CD4+ T cells induces preterm labor and birth. Therefore, it is possible that, in humans, activated fetal T cells induce preterm parturition as proposed herein. It is worth mentioning that, due to the difficulty of obtaining a sufficient number of amniotic fluid cells, we used cord blood T cells from neonates born to women with idiopathic or intra-amniotic inflammation-associated preterm labor to test their functionality in these two subsets of preterm birth. Yet, cord blood immune responses are distinct from those of amniotic fluid immune cells (Fig. 1); therefore, further investigation is needed to evaluate whether amniotic fluid immune responses differ between these two subsets of preterm birth.
In summary, the findings presented in this report allow us to suggest a new mechanism of disease for a subset of idiopathic preterm labor, in which fetal T cells mediate a unique inflammatory response in the amniotic cavity, partially mediated by IL-2, IL-4, and IL-13, which precedes preterm birth (Visual Abstract, A). Such a mechanism of disease is different from that observed in women with preterm labor and intra-amniotic inflammation, in which maternal and fetal innate immune cells (neutrophils and macrophages) and T cells respond towards microbial products, involving the prototypical cytokines IL-6, IL-1β, and IL-8 (Visual Abstract, B). Finally, we showed that the intra-amniotic administration of activated neonatal CD4+ T cells induces preterm birth in a subset of dams. Collectively, these findings provide insight into a potential new mechanism implicated in the pathogenesis of idiopathic preterm labor and birth, the leading cause of perinatal morbidity and mortality worldwide.
Supplementary Material
Key Points.
Amniotic fluid T cells are of fetal origin and functional in preterm gestations
Amniotic fluid T cells are increased in women with idiopathic preterm labor and birth
Intra-amniotic injection of activated neonatal T cells induces preterm birth in mice
ACKNOWLEDGMENTS
We thank the physicians, nurses, and research assistants from the Center for Advanced Obstetrical Care and Research, Intrapartum Unit, PRB Clinical Laboratory, and PRB Perinatal Translational Science Laboratory for their help with collecting and processing samples. We would especially like to thank George Schwenkel for his assistance with the animal experiments. We are grateful to Dr. Tippi C. MacKenzie for helpful discussions of the findings.
This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health.
3. Non-standard abbreviations
- IAI
intra-amniotic inflammation
- PTL
preterm labor
- PTB
preterm birth
- TB
term birth
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, and Lawn JE 2012. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (London, England) 379: 2162–2172. [DOI] [PubMed] [Google Scholar]
- 2.Monier I, Ancel PY, Ego A, Jarreau PH, Lebeaux C, Kaminski M, Goffinet F, and Zeitlin J 2017. Fetal and neonatal outcomes of preterm infants born before 32 weeks of gestation according to antenatal vs postnatal assessments of restricted growth. American journal of obstetrics and gynecology 216: 516.e511–516.e510. [DOI] [PubMed] [Google Scholar]
- 3.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, Lewis C, Rattanakanokchai S, Teng DN, Thinkhamrop J, Watananirun K, Zhang J, Zhou W, and Gulmezoglu AM 2018. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. The Lancet. Global health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travers CP, Carlo WA, McDonald SA, Das A, Bell EF, Ambalavanan N, Jobe AH, Goldberg RN, D’Angio CT, Stoll BJ, Shankaran S, Laptook AR, Schmidt B, Walsh MC, Sanchez PJ, Ball MB, Hale EC, Newman NS, and Higgins RD 2018. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. American journal of obstetrics and gynecology 218: 130.e131–130.e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubow JM, How HY, Habli M, Maxwell R, and Sibai BM 2009. Indications for delivery and short-term neonatal outcomes in late preterm as compared with term births. American journal of obstetrics and gynecology 200: e30–33. [DOI] [PubMed] [Google Scholar]
