Abstract
Background:
Critical limb ischemia (CLI) is diagnosed by the presence of rest pain, tissue ulceration, or gangrene due to chronic arterial insufficiency. It is unclear what fraction of patients with suspected CLI have severe peripheral artery disease (PAD) on non-invasive functional testing.
Objectives:
To describe the distribution of pre-intervention treated limb ankle-brachial indices (ABIs) among patients with CLI undergoing percutaneous vascular intervention (PVI) or surgical revascularization (SR).
Methods:
We included patients who underwent lower extremity revascularization for CLI in a multicenter registry in Michigan from January 2012 through June 2015. ABIs were classified as normal (0.91–1.40), mild-moderate (0.41–0.90), and severe (≤0.40). Pre- and post-intervention Peripheral Artery Questionnaire (PAQ) summary scores were assessed in a subset of patients.
Results:
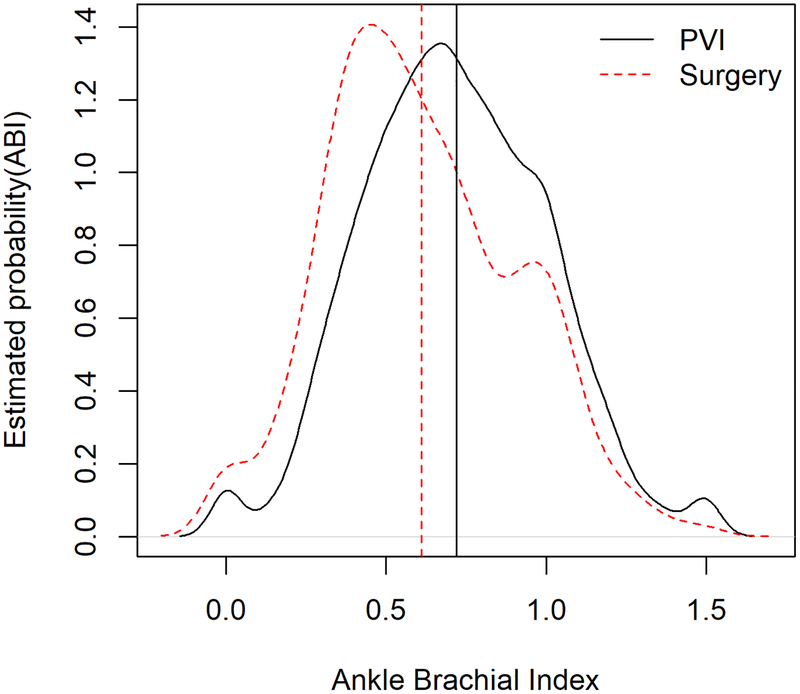
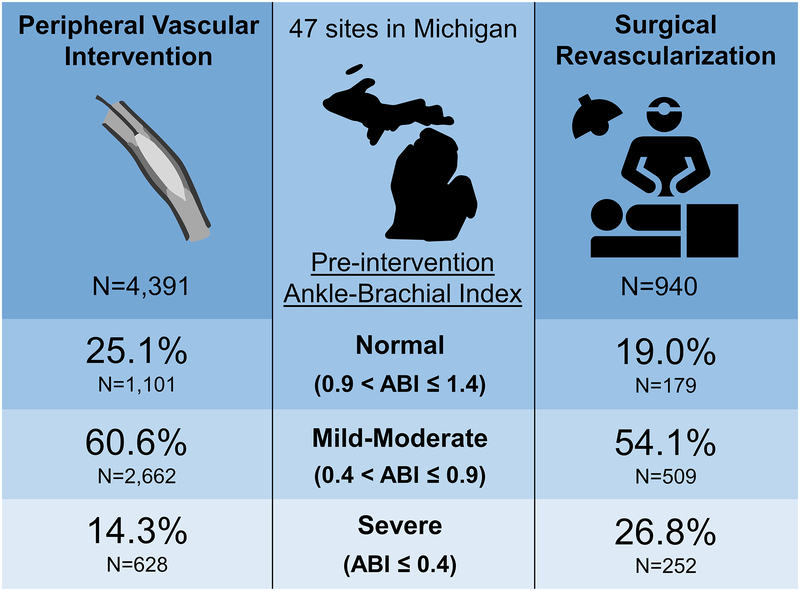
Among 10,756 patients with signs or symptoms of CLI, 9,113 (84.7%) underwent PVI and 1,643 (15.3%) underwent SR. ABIs were recorded in 4,972 (54.6%) PVI and 1,012 (61.6%) SR patients. Patients undergoing PVI had higher ABIs than those undergoing SR, with substantial variation in both groups [mean±SD, PVI: 0.72±0.29 vs. SR: 0.61±0.29; P<0.001]. Nearly a quarter of patients with compressible arteries had normal ABIs (24.0%), whereas severe PAD was uncommon (16.5%). A significant improvement in PAQ scores was noted after intervention across all ABI categories.
Conclusions:
Among patients undergoing revascularization for CLI in contemporary practice, we found substantial heterogeneity in pre-intervention ABIs. The disconnect between ABI results and clinical diagnosis calls into question the utility of ABIs in this population and suggests the need for standardization of functional PAD testing.
Keywords: peripheral artery disease, critical limb ischemia, ankle-brachial index, percutaneous vascular intervention, surgical bypass graft
Condensed Abstract:
It is unclear what fraction of patients with suspected CLI have severe peripheral artery disease (PAD) on non-invasive functional testing. We sought to describe the distribution of pre-intervention treated limb ankle-brachial indices (ABIs) among patients with CLI undergoing percutaneous vascular intervention (PVI) or surgical revascularization (SR) in the state of Michigan. Nearly a quarter of patients with compressible arteries had normal ABIs (24.0%), whereas severe PAD was uncommon (16.5%). The disconnect between ABI results and clinical diagnosis calls into question the utility of ABIs in this population and suggests the need for standardization of functional PAD testing.
