Abstract
Background and Purpose—
Simple and rapid measures of intraventricular hemorrhage (IVH) volume are lacking. We developed and validated a modification of the original Graeb scale to facilitate rapid assessment of IVH over time.
Methods—
We explored the relationship between the modified Graeb scale (mGS), original Graeb scale, measured IVH volume, and outcome using data from the Clot Lysis: Evaluating Accelerated Resolution of Hemorrhage with rtPA B (CLEAR B) study. We also explored its reliability. We then evaluated the relationship between mGS and outcome in a large sample of participants with IVH using data contained within the Virtual International Stroke Trials Archive (VISTA). We defined outcome using the modified Rankin scale (>3 signifying poor outcome).
Results—
The CLEAR B study included 360 scans from 36 subjects. The mGS score and IVH volume were highly correlated (R = 0.80, P<0.0001, R2 0.65). Baseline mGS was predictive of poor outcome (area under receiving operating characteristic curve 0.74, 95% confidence interval, 0.57–0.91), whereas the original Graeb scale was not. The VISTA study included 399 participants. Each unit increase in the mGS led to a 12% increase in the odds of a poor outcome (odds ratio, 1.12; 95% confidence interval, 1.05–1.19). Measures of reliability (intra- and interreader) were good in both studies.
Conclusions—
The mGS, a semiquantitative scale for IVH volume measurement, is a reliable measure with prognostic validity suitable for rapid use in clinical practice and in research.
Keywords: cerebral hemorrhage, interobserver variation, intraventricular pressure, outcomes assessment
Intraventricular hemorrhage (IVH) secondary to spontaneous intracerebral hemorrhage (ICH) results in death in 32% to 43% of cases1–8 and poor functional outcome in most survivors.5,7,9–15 In severe IVH with obstruction of the third or fourth ventricle, the placement of an extraventricular drain can help lower raised intracranial pressure.12 Meta-analysis shows this reduces mortality, but poor outcomes remain commonplace.16 In addition to extraventricular drain placement, the use of intraventricular thrombolytic therapy may improve outcomes and is being evaluated in the Clot Lysis Evaluating Accelerated Resolution of Intraventricular Hemorrhage trial program.17 A pivotal phase III study (Clot Lysis: Evaluating Accelerated Resolution of Hemorrhage with rtPA III [CLEAR III]) of the effect of thrombolytic based removal of ventricular blood on functional outcome is underway.18
The use of thrombolytic drugs in this setting is associated with bleeding risk.19 To reduce this risk, the CLEAR study criteria include ICH and IVH clot stability, defined by consecutive computed tomography scans of brain (CT) no less than 6 hours apart showing no increase in ICH and IVH volume.20 Although rapid, reliable, and well validated means of assessing ICH volume are available,21,22 IVH volume assessment remains laborious, time consuming, and subject to variability between readers.23 A reliable, simple, quick, and clinically meaningful approximation of IVH volume is needed not only for clinical trials such as CLEAR, but also clinical practice more widely. The Graeb score is a semiquantative score ranging from 0 to 12, which could be used for this purpose. However, it lacks the ability to differentiate specific regions of the ventricular system, which may limit its relationship with true IVH volume, its ability to detect IVH growth or removal in subcompartments of the ventricular system, and its ability to predict outcome.
We report a program of research in which trained assessors evaluated and validated a modification of the original Graeb score (oGS),24 called the modified Graeb Scale score (mGS), which we designed to address the above issues. Also described as the expanded Graeb scale, Hinson et al25 provide an excellent description of the mGS. However, this study (to our knowledge) is the first validation of this modified Graeb scale. In Study One we explored the relationship between the mGs, oGS, volumetrically measured IVH, and outcome using data from the CLEAR B trial. We then explored in Study Two the relationship between mGS and 90-day functional outcome in a large sample of patients with IVH using data from the Virtual International Stroke Trials Archive (VISTA).26
Methods
Development of the Modified Graeb Scale
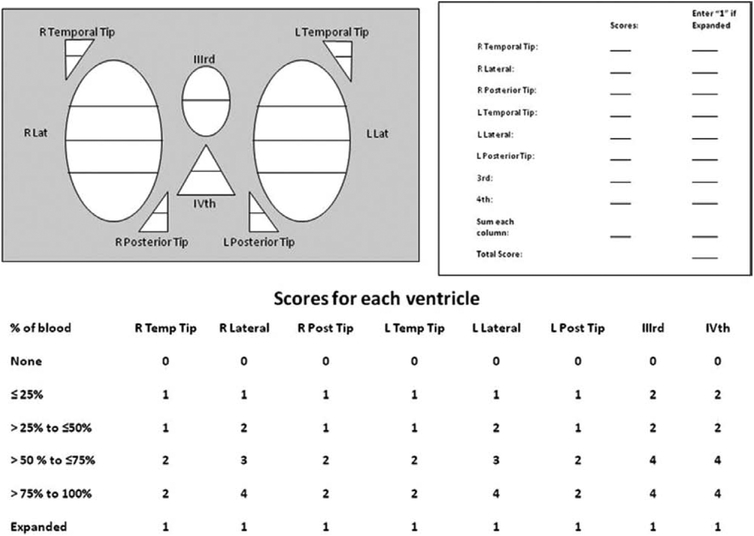
The oGS was based on only the third, fourth, right and left lateral ventricles.24 A maximum score of 4 is given for each lateral ventricle, where it is expanded and filled with blood and a maximum score of 2 is given for the third and fourth ventricles if they are similarly filled. The maximum possible score is therefore 12. For the mGS, we introduced scores for separate ventricular compartments to better reflect total IVH volume and selective regional accumulation or removal of blood. The mGS is thus based on the fourth ventricle (maximum score 4), third (maximum score 4), right and left lateral ventricles (maximum score 4 for each), right and left occipital horns (maximum score 2 for each), and the right and left temporal horns (maximum score 2 for each). An additional score of +1 is given to each compartment if it is expanded beyond normal anatomic limits attributable to clot. The boundaries between the lateral ventricle, the occipital horn, and the temporal horn are composed of 3 planes that intersect in (and project outwardly from) the trigone, or the central region where the 3 compartments converge. The maximum possible score is 32, in which every compartment is filled with blood and expanded. A score of 0 denotes no intraventricular blood. The scoring rubric is shown in Figure 1.