- 6.Mwaniki MK, Atieno M, Lawn JE, and Newton CR 2012. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet (London, England) 379: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, Thorp JM, Caritis SN, Prasad M, Tita AT, Saade GR, Sorokin Y, Rouse DJ, Blackwell SC, and Tolosa JE 2016. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. American journal of obstetrics and gynecology 215: 103.e101–103.e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevallier M, Debillon T, Pierrat V, Delorme P, Kayem G, Durox M, Goffinet F, Marret S, and Ancel PY 2017. Leading causes of preterm delivery as risk factors for intraventricular hemorrhage in very preterm infants: results of the EPIPAGE 2 cohort study. American journal of obstetrics and gynecology 216: 518.e511–518.e512. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg RL, Culhane JF, Iams JD, and Romero R 2008. Epidemiology and causes of preterm birth. Lancet (London, England) 371: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Dey SK, and Fisher SJ 2014. Preterm labor: one syndrome, many causes. Science 345: 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravett MG, Hummel D, Eschenbach DA, and Holmes KK 1986. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstetrics and gynecology 67: 229–237. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, and Hobbins JC 1988. Infection in the pathogenesis of preterm labor. Seminars in perinatology 12: 262–279. [PubMed] [Google Scholar]
- 13.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, and Hobbins JC 1989. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. American journal of obstetrics and gynecology 161: 817–824. [DOI] [PubMed] [Google Scholar]
- 14.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, and Novy MJ 1994. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. American journal of obstetrics and gynecology 171: 1660–1667. [DOI] [PubMed] [Google Scholar]
- 15.Gomez R, Romero R, Edwin SS, and David C 1997. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infectious disease clinics of North America 11: 135–176. [DOI] [PubMed] [Google Scholar]
- 16.Kallapur SG, Willet KE, Jobe AH, Ikegami M, and Bachurski CJ 2001. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 280: L527–536. [DOI] [PubMed] [Google Scholar]
- 17.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, and Waites KB 2009. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reproductive sciences (Thousand Oaks, Calif.) 16: 56–70. [DOI] [PubMed] [Google Scholar]
- 18.Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, Aravind L, Hitti J, Adams Waldorf KM, and Rajagopal L 2013. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J Exp Med 210: 1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, Rael J, Grove T, Morgan TK, Clewell W, Miller H, Luthy D, Pereira L, Nageotte M, Robilio PA, Fortunato S, Simhan H, Baxter JK, Amon E, Franco A, Trofatter K, and Heyborne K 2014. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. American journal of obstetrics and gynecology 210: 125.e121–125.e115. [DOI] [PubMed] [Google Scholar]
- 20.Cobo T, Kacerovsky M, and Jacobsson B 2014. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. American journal of obstetrics and gynecology 211: 708. [DOI] [PubMed] [Google Scholar]
- 21.Bobitt JR, and Ledger WJ 1977. Unrecognized amnionitis and prematurity: a preliminary report. The Journal of reproductive medicine 19: 8–12. [PubMed] [Google Scholar]
- 22.Bobitt JR, Hayslip CC, and Damato JD 1981. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. American journal of obstetrics and gynecology 140: 947–952. [DOI] [PubMed] [Google Scholar]
- 23.Wallace RL, and Herrick CN 1981. Amniocentesis in the evaluation of premature labor. Obstetrics and gynecology 57: 483–486. [PubMed] [Google Scholar]
- 24.Wahbeh CJ, Hill GB, Eden RD, and Gall SA 1984. Intra-amniotic bacterial colonization in premature labor. American journal of obstetrics and gynecology 148: 739–743. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, and Mazor M 1988. Infection and preterm labor. Clinical obstetrics and gynecology 31: 553–584. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Avila C, Brekus CA, and Morotti R 1991. The role of systemic and intrauterine infection in preterm parturition. Annals of the New York Academy of Sciences 622: 355–375. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, and Sweet RL 1992. A review of premature birth and subclinical infection. American journal of obstetrics and gynecology 166: 1515–1528. [DOI] [PubMed] [Google Scholar]
- 28.Watts DH, Krohn MA, Hillier SL, and Eschenbach DA 1992. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstetrics and gynecology 79: 351–357. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, and Kim YM 2001. The role of infection in preterm labour and delivery. Paediatric and perinatal epidemiology 15 Suppl 2: 41–56. [DOI] [PubMed] [Google Scholar]
- 30.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, and Jun JK 2001. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology 185: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Espinoza J, Chaiworapongsa T, and Kalache K 2002. Infection and prematurity and the role of preventive strategies. Seminars in neonatology : SN 7: 259–274. [DOI] [PubMed] [Google Scholar]
- 32.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, McCormack A, Lapidus JA, Hitti J, Eschenbach DA, Roberts CT Jr., and Nagalla SR 2004. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. Jama 292: 462–469. [DOI] [PubMed] [Google Scholar]