Introduction
Peripheral artery disease (PAD) affects approximately 8.5 million Americans (1,2), and the worldwide prevalence continues to increase (3,4). Critical limb ischemia (CLI), a condition characterized by severe chronic arterial insufficiency resulting in rest pain, tissue ulceration, or gangrene (5), represents only a minority of patients with PAD (3,6,7); however, it is associated with significant morbidity and mortality (5,8). Because the signs and symptoms of CLI may be due to non-vascular etiologies (8), current guidelines recommend assessment of vascular insufficiency using objective functional testing such as the ankle-brachial index (ABI), toe-brachial index (TBI), tissue oxygen pressure (TcPO2), or skin perfusion pressure (5).
The ankle-brachial index (ABI) is a cost-effective, non-invasive, office-based assessment of arterial perfusion to the lower extremities and is recommended as the first-line investigation for assessment of arterial insufficiency. Although clinical practice guidelines have recommended the use of ABIs in the diagnosis of CLI (5), recent research from a single-center tertiary care institution and a secondary analysis of a randomized controlled trial have called into question the utility of ABI testing for the diagnosis of PAD among patients with suspected CLI (9,10).
It remains unclear what fraction of patients in real-world practice undergoing revascularization for CLI have severe PAD by functional testing. Therefore, using a multicenter, multispecialty, statewide vascular interventions registry in Michigan, we sought to describe the distribution of pre-intervention ABI results among patients undergoing revascularization for CLI.
Methods
Study population
Our study population comprised patients undergoing percutaneous vascular intervention (PVI) or surgical revascularization (SR) for lower-extremity CLI between January 1, 2012 and June 30, 2015 at 47 medical centers in Michigan participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Interventions Collaborative (BMC2 VIC). Thirty-three sites performed both PVI and SR, thirteen performed PVI only, and one performed SR only. Briefly, BMC2 VIC is a statewide, prospective, multicenter, multispecialty quality improvement registry founded in October 2007. A more detailed description of this registry, including data collection and auditing practices, has been previously described (11). PVI was defined as endovascular treatment with angioplasty, atherectomy, or stent implantation. SR was defined as vascular bypass graft surgery.
Patients were included if they had symptoms of CLI, defined as rest pain, tissue ulceration, or gangrene. We excluded patients who were asymptomatic or experienced claudication only; presented with acute limb ischemia, threatened vascular bypass graft(s), or trauma; and patients undergoing intervention for other reasons including a failed endovascular procedure, high stent or stent graft velocities on surveillance Doppler study, complications from a prior procedure, facilitation of a future procedure, and impaired work function. In patients with multiple interventions during the study period, we randomly selected one intervention for inclusion in the study cohort to avoid violating the statistical assumption of independence. Patients without a recorded pre-intervention treated-limb ABI result were excluded from this analysis.
Study measures
Baseline demographic, pre-procedural, and procedural variables were collected for all patients. Pre-intervention treated limb ABI results were categorized into the following groups: severe (≤0.40); mild-moderate (0.41–0.90); and normal (0.91–1.4) (5,12). Although ABIs between 0.91 and 1.00 are often classified as “borderline,” for this study we considered these values to be “normal” since they are not diagnostic of PAD. For a subset of patients, toe-brachial indices (TBIs) were also recorded.
We evaluated changes in disease-specific health status using the Peripheral Artery Questionnaire (PAQ). Briefly, the PAQ is a validated 20-item questionnaire developed to quantify symptoms, function, and quality of life in patients experiencing claudication related to PAD (13,14). A summary score is derived for each patient as the sum of scores from the physical limitation, symptom frequency/burden, social function, and quality-of-life domains divided by four. Scores range from 0 to 100 with higher scores indicating better health status. We attempted to collect pre-intervention PAQs on all patients, and 6-month or 1-year post-intervention PAQs on patients undergoing PVI or SR, respectively.
Finally, we compared rates of post-procedural discharge outcomes including mortality and amputation across ABI categories.
Statistical analysis
Differences in baseline demographic, pre-procedural, and procedural variables were evaluated across ABI categories. We used ANOVA for continuous variables and a chi-square test for categorical variables to identify statistically significant differences across the three categories. If a statistically significant difference was found, pairwise comparisons across categories was conducted using t-tests for continuous variables and chi-square tests for categorical variables with a Bonferroni correction of the p-values for multiple testing. The mean ABI and TBI for patients undergoing PVI versus SR were compared using Student t-test.
Multivariable ordinal logistic regression was used to identify independent predictors of PAD severity by ABI testing. As PAD severity was defined by three ranked categories of ABI values, the proportional odds model assumes that the effect of the predictors on the odds of severity category membership in every subsequent category is the same (15). We assessed the proportionality assumption using the graphical technique described by Harrell (16). To create a parsimonious model, backward stepwise selection of predictors with Akaike’s information criterion was conducted. A detailed description of the candidate predictor variables included in the model along and assessment of model performance can be found in the Supplementary Appendix. As patients were clustered within hospitals and outpatient centers, they could not be assumed to be independent of one another, therefore a “robust” Huber-White covariance matrix was utilized for the calculation of confidence intervals and statistical tests (17).
To evaluate the change in PAQ scores in patients across each ABI category, a generalized least-squares model (GLS) was used as it allows for correlation of pre- and post-intervention PAQ scores within subjects (18). Separate GLS models were created for PVI and SR patients. Initially models included all potential confounders but we ultimately simplified them to include only ABI severity category and pre-/post-intervention time, as additional confounders did not improve model fit. A Huber-White covariance matrix was utilized for the calculation of confidence intervals and statistical tests to account for clustering. Comparisons across ABI categories or across time in the GLS models were adjusted for multiple comparisons with the Bonferroni correction. All analyses were conducted with the R statistical program, version 3.3.1 (19).
Results
Study population
Between January 1, 2012 and June 30, 2015, 32,846 lower extremity interventions were performed at 47 sites. In total, 18,469 (56.2%) interventions were excluded from analysis, many of which were performed for symptoms of intermittent claudication (n=14,551; 44.3%) or in patients with acute limb ischemia (n=3,010; 9.2%). Of the remaining 14,377 lower extremity interventions performed for symptoms of rest pain or signs of tissue ulceration or gangrene, 3,621 interventions were randomly removed from 2,454 patients who had multiple interventions during the study period to avoid violating the statistical assumption of independence. Of the remaining 10,756 patients, 9,113 (84.7%) underwent PVI and 1,643 (15.3%) underwent SR. Pre-intervention ABIs were recorded in 4,972 (54.6%) patients who underwent PVI and in 1,012 (61.6%) patients who underwent SR forming the two study groups of interest.
Patients with ABIs recorded were more frequently white, female, and current tobacco users than patients who did not have ABIs recorded. They also less frequently had a history of diabetes mellitus, renal failure requiring dialysis, and cardiovascular comorbidities (Supplementary Table S1). There was substantial site-level variation in the rate of recorded pre-intervention ABIs (Supplementary Figure S1). Among sites performing PVI, the mean rate of pre-intervention documented ABIs was 50.5% (range: 13.3% to 83.3%), whereas among sites performing SR, the mean rate of ABI documentation was 52.2% (range: 11.3% to 90.0%).
Ankle-brachial indices
Patients undergoing PVI had higher ABIs compared with patients undergoing SR (mean±SD: 0.72±0.29 vs. 0.61±0.29, respectively; P<0.001; Figure 1). Among patients with recorded pre-intervention ABIs, 581 (11.7%) PVI patients and 72 (7.1%) SR patients had ABIs >1.4, indicative of non-compressible arteries. The demographic, clinical, and procedural characteristics of patients with non-compressible ABIs are shown in Tables 1 and 2. These subjects were excluded from further analyses as our primary objective was to evaluate the heterogeneity of diagnostic results, thus leaving 4,391 PVI patients and 940 SR patients in the two study groups. There was substantial heterogeneity in ABI results regardless of the mode of revascularization (Central Illustration). Overall, nearly a quarter of patients (24.0%) had normal ABIs, whereas a small fraction (16.5%) had severe ABIs.
Figure 1: Distribution of ankle-brachial indices by mode of revascularization.
The probability density function for ABI results for patients undergoing percutaneous vascular intervention or surgical revascularization for critical limb ischemia are demonstrated by the solid black and dashed red lines, respectively. The dashed vertical red line indicates the mean ABI for surgical revascularization (mean±SD: 0.61±0.29), whereas the solid black line indicates the mean ABI for PVI (0.72±0.29).
Abbreviations: ABI = ankle-brachial index; PVI = peripheral vascular intervention.
Table 1:
Patient demographic and clinical history characteristics by PAD severity category as defined by the ankle-brachial index.
| Severe | Mild-moderate | Normal | Non-compressible | ||
|---|---|---|---|---|---|
| ABI ≤ 0.4 | 0.4 < ABI ≤ 0.9 | 0.9 < ABI ≤ 1.4 | ABI > 1.4 | ||
| N=880 | N=3,171 | N=1,280 | N=653 | P-value* | |
| Demographics | |||||
| Male gender | 464 (52.7%) | 1780 (56.1%) | 788 (61.6%) | 429 (65.7%) | < 0.001†‡§‖ |
| Age | 70.13 (11.80) | 69.45 (11.69) | 67.79 (12.22) | 71.36 (12.19) | < 0.001†§‖# |
| Body mass index | 27.23 (5.90) | 28.11 (6.06) | 28.54 (6.01) | 28.89 (6.77) | < 0.001*†‡‖ |
| Race | 0.641 | ||||
| White | 682 (77.5%) | 2401 (75.7%) | 954 (74.5%) | 502 (76.9%) | |
| Black | 174 (19.8%) | 676 (21.3%) | 292 (22.8%) | 130 (19.9%) | |
| Other | 24 (2.7%) | 94 (3.0%) | 34 (2.7%) | 21 (3.2%) | |
| ZIP poverty rate | 0.940 | ||||
| 10% or less | 197 (24.0%) | 671 (22.6%) | 290 (24.1%) | 147 (23.8%) | |
| 11% to 19% | 313 (38.1%) | 1128 (38.0%) | 449 (37.3%) | 231 (37.4%) | |
| 20% or more | 311 (37.9%) | 1169 (39.4%) | 464 (38.6%) | 240 (38.8%) | |
| ZIP population density | 0.028† | ||||
| Urban | 499 (60.8%) | 1969 (66.3%) | 816 (67.8%) | 99 (16.0%) | |
| Suburban | 167 (20.3%) | 505 (17.0%) | 195 (16.2%) | 122 (19.7%) | |
| Rural | 155 (18.9%) | 495 (16.7%) | 192 (16.0%) | 397 (64.2%) | |
| Clinical history | |||||
| Smoking status | < 0.001*†‡§‖# | ||||
| Never | 124 (14.1%) | 541 (17.1%) | 276 (21.6%) | 214 (32.8%) | |
| Past | 400 (45.5%) | 1468 (46.3%) | 561 (43.8%) | 336 (51.5%) | |
| Current | 356 (40.5%) | 1162 (36.6%) | 443 (34.6%) | 103 (15.8%) | |
| Hypertension | 815 (92.6%) | 2890 (91.1%) | 1154 (90.2%) | 614 (94.0%) | 0.017# |
| Hyperlipidemia | 732 (83.2%) | 2683 (84.6%) | 1062 (83.0%) | 556 (85.1%) | 0.402 |
| Diabetes mellitus | 447 (50.8%) | 1716 (54.1%) | 692 (54.1%) | 480 (73.5%) | < 0.001‡‖# |
| Chronic obstructive pulmonary disease (COPD) | 293 (33.3%) | 1043 (32.9%) | 348 (27.2%) | 173 (26.5%) | < 0.001†‡§‖ |
| History of TIA/CVA | 304 (34.5%) | 958 (30.2%) | 328 (25.6%) | 199 (30.5%) | < 0.001†§ |
| Renal failure currently requiring dialysis | 58 (6.6%) | 165 (5.2%) | 78 (6.1%) | 143 (21.9%) | < 0.001‡‖# |
| History of renal transplant | 3 (0.3%) | 24 (0.8%) | 13 (1.0%) | 32 (4.9%) | < 0.001‡‖# |
| Family hist. of premature CAD | 135 (15.3%) | 510 (16.1%) | 218 (17.0%) | 92 (14.1%) | 0.378 |
| History of CAD | 462 (52.5%) | 1679 (52.9%) | 682 (53.3%) | 390 (59.7%) | 0.013‡‖# |
| Prior PCI | 206 (23.4%) | 844 (26.6%) | 353 (27.6%) | 186 (28.5%) | 0.094 |
| Prior CABG | 193 (21.9%) | 691 (21.8%) | 255 (19.9%) | 188 (28.8%) | < 0.001‡‖# |
| Prior CHF | 213 (24.2%) | 743 (23.4%) | 289 (22.6%) | 253 (38.7%) | < 0.001‡‖# |
| Significant valve disease | 62 (7.0%) | 231 (7.3%) | 75 (5.9%) | 78 (11.9%) | < 0.001‡‖# |
| Prior PVI | 116 (17.6%) | 484 (20.0%) | 197 (20.2%) | 97 (18.5%) | 0.495 |
| Prior surgical revascularization | 92 (12.1%) | 222 (7.6%) | 86 (7.3%) | 38 (6.7%) | < 0.001*†‡ |
P-values provide ANOVA or chi-square test across all symptom categories. Symbols indicate statistical significance for pairwise Bonferroni corrected (P < 0.008) comparisons:
severe vs. mild-moderate;
severe vs. normal;
severe vs. noncompressible;
mild-moderate vs. normal;
mild-moderate vs. noncompressible;
normal vs. noncompressible.