- 33.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, and Dinarello CA 1992. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. American journal of reproductive immunology (New York, N.Y. : 1989) 27: 117–123. [DOI] [PubMed] [Google Scholar]
- 34.Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, Kusanovic JP, Tolosa JE, Hassan SS, and Espinoza J 2007. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. Journal of perinatal medicine 35: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, Edwin S, Gomez R, Hassan SS, and Romero R 2008. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 21: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, and Hassan SS 2011. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 24: 1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, and Hassan SS 2016. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. American journal of reproductive immunology (New York, N.Y. : 1989) 75: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, and Yeo L 2014. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. American journal of reproductive immunology (New York, N.Y. : 1989) 72: 458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, and Kim YM 2015. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 28: 1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibbs RS, and Duff P 1991. Progress in pathogenesis and management of clinical intraamniotic infection. American journal of obstetrics and gynecology 164: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 41.Oh KJ, Kim SM, Hong JS, Maymon E, Erez O, Panaitescu B, Gomez-Lopez N, Romero R, and Yoon BH 2017. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. American journal of obstetrics and gynecology 216: 604 e601–604 e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Flores V, Romero R, Miller D, Xu Y, Done B, Veerapaneni C, Leng Y, Arenas-Hernandez M, Khan N, Panaitescu B, Hassan SS, Alvarez-Salas LM, and Gomez-Lopez N 2018. Inflammation-Induced Adverse Pregnancy and Neonatal Outcomes Can Be Improved by the Immunomodulatory Peptide Exendin-4. Frontiers in immunology 9: 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, and Hobbins JC 1991. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. American journal of obstetrics and gynecology 165: 821–830. [DOI] [PubMed] [Google Scholar]
- 44.Hsu CD, Meaddough E, Aversa K, Hong SF, Lee IS, Bahodo-Singh RO, Lu LC, and Copel JA 1998. Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection. American journal of perinatology 15: 683–687. [DOI] [PubMed] [Google Scholar]
- 45.Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, and Edwin S 2001. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. American journal of obstetrics and gynecology 185: 1143–1148. [DOI] [PubMed] [Google Scholar]
- 46.Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, Ohlsson K, and Uldbjerg N 2002. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 12: 237–246. [DOI] [PubMed] [Google Scholar]
- 47.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, Blackwell S, Whitty J, Berman S, Redman M, Yoon BH, and Sorokin Y 2003. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 13: 2–21. [DOI] [PubMed] [Google Scholar]
- 48.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, and Romero R 2007. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 20: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, and Margolis L 2015. Evidence of perturbations of the cytokine network in preterm labor. American journal of obstetrics and gynecology 213: 836 e831–836 e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A, Hassan SS, and Panaitescu B 2017. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. American journal of reproductive immunology (New York, N.Y. : 1989) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, Jacques SM, Hassan SS, Faro J, Alsamsam A, Alhousseini A, Gomez-Roberts H, Panaitescu B, Yeo L, and Maymon E 2017. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? American journal of obstetrics and gynecology 217: 693 e691–693 e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, Shaman M, Jacques SM, Panaitescu B, Garcia-Flores V, and Hassan SS 2017. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reproductive sciences (Thousand Oaks, Calif.) 24: 1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nadeau-Vallee M, Chin PY, Belarbi L, Brien ME, Pundir S, Berryer MH, Beaudry-Richard A, Madaan A, Sharkey DJ, Lupien-Meilleur A, Hou X, Quiniou C, Beaulac A, Boufaied I, Boudreault A, Carbonaro A, Doan ND, Joyal JS, Lubell WD, Olson DM, Robertson SA, Girard S, and Chemtob S 2017. Antenatal Suppression of IL-1 Protects against Inflammation-Induced Fetal Injury and Improves Neonatal and Developmental Outcomes in Mice. J Immunol 198: 2047–2062. [DOI] [PubMed] [Google Scholar]