Abbreviations: CABG = coronary artery bypass grafting; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; CHF = congestive heart failure; PCI = percutaneous coronary intervention; PVI = percutaneous vascular intervention; TIA = transient ischemic attack.
Table 2:
Patient symptoms, indications, medications, and test results by ABI severity.
| Severe | Mild-moderate | Normal | Non-compressible | ||
|---|---|---|---|---|---|
| ABI ≤ 0.4 | 0.4 < ABI ≤ 0.9 | 0.9 < ABI ≤ 1.4 | ABI > 1.4 | ||
| N=880 | N=3,171 | N=1280 | N=653 | P-value | |
| Pre-intervention medications | |||||
| Any anti-platelet | 700 (84.0%) | 2538 (84.3%) | 1021 (83.6%) | 522 (84.5%) | 0.945 |
| Aspirin | 654 (77.4%) | 2390 (78.3%) | 975 (78.4%) | 481 (76.8%) | 0.809 |
| Statin | 612 (72.2%) | 2259 (73.6%) | 871 (70.1%) | 462 (74.2%) | 0.099 |
| Beta blockers | 537 (63.0%) | 1890 (61.0%) | 729 (57.9%) | 422 (66.9%) | 0.001‖# |
| ACE inhibitor | 500 (59.7%) | 1854 (61.3%) | 699 (57.5%) | 341 (56.5%) | 0.039 |
| Symptoms/Indications | |||||
| Anatomic level of treatment: | < 0.001*†‡§‖# | ||||
| Aorto-iliac | 241 (27.5%) | 702 (22.2%) | 190 (14.8%) | 50 (7.7%) | |
| Femoral popliteal | 441 (50.4%) | 1566 (49.5%) | 603 (47.1%) | 252 (38.7%) | |
| Below the knee | 193 (22.1%) | 898 (28.4%) | 487 (38.0%) | 349 (53.6%) | |
| Surgical revascularization | 252 (28.6%) | 509 (16.1%) | 179 (14.0%) | 72 (11.0%) | < 0.001*†‡‖ |
| Repeat procedure | 50 (6.5%) | 88 (3.0%) | 38 (3.1%) | 22 (3.6%) | < 0.001*† |
| Urgent/emergent procedure | 138 (15.7%) | 386 (12.2%) | 158 (12.3%) | 105 (16.1%) | 0.004*‖ |
| Symptom severity | < 0.001‡‖# | ||||
| Rest pain only | 430 (48.9%) | 1585 (50.0%) | 635 (49.6%) | 121 (18.5%) | |
| Ulcer/Gangrene | 450 (51.1%) | 1586 (50.0%) | 645 (50.4%) | 532 (81.5%) | |
| Tests/Labs | |||||
| TBI ≤0.7¥ | 265/268 (98.9%) | 953/1011 (94.3%) | 300/454 (66.1%) | 238/268 (88.8%) | < 0.001*†‡§‖# |
| PAQ summary score | 22.98 (19.72) | 29.04 (21.19) | 34.58 (24.43) | 29.92 (22.87) | < 0.001*†‡§# |
| Contrast cineangiography¥ | 261/262 (99.6%) | 849/852 (99.6%) | 289/291 (99.3%) | 185/185 (100%) | 0.692 |
| CT angiography¥ | 269/270 (99.6%) | 747/760 (98.3%) | 287/291 (98.6%) | 130/133 (97.7%) | 0.363 |
| Exercise ABI¥ | 31/32 (96.9%) | 176/177 (99.4%) | 63/67 (94%) | 7/7 (100%) | 0.067 |
| MR angiography¥ | 20/23 (87.0%) | 97/100 (97.0%) | 39/41 (95.1%) | 18/21 (85.7%) | 0.097 |
| Hemoglobin (mg/dL) | 12.22 (2.12) | 12.5 (2.02) | 12.62 (2.01) | 11.44 (1.96) | < 0.001*†‡‖# |
| Creatinine (mg/dL) | 1.06 (0.39) | 1.07 (0.40) | 1.05 (0.35) | 1.18 (0.49) | < 0.001‡‖# |
P-values provide ANOVA or chi-square test across all symptom categories. Symbols indicate statistical significance for pairwise Bonferroni corrected (P < 0.008) comparisons:
severe vs. mild-moderate;
severe vs. normal;
severe vs. noncompressible;
mild-moderate vs. normal;
mild-moderate vs. noncompressible;
normal vs. noncompressible,
the number of subjects with abnormal test results divided by the number of subjects who had the test performed.
Abbreviations: ABI = ankle-brachial index; ACE = angiotensin-converting enzyme; CT = computed tomography; MR = magnetic resonance; PAQ = Peripheral Artery Questionnaire; TBI = toe-brachial index.
Central Illustration: Heterogeneity of pre-intervention ankle-brachial indices among patients undergoing revascularization for critical limb ischemia.
Frequency of normal, mild-moderate, and severe pre-intervention treated limb ankle-brachial index results among patients with compressible arteries undergoing percutaneous vascular intervention or surgical revascularization for treatment of critical limb ischemia.
Demographic and clinical characteristics across ABI categories are shown in Table 1. Patients with normal ABIs were more likely to be younger and male, and less likely to have chronic lung disease and have undergone prior SR compared with patients with mild-moderate or severe ABIs. As ABI severity increased, there was a clear ordering across the ABI categories in terms of decreased body mass index and increased likelihood of being a current smoker.
When comparing patients between ABI severity categories (Table 2), patients with severe ABIs were more likely to undergo SR, have the current procedure treat a previously treated site, and have lower hemoglobin levels than patients in the mild-moderate or normal ABIs. The anatomic level of the targeted lesion also differed across ABI categories, as patients with severe ABIs were more likely to have a procedure treating the aorto-iliac level while patients with normal ABIs were more likely to have a procedure treating a lesion below the knee. There was no difference in the frequency of rest pain only and tissue ulceration or gangrene among patients with normal, mild-moderate, or severe ABIs; however, patient quality of life, as measured by the pre-intervention PAQ summary score, was significantly lower as ABI severity increased (Figure 2).
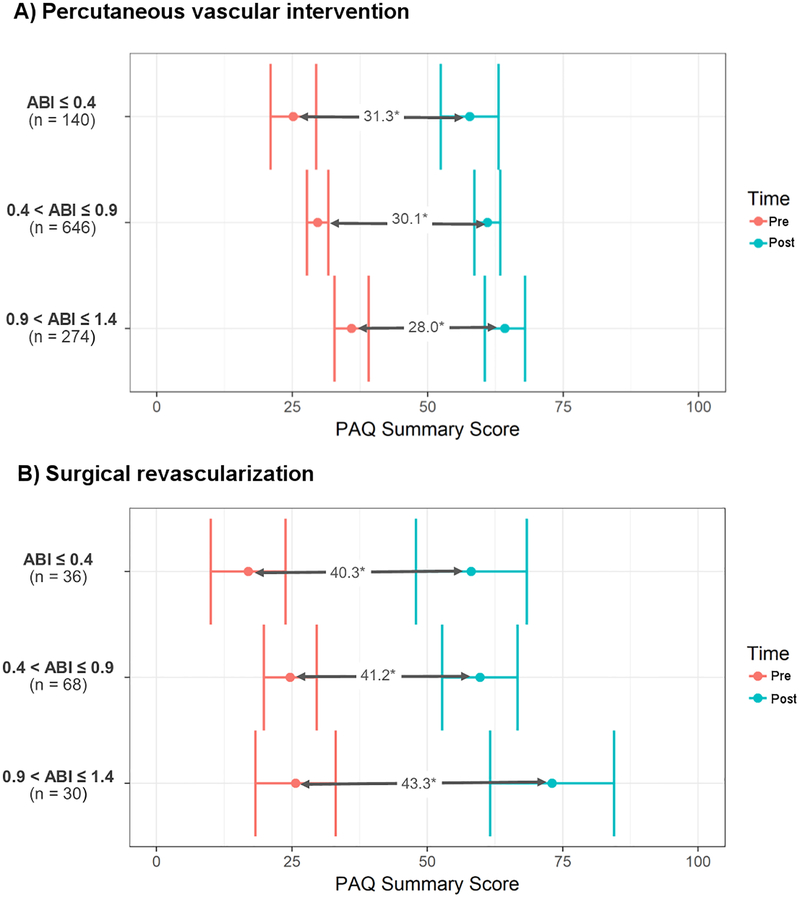
Figure 2: Pre- and post-intervention peripheral artery questionnaire summary scores by mode of revascularization.
Pre-intervention and post-intervention Peripheral Artery Questionnaire (PAQ) summary scores are demonstrated for patients undergoing percutaneous vascular intervention (A) and surgical revascularization (B). Patients who underwent percutaneous vascular intervention had 6-month post-intervention PAQ scores collected, whereas those who underwent surgical revascularization had 1-year post-intervention PAQ scores collected.
Abbreviations: PAQ = Peripheral Artery Questionnaire.
* All pre- and post-intervention differences are statistically significant at P<0.001.
Toe-brachial indices
Among patients undergoing revascularization for CLI, 20.7% (n/N=2,225/10,756) had documented TBIs prior to revascularization. Like the ABI findings above, patients undergoing PVI had less severe PAD compared with patients undergoing SR as demonstrated by higher TBIs (mean±SD; PVI: 0.41±0.26 vs. SR: 0.34±0.25; P <0.001). Most patients who underwent TBI testing had evidence of PAD with a TBI <0.7 (PVI 87.5%; SR 91.5%).
Independent Predictors of ABI severity
Using multivariable ordinal logistic regression, we found eight patient characteristics that were independent predictors of increased ABI severity (Table 3). Characteristics that increased the odds of ABI severity included increasing age, past or current smoking, history of transient ischemic attack or cerebrovascular accident, previous SR, and decreased pre-intervention hemoglobin levels. For example, a patient with a pre-intervention hemoglobin of 11 mg/dL has 1.20 times the odds (95% CI 1.10–1.34; P<0.001) of a more severe ABI category compared with a patient with a pre-intervention hemoglobin of 14 mg/dL, after adjusting for other factors.
Table 3:
Reduced ordinal logistic regression of patient characteristics and anatomic level of treatment on increased ankle-brachial index severity.*
| Odds Ratio (95% Confidence Interval) | P-value | |
|---|---|---|
| Male gender | 0.85 (0.78–0.94) | < 0.001 |
| Age (change from age 60 to 70) | 1.17 (1.11–1.23) | < 0.001 |
| ZIP population density | ||
| Urban | Reference | |
| Suburban | 1.22 (0.96–1.43) | 0.119 |
| Rural | 1.18 (0.95–1.52) | 0.130 |
| Body mass index (change from 25 to 30 kg/m2) | 0.95 (0.92–0.99) | 0.004 |
| Smoking status | ||
| Never | Reference | |
| Past | 1.31 (1.15–1.49) | < 0.001 |
| Current | 1.58 (1.31–1.93) | < 0.001 |
| History of TIA/CVA | 1.22 (1.08–1.39) | 0.002 |
| Prior surgical revascularization | 1.33 (1.11–1.62) | 0.002 |
| Anatomic level of treatment: | ||
| Aorto-iliac | Reference | |
| Femoral-popliteal | 0.70 (0.58–0.84) | < 0.001 |
| Below the knee | 0.48 (0.41–0.57) | < 0.001 |
| Hemoglobin (change from 14 to 11 mg/dL) | 1.20 (1.10–1.34) | < 0.001 |
Models odds of increased ABI severity category: normal to mild-moderate to severe.