- 54.Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, Faro J, Pacora P, Hassan SS, and Hsu CD 2018. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. American journal of reproductive immunology (New York, N.Y. : 1989) 80: e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varrey A, Romero R, Panaitescu B, Miller D, Chaiworapongsa T, Patwardhan M, Faro J, Pacora P, Hassan SS, Hsu CD, and Gomez-Lopez N 2018. Human beta-defensin-1: A natural antimicrobial peptide present in amniotic fluid that is increased in spontaneous preterm labor with intra-amniotic infection. American journal of reproductive immunology (New York, N.Y. : 1989) 80: e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, and Bianchi DW 1995. Premature parturition is characterized by in utero activation of the fetal immune system. American journal of obstetrics and gynecology 173: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 57.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, and Berry SM 1998. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. American journal of obstetrics and gynecology 179: 186–193. [DOI] [PubMed] [Google Scholar]
- 58.Yoon BH, Romero R, Jun JK, Maymon E, Gomez R, Mazor M, and Park JS 1998. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. American journal of obstetrics and gynecology 179: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 59.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, and Hassan SS 2007. The fetal inflammatory response syndrome. Clinical obstetrics and gynecology 50: 652–683. [DOI] [PubMed] [Google Scholar]
- 60.Lee SE, Romero R, Jung H, Park CW, Park JS, and Yoon BH 2007. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. American journal of obstetrics and gynecology 197: 294 e291–296. [DOI] [PubMed] [Google Scholar]
- 61.Melville JM, Bischof RJ, Meeusen EN, Westover AJ, and Moss TJ 2012. Changes in fetal thymic immune cell populations in a sheep model of intrauterine inflammation. Reproductive sciences (Thousand Oaks, Calif.) 19: 740–747. [DOI] [PubMed] [Google Scholar]
- 62.Kallapur SG, Presicce P, Rueda CM, Jobe AH, and Chougnet CA 2014. Fetal immune response to chorioamnionitis. Semin Reprod Med 32: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rueda CM, Presicce P, Jackson CM, Miller LA, Kallapur SG, Jobe AH, and Chougnet CA 2016. Lipopolysaccharide-Induced Chorioamnionitis Promotes IL-1-Dependent Inflammatory FOXP3+ CD4+ T Cells in the Fetal Rhesus Macaque. J Immunol 196: 3706–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jackson CM, Wells CB, Tabangin ME, Meinzen-Derr J, Jobe AH, and Chougnet CA 2017. Pro-inflammatory immune responses in leukocytes of premature infants exposed to maternal chorioamnionitis or funisitis. Pediatr Res 81: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matta P, Sherrod SD, Marasco CC, Moore DJ, McLean JA, and Weitkamp JH 2017. In Utero Exposure to Histological Chorioamnionitis Primes the Exometabolomic Profiles of Preterm CD4(+) T Lymphocytes. J Immunol 199: 3074–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frascoli M, Coniglio L, Witt R, Jeanty C, Fleck-Derderian S, Myers DE, Lee TH, Keating S, Busch MP, Norris PJ, Tang Q, Cruz G, Barcellos LF, Gomez-Lopez N, Romero R, and MacKenzie TC 2018. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Science translational medicine 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.St Louis D, Romero R, Plazyo O, Arenas-Hernandez M, Panaitescu B, Xu Y, Milovic T, Xu Z, Bhatti G, Mi QS, Drewlo S, Tarca AL, Hassan SS, and Gomez-Lopez N 2016. Invariant NKT Cell Activation Induces Late Preterm Birth That Is Attenuated by Rosiglitazone. J Immunol 196: 1044–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, Garcia-Flores V, Hassan SS, Xu Z, Tarca AL, Drewlo S, and Gomez-Lopez N 2016. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J Immunol 196: 2476–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cross SN, Potter JA, Aldo P, Kwon JY, Pitruzzello M, Tong M, Guller S, Rothlin CV, Mor G, and Abrahams VM 2017. Viral Infection Sensitizes Human Fetal Membranes to Bacterial Lipopolysaccharide by MERTK Inhibition and Inflammasome Activation. J Immunol 199: 2885–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Powell RM, Lissauer D, Tamblyn J, Beggs A, Cox P, Moss P, and Kilby MD 2017. Decidual T Cells Exhibit a Highly Differentiated Phenotype and Demonstrate Potential Fetal Specificity and a Strong Transcriptional Response to IFN. J Immunol 199: 3406–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, Than NG, Chaiworapongsa T, Panaitescu B, Dong Z, Tarca AL, Abrahams VM, Yeo L, and Hassan SS 2017. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reproductive sciences (Thousand Oaks, Calif.) 24: 1382–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marcellin L, Schmitz T, Messaoudene M, Chader D, Parizot C, Jacques S, Delaire J, Gogusev J, Schmitt A, Lesaffre C, Breuiller-Fouche M, Caignard A, Vaiman D, Goffinet F, Cabrol D, Gorochov G, and Mehats C 2017. Immune Modifications in Fetal Membranes Overlying the Cervix Precede Parturition in Humans. J Immunol 198: 1345–1356. [DOI] [PubMed] [Google Scholar]