Model performance measures: Concordance statistic 0.589; calibration intercept 0.091; calibration slope 0.914.
Abbreviations: CVA = cerebrovascular accident; TIA = transient ischemic attack
Characteristics that decreased the odds of a more severe ABI category included male gender, increased body mass index, and a more distal anatomic level of treatment. In fact, the strongest predictor of ABI severity was the anatomic level of treatment, where having a targeted lesion in a more distal vascular bed significantly decreased the odds of increased ABI severity. For instance, compared with patients whose targeted lesion was in the aorto-iliac vascular bed, patients with a treated lesion in the femoral-popliteal vascular bed has 0.70 the odds of increased ABI severity (95% CI 0.58–0.84; P<0.001), while patients with the targeted lesion below the knee has 0.48 the odds of increased PAD severity (95% CI 0.41–0.57; P<0.001). This relationship holds when comparing patients with a targeted lesion location below the knee compared with patients with a targeted lesion location in the femoral-popliteal region (odds ratio 0.69; 95% CI 0.60–0.79; P<0.001).
Outcomes
Of 4,391 patients with pre-intervention compressible ABIs who underwent PVI, 1,060 (24.1%) had pre-intervention and 6-month post-intervention PAQ summary scores documented. Pre-intervention PAQ summary scores, shown in Figure 2A, were significantly different across all ABI categories (Wald test, P<0.001). Baseline PAQ summary scores among patients with severe ABIs were, on average, 4.6 points lower than those with mild-moderate ABIs (Bonferroni corrected Wald test, P=0.004) and 11.07 points lower than those with normal ABIs (P<0.001). Baseline PAQ summary scores were 6.47 points lower among those with mild-moderate ABIs as compared with those with normal ABIs (P<0.001). Importantly, there were significant increases in PAQ scores after intervention across all three ABI categories (P<0.001; Figure 2A). There were no significant differences in the degree of improvement when comparing the three ABI groups (P=0.137).
Of 940 patients with pre-intervention compressible ABI results who underwent SR, 134 (14.3%) had pre-intervention and 1-year post-intervention PAQ summary scores recorded (Figure 2B). Among patients with severe ABIs, baseline PAQ scores were 7.88 points lower than patients with mild-moderate ABIs (P=0.001), and 8.65 points lower than patients with normal ABIs (P=0.011). Significant improvements in PAQ scores were seen across all three ABI categories (P<0.001; Figure 2B). As above, there were no significant differences in the magnitude of improvement in PAQ scores between the three categories (Wald test, P=0.32).
Lastly, we compared discharge outcomes across ABI categories for patients treated with PVI and SR. We found no significant differences in the rates of amputation or death across both modes of revascularization (Supplementary Table S2).
Discussion
To our knowledge, this is the largest multicenter and multispecialty assessment of ABI results in patients undergoing revascularization for CLI. The present study has 2 principal findings. First, there was remarkable heterogeneity in pre-intervention ABI results in these patients, regardless of the mode of revascularization or the patient’s presenting signs and symptoms. Remarkably, nearly a quarter of patients undergoing revascularization for CLI had normal ABIs, whereas only 16% of patients had severe PAD by ABI testing. Second, despite this variation in ABI results, significant symptomatic improvement after intervention was seen across all levels of PAD severity, suggesting that a component of patients’ poor baseline health status assessments was due to vascular insufficiency despite the pre-intervention ABI results. These findings raise concerns regarding the role of ABI testing in this population.
In fact, recent studies have called into question the diagnostic utility of ABI testing in the diagnosis of PAD among patients presenting with CLI (9,10). In a single-center study of 89 patients, Bunte and colleagues found 29% of patients with CLI to have near-normal or normal ABIs despite significant infrapopliteal arterial disease on angiography (9). Furthermore, there was a paradoxical relationship between ABI results and infrapopliteal arterial runoff, with the highest ABI results in those with the fewest patent infrapopliteal arteries. Also, in a recent secondary analysis of the IN.PACT DEEP trial, Shishehbor et al discovered that among 237 patients with angiographically-confirmed isolated infrapopliteal disease, only 6% had severe PAD by pre-intervention ABI testing (10).
The present study confirms and extends these findings by demonstrating substantial heterogeneity in pre-intervention ABIs among a large, multicenter, real-world population of patients undergoing revascularization. Similar to prior work, we found that more distal PAD was independently associated with less severe ABIs (i.e. higher ABI values). Furthermore, male gender and elevated body mass index were also independently associated with less severe ABIs. Previous research demonstrated that increased baseline obesity was associated with mean increases in ABI over time and the development of new-onset high-ABI measurements (ABI≥1.3) (20). Aboyans and colleagues also found that female gender was associated with lower baseline ABI values. The mechanisms underlying these findings remains incompletely understood (21).
Prior studies have demonstrated that ABI testing does not correlate well with arterial disease determined by angiographic assessment among patients with suspected CLI (9,10). However, angiographic imaging may only point to an anatomic obstruction, and not necessarily functional hypoperfusion. The current study goes a step beyond solely demonstrating that a substantial proportion of patients with anatomic PAD undergoing revascularization had normal ABIs; we also found a similar improvement in the PAQ summary score after intervention across all ABI categories. This suggests that some degree of the patient’s improved health status was likely due to treatment directed at their underlying vascular insufficiency regardless of their pre-intervention ABI. Of note, the improvement in PAQ scores was not solely a reflection of the benefits of revascularization, but also incorporates benefits derived from being under the care of vascular specialists who are likely providing optimal non-procedural treatment including medical therapy, management of comorbid illnesses (i.e. diabetes mellitus), wound care, and exercise therapy.