- 73.Xu Y, Romero R, Miller D, Silva P, Panaitescu B, Theis KR, Arif A, Hassan SS, and Gomez-Lopez N 2018. Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. American journal of reproductive immunology (New York, N.Y. : 1989) 79: e12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, Hsu CD, and Panaitescu B 2018. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth and adverse neonatal outcomes. Biol Reprod. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rogers LM, Anders AP, Doster RS, Gill EA, Gnecco JS, Holley JM, Randis TM, Ratner AJ, Gaddy JA, Osteen K, and Aronoff DM 2018. Decidual stromal cell-derived PGE2 regulates macrophage responses to microbial threat. American journal of reproductive immunology (New York, N.Y. : 1989) 80: e13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Faro J, Romero R, Schwenkel G, Garcia-Flores V, Arenas-Hernandez M, Leng Y, Xu Y, Miller D, Hassan SS, and Gomez-Lopez N 2018. Intra-Amniotic inflammation induces preterm birth by Activating the NLRP3 inflammasome. Biol Reprod. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Zwan A, Bi K, Norwitz ER, Crespo AC, Claas FHJ, Strominger JL, and Tilburgs T 2018. Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc Natl Acad Sci U S A 115: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leng Y, Romero R, Xu Y, Galaz J, Slutsky R, Arenas-Hernandez M, Garcia-Flores V, Motomura K, Hassan SS, Reboldi A, and Gomez-Lopez N 2019. Are B cells altered in the decidua of women with preterm or term labor? American journal of reproductive immunology (New York, N.Y. : 1989) 81: e13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arenas-Hernandez M, Romero R, Xu Y, Panaitescu B, Garcia-Flores V, Miller D, Ahn H, Done B, Hassan SS, Hsu CD, Tarca AL, Sanchez-Torres C, and Gomez-Lopez N 2019. Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone. J Immunol 202: 2585–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barros FC, Papageorghiou AT, Victora CG, Noble JA, Pang R, Iams J, Cheikh Ismail L, Goldenberg RL, Lambert A, Kramer MS, Carvalho M, Conde-Agudelo A, Jaffer YA, Bertino E, Gravett MG, Altman DG, Ohuma EO, Purwar M, Frederick IO, Bhutta ZA, Kennedy SH, Villar J, International F, and Newborn C Growth Consortium for the 21st. 2015. The distribution of clinical phenotypes of preterm birth syndrome: implications for prevention. JAMA Pediatr 169: 220–229. [DOI] [PubMed] [Google Scholar]
- 81.Sampson JE, Theve RP, Blatman RN, Shipp TD, Bianchi DW, Ward BE, and Jack RM 1997. Fetal origin of amniotic fluid polymorphonuclear leukocytes. American journal of obstetrics and gynecology 176: 77–81. [DOI] [PubMed] [Google Scholar]
- 82.Macias AE, Wong SW, Sadowsky DW, Luetjens CM, Axthelm MK, Gravett MG, Haluska GJ, and Novy MJ 2001. Maternal or fetal origin of rhesus monkey (Macaca mulatta) amniotic fluid leukocytes can be identified by polymerase chain reaction using the zinc finger Y gene. Am J Primatol 55: 159–170. [DOI] [PubMed] [Google Scholar]
- 83.Marquardt N, Ivarsson MA, Sundstrom E, Akesson E, Martini E, Eidsmo L, Mjosberg J, Friberg D, Kublickas M, Ek S, Tegerstedt G, Seiger A, Westgren M, and Michaelsson J 2016. Fetal CD103+ IL-17-Producing Group 3 Innate Lymphoid Cells Represent the Dominant Lymphocyte Subset in Human Amniotic Fluid. J Immunol 197: 3069–3075. [DOI] [PubMed] [Google Scholar]
- 84.Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B, Silva P, Faro J, Alhousseini A, Gill N, Hassan SS, and Hsu CD 2018. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. American journal of reproductive immunology (New York, N.Y. : 1989) 79: e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, and Edberg S 1988. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. American journal of obstetrics and gynecology 159: 114–119. [DOI] [PubMed] [Google Scholar]
- 86.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, and Diamond MP 1990. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. American journal of obstetrics and gynecology 163: 968–974. [DOI] [PubMed] [Google Scholar]