From a population health perspective, we do not know the number of patients who present with signs or symptoms of CLI and are found to have normal ABIs, ultimately leading to delayed diagnosis or misdiagnosis. Given that prompt revascularization is the cornerstone of treatment in CLI with the aim of preventing amputation and improving symptoms, if 25% of patients suspected of having CLI do not undergo revascularization because of normal ABIs, we are likely to find an alarming number of patients undergoing preventable amputation – making this issue a priority for further research and evaluation. Indeed, the most recent American Heart Association/American College of Cardiology Lower Extremity PAD guidelines state that it is reasonable to diagnose CLI by using TBI with waveforms, TcPO2, or skin perfusion pressure among patients with nonhealing wounds or gangrene who have normal or borderline ABI results (5). We found a similar proportion of normal ABI results among patients with rest pain only compared with those with evidence of tissue necrosis, suggesting that there may be utility in further perfusion assessment among patients with rest pain only as well.
Previous research has demonstrated that the TBI may be a more sensitive test in diagnosing PAD than the ABI, particularly among patients with diabetes and calcified vessels (22). Similarly, in a small subset of patients with recorded TBIs, we discovered a lower mean value for TBIs than ABIs, and a larger proportion of patients having a diagnosis of PAD by TBI testing.
Finally, with increasing interest in the wound-directed angiosome revascularization approach for CLI (6,23,24), substantial promise may lie in the clinical application of technologies focused on assessing microcirculatory perfusion such as blood oxygenation level-dependent cardiovascular magnetic resonance (25), TcPO2 (26), laser Doppler (27), and indocyanine green angiography (28,29). Also, newer classification schemes, such as the wound, ischemia, and foot infection classification system (WIfI) may generate new diagnostic and treatment paradigms (30). Although the WIfI criteria still relies on ABI testing in assessing the domain of “ischemia,” it is clearly noted that if arterial calcification precludes reliable hemodynamic assessment, TcPO2, skin perfusion pressure, or pulse volume recordings should be used. We hope that the process of creating and evaluating these classification schemes will ultimately result in important innovations and advances in the field of vascular medicine and better outcomes for patients with CLI.
This study should be interpreted in the context of several important limitations. First, these are patients undergoing revascularization for signs and symptoms of CLI, therefore, despite normal ABIs, the treating physicians believed that revascularization would be beneficial. Exactly how each physician arrived at this conclusion is beyond the scope of this study. Second, nearly 50% of patients undergoing revascularization for CLI did not have ABIs recorded in our registry, thus potentially making our findings susceptible to selection bias. The specific reasons why a patient may not have had an ABI performed or did not have it recorded in the registry are unknown. Despite attempts to obtain office-based records including ABIs, it is possible that these were not received for several patients. Furthermore, patients with CLI may not have had ABIs performed due to the presence of wounds or limb pain limiting the ability to physically perform the diagnostic test. Third, the PAQ was validated in patients experiencing intermittent claudication (i.e. Rutherford class 2 and 3), not CLI (14). To our knowledge, there is a paucity of quality-of-life assessment tools for patients with CLI (31), therefore we believe that using the PAQ in an exploratory manner may still provide important information in this population. Lastly, the exact method or technique used to perform ABIs at each site was not collected, therefore we could not account for the effect of operator variability on our results. Although prior work has demonstrated a relationship between examiner experience and ABI reproducibility (12), our results represent ABIs that are obtained and used in real-world practice to inform clinical decisions, not a controlled research setting, and are thus more generalizable.
Conclusion
A small fraction of patients undergoing revascularization for CLI in contemporary practice had severe PAD by ABI testing. Despite this heterogeneity in ABIs, symptomatic improvement after intervention was seen across all levels of PAD severity. Notably, patients who had more distal anatomic sites of treatment were less likely to have severe ABIs. Although the ABI remains an easily administered, non-invasive, functional assessment of PAD, the disconnect between ABI severity and the clinical diagnosis of CLI calls into question the utility of ABIs in this population and suggests the need for standardization of functional PAD testing in patients with signs or symptoms concerning for CLI.
Supplementary Material
Clinical Perspectives: Core Clinical Competencies and Translational Outlook implications.
What’s known?
The ankle-brachial index (ABI) is recommended as the first-line investigation for assessment of arterial insufficiency among patients with signs and symptoms of critical limb ischemia (CLI).
What’s new?
Among patients with compressible arteries undergoing revascularization for signs or symptoms of critical limb ischemia with recorded pre-intervention ABIs, nearly a quarter of the ABI results were normal (ABI: 0.91–1.4).
What’s next?
With the development of newer technologies and imaging modalities to assess functional limb perfusion, further research is needed to evaluate the diagnostic performance of these tests. This should ultimately lead to standardization of functional peripheral artery disease testing among patients with suspected critical limb ischemia.
Acknowledgements:
The authors are indebted to all the study coordinators, investigators, and patients who participated in the BMC2 registry.
Funding:
Dr. Sukul is supported by the National Institutes of Health T32 postdoctoral research training grant (T32-HL007853). This work was supported by the Blue Cross Blue Shield of Michigan and Blue Care Network as part of the Blue Cross Blue Shield of Michigan Value Partnerships program. The funding source supported data collection at each site and funded the data-coordinating center but had no role in study concept, interpretation of findings, or in the preparation, final approval or decision to submit the manuscript.
Disclosures: P. Michael Grossman receives research funding from Blue Cross Blue Shield of Michigan, the National Institutes of Health, and Medtronic Cardiovascular and was a research investigator in the Edwards Sapien clinical trial. Scott F. Grey receives funding from Blue Cross Blue Shield of Michigan. Peter K. Henke receives funding from the National Institutes of Health and Blue Cross Blue Shield of Michigan. Hitinder S. Gurm receives research funding from Blue Cross Blue Shield of Michigan, the National Institutes of Health and is a consultant for Osprey Medical. None of the authors have any conflicts directly relevant to this study.