- 87.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, and Kim YM 2015. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. American journal of obstetrics and gynecology 213: S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Redline RW 2015. Classification of placental lesions. American journal of obstetrics and gynecology 213: S21–28. [DOI] [PubMed] [Google Scholar]
- 89.Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, and Gomez-Lopez N 2016. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol 13: 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoyes AD 1968. Ultrastructure of the cells of the amniotic fluid. The Journal of obstetrics and gynaecology of the British Commonwealth 75: 164–171. [DOI] [PubMed] [Google Scholar]
- 91.Casadei R, D’Ablaing G 3rd, Kaplan BJ, and Schwinn CP 1973. A cytologic study of amniotic fluid. Acta cytologica 17: 289–298. [PubMed] [Google Scholar]
- 92.Sutherland GR, Bauld R, and Bain AD 1974. Observations on human amniotic fluid cell strains in serial culture. Journal of medical genetics 11: 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cutz E, and Conen PE 1978. Macrophages and epithelial cells in human amniotic fluid: transmission and scanning electron microscopic study. The American journal of anatomy 151: 87–101. [DOI] [PubMed] [Google Scholar]
- 94.Frascoli M, Jeanty C, Fleck S, Moradi PW, Keating S, Mattis AN, Tang Q, and MacKenzie TC 2016. Heightened Immune Activation in Fetuses with Gastroschisis May Be Blocked by Targeting IL-5. J Immunol 196: 4957–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, and Oppenheim JJ 1993. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med 177: 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, and Neote K 1998. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med 187: 2009–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, and Kim JS 2010. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 23: 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Romero R, Chaemsaithong P, Chaiyasit N, Docheva N, Dong Z, Kim CJ, Kim YM, Kim JS, Qureshi F, Jacques SM, Yoon BH, Chaiworapongsa T, Yeo L, Hassan SS, Erez O, and Korzeniewski SJ 2017. CXCL10 and IL-6: Markers of two different forms of intra-amniotic inflammation in preterm labor. American journal of reproductive immunology (New York, N.Y. : 1989) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Romero R, Ceska M, Avila C, Mazor M, Behnke E, and Lindley I 1991. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. American journal of obstetrics and gynecology 165: 813–820. [DOI] [PubMed] [Google Scholar]
- 100.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, and Durum SK 1989. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. American journal of obstetrics and gynecology 160: 1117–1123. [DOI] [PubMed] [Google Scholar]
- 101.Revello R, Alcaide MJ, Dudzik D, Abehsera D, and Bartha JL 2016. Differential amniotic fluid cytokine profile in women with chorioamnionitis with and without funisitis. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 29: 2161–2165. [DOI] [PubMed] [Google Scholar]
- 102.Xue L, Cai JY, Ma J, Huang Z, Guo MX, Fu LZ, Shi YB, and Li WX 2013. Global expression profiling reveals genetic programs underlying the developmental divergence between mouse and human embryogenesis. BMC Genomics 14: 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li N, van Unen V, Abdelaal T, Guo N, Kasatskaya SA, Ladell K, McLaren JE, Egorov ES, Izraelson M, Chuva SM de Sousa Lopes, Hollt T, Britanova OV, Eggermont J, de Miranda N, Chudakov DM, Price DA, Lelieveldt BPF, and Koning F 2019. Memory CD4(+) T cells are generated in the human fetal intestine. Nat Immunol 20: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Condon JC, Jeyasuria P, Faust JM, and Mendelson CR 2004. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A 101: 4978–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mendelson CR 2009. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Molecular endocrinology (Baltimore, Md.) 23: 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mendelson CR, Montalbano AP, and Gao L 2017. Fetal-to-maternal signaling in the timing of birth. The Journal of steroid biochemistry and molecular biology 170: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luciano AA, Yu H, Jackson LW, Wolfe LA, and Bernstein HB 2011. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS One 6: e16698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berry SM, Fine N, Bichalski JA, Cotton DB, Dombrowski MP, and Kaplan J 1992. Circulating lymphocyte subsets in second- and third-trimester fetuses: comparison with newborns and adults. American journal of obstetrics and gynecology 167: 895–900. [DOI] [PubMed] [Google Scholar]
- 109.Gomez-Lopez N, Romero R, Galaz J, Xu Y, Panaitescu B, Slutsky R, Motomura K, Gill N, Para R, Pacora P, Jung E, and Hsu CD 2019. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.