Abbreviations:
- PAD
peripheral artery disease
- CLI
critical limb ischemia
- ABI
ankle-brachial index
- TBI
toe-brachial index
- TcPO2
transcutaneous oxygen pressure
- BMC2 VIC
Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Interventions Collaborative
- PVI
percutaneous vascular intervention
- SR
surgical revascularization
- PAQ
Peripheral Artery Questionnaire
- GLS
generalized least squares
Footnotes
This study was presented at the 27th Annual Society of Vascular Medicine Scientific Sessions in June 2016 and Dr. Devraj Sukul was the recipient of a Young Investigator Award.
Publisher's Disclaimer: Disclaimer: Although Blue Cross Blue Shield of Michigan (BCBSM) and BMC2 work collaboratively, the opinions, beliefs and viewpoints expressed by the author do not necessarily reflect the opinions, beliefs and viewpoints of BCBSM or any of its employees.
References
- 1.Allison MA, Ho E, Denenberg JO et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med 2007;32:328–33. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2015. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–26. [DOI] [PubMed] [Google Scholar]
- 4.Fowkes FG, Rudan D, Rudan I et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40. [DOI] [PubMed] [Google Scholar]
- 5.Gerhard-Herman MD, Gornik HL, Barrett C et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunte MC, Shishehbor MH. Treatment of infrapopliteal critical limb ischemia in 2013: the wound perfusion approach. Curr Cardiol Rep 2013;15:363. [DOI] [PubMed] [Google Scholar]
- 7.Nehler MR, Duval S, Diao L et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. Journal of vascular surgery 2014;60:686–95 e2. [DOI] [PubMed] [Google Scholar]
- 8.Norgren L, Hiatt WR, Dormandy JA et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg 2007;33 Suppl 1:S1–75. [DOI] [PubMed] [Google Scholar]
- 9.Bunte MC, Jacob J, Nudelman B, Shishehbor MH. Validation of the relationship between ankle-brachial and toe-brachial indices and infragenicular arterial patency in critical limb ischemia. Vasc Med 2015;20:23–9. [DOI] [PubMed] [Google Scholar]
- 10.Shishehbor MH, Hammad TA, Zeller T, Baumgartner I, Scheinert D, Rocha-Singh KJ. An analysis of IN.PACT DEEP randomized trial on the limitations of the societal guidelines-recommended hemodynamic parameters to diagnose critical limb ischemia. Journal of vascular surgery 2016;63:1311–7. [DOI] [PubMed] [Google Scholar]
- 11.Renard BM, Seth M, Share D et al. If not now, when? Prescription of evidence-based medical therapy prior to hospital discharge increases utilization at 6 months in patients with symptomatic peripheral artery disease. Vasc Med 2015;20:544–50. [DOI] [PubMed] [Google Scholar]
- 12.Aboyans V, Criqui MH, Abraham P et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–909. [DOI] [PubMed] [Google Scholar]
- 13.Hoeks SE, Smolderen KG, Scholte Op Reimer WJ, Verhagen HJ, Spertus JA, Poldermans D. Clinical validity of a disease-specific health status questionnaire: the peripheral artery questionnaire. Journal of vascular surgery 2009;49:371–7. [DOI] [PubMed] [Google Scholar]
- 14.Spertus J, Jones P, Poler S, Rocha-Singh K. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. American heart journal 2004;147:301–8. [DOI] [PubMed] [Google Scholar]
- 15.McCullagh P Regression Models for Ordinal Data. J R Stat Soc B 1980;42:109–142. [Google Scholar]
- 16.Harrel FE. Regression Modeling Strategies. New York: Springer-Verlag, 2001. [Google Scholar]
- 17.Zeileis A Object-oriented computation of sandwich estimators. Journal of Statistical Software 2006;16:1–16. [Google Scholar]
- 18.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York: Springer-Verlag, 2000. [Google Scholar]
- 19.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2015. [Google Scholar]
- 20.Tison GH, Ndumele CE, Gerstenblith G, Allison MA, Polak JF, Szklo M. Usefulness of baseline obesity to predict development of a high ankle brachial index (from the Multi-Ethnic Study of Atherosclerosis). The American journal of cardiology 2011;107:1386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aboyans V, Criqui MH, McClelland RL et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA). Journal of vascular surgery 2007;45:319–27. [DOI] [PubMed] [Google Scholar]
- 22.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. Journal of vascular surgery 2008;48:1197–203. [DOI] [PubMed] [Google Scholar]
- 23.Huang TY, Huang TS, Wang YC, Huang PF, Yu HC, Yeh CH. Direct Revascularization With the Angiosome Concept for Lower Limb Ischemia: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94:e1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinlay S Management of Critical Limb Ischemia. Circulation Cardiovascular interventions 2016;9:e001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajwa A, Wesolowski R, Patel A et al. Blood Oxygenation Level-Dependent CMR-Derived Measures in Critical Limb Ischemia and Changes With Revascularization. Journal of the American College of Cardiology 2016;67:420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White RA, Nolan L, Harley D et al. Noninvasive evaluation of peripheral vascular disease using transcutaneous oxygen tension. The American Journal of Surgery 1982;144:68–75. [DOI] [PubMed] [Google Scholar]
- 27.Ambrozy E, Waczulikova I, Willfort-Ehringer A, Ehringer H, Koppensteiner R, Gschwandtner ME. Microcirculation in mixed arterial/venous ulcers and the surrounding skin: clinical study using a laser Doppler perfusion imager and capillary microscopy. Wound Repair Regen 2009;17:19–24. [DOI] [PubMed] [Google Scholar]
- 28.Braun JD, Trinidad-Hernandez M, Perry D, Armstrong DG, Mills JL, Sr. Early quantitative evaluation of indocyanine green angiography in patients with critical limb ischemia. Journal of vascular surgery 2013;57:1213–8. [DOI] [PubMed] [Google Scholar]
- 29.Benitez E, Sumpio BJ, Chin J, Sumpio BE. Contemporary assessment of foot perfusion in patients with critical limb ischemia. Semin Vasc Surg 2014;27:3–15. [DOI] [PubMed] [Google Scholar]
- 30.Mills JL Sr., Conte MS, Armstrong DG et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). Journal of vascular surgery 2014;59:220–34 e1–2. [DOI] [PubMed] [Google Scholar]
- 31.Patel MR, Conte MS, Cutlip DE et al. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC). Journal of the American College of Cardiology 2015;65:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.