Figure 1.
The modified Graeb scale.
Observer Training Before Review of CT Images
Observers (J.D., N.M., T.M., D.S.) were trained in application of the mGS score using a set of training scans (n=80) before review of any scans included in this study. Written guidance could be consulted27 and, after review, a telephone conference was convened in which questions were addressed and scoring rules were clarified.
Study One – Relationship Between oGS, mGS, IVH Volume, and Outcome in Patients Undergoing Thrombolytic Therapy
Data from the CLEAR B trial (a multicenter trial coordinated by Johns Hopkins University) were used. This was a dose-finding phase II clinical trial to evaluate the efficacy of intraventricular administration of thrombolytic agent (recombinant tissue plasminogen activator [rt-PA]) for patients with severe IVH secondary to spontaneous ICH.18 Inclusion criteria included complete obstruction of the third or fourth ventricle(s) requiring the placement of an extraventricular drain, small ICH (< 30 mL) at time of enrolment, IVH and ICH clot stability at time of enrollment, and acquisition of the first CT scan within 24 hours of symptom onset. Additional details regarding the CLEAR eligibility specifications are available elsewhere.17
Each scan was scored using the both the oGS and mGS scoring systems. Two independent assessors scored the data set using the mGS to assess inter-reader variability. Additionally, 1 of the 2 readers rescored the data set 18 months later to assess intrareader reliability. Two readers calculated the IVH volumes by a computed free-hand tracing technique using medical imaging software (Alice, Perceptive Informatics, Boston, MA), which is consistent with established methods.23
Functional outcome was measured using the modified Rankin scale score (mRS) at 180 days (the primary end point in the CLEAR trial program).
Statistical Analyses
Analyses were conducted using SPSS version 19 (SPSS Inc, Chicago, IL). A correlation was assessed between the oGS/mGS score and IVH volumetric measurements using Pearson correlation coefficient. The significance of the difference between the 2 correlation coefficients was assessed using the Fishers r to z transformation (2-tailed test). The change in oGS and mGS between baseline and study end were similarly correlated to change in IVH volumetric measurements. Receiver operating characteristic (ROC) analysis was used to establish whether baseline oGS and mGS were predictive of day-180 functional outcome. The intraclass correlation coefficient (ICC), which is equivalent to quadratic weighted kappa statistics, was calculated to evaluate inter- and intraobserver variability for the mGS, as well as the agreement for IVH volume measurement.
Study Two - Relationship Between mGS and 90-Day Functional Outcome
Data from VISTA were used for this study as the large sample size would permit more definitive evaluation of the relationship between mGS and outcome. The study was performed in the Institute of Cardiovascular and Medical Sciences at the University of Glasgow. For all patients with ICH, a baseline CT brain scan and a 90-day mRS score were included. VISTA has been described in detail elsewhere•• and currently holds data for 1829 patients with ICH and CT scans for 1250 of these.26
These 1250 cases were reviewed, and those with IVH were included in further analyses. The presence of IVH, mGS score, and ICH volume and location were recorded independently by 2 blinded observers (J.D. and N.M.). Where mGS scores differed between observers, the mean score was used unless there was disagreement concerning the presence of IVH. In such cases, scans were rereviewed and disputes resolved by consensus. ICH volumes were calculated by M.R. and N.M. Computer assisted volumetric analysis of ICH volume was performed (using IMAGE J software [http://rsbweb.nih.gov/ij/]), except for 136 participants, for which only a hard copy of the scan was available. For these scans the ABC/2 method21 was used, with the aid of digital callipers.
Statistical Analysis
The primary end point was poor outcome, defined as day 90 mRS score >3. Analyses were conducted using SPSS version 19 (SPSS Inc, Chicago, IL). Variables associated with poor outcome were identified (using a chi-squared test for dichotomized variables or regression analysis for continuous variables). These variables were then included in a binary multiple logistic regression models to evaluate the relationship between mGS and outcome. Baseline Glasgow Coma Scale score, baseline blood glucose level, and use of anticoagulant drugs before ICH were not available for all participants. Analyses were repeated including only patients with these variables recorded to explore sensitivity of our findings. The ICC was used to assess interobserver agreement using the mGS.
Results
Study One (CLEAR B Analysis)
The sample included 360 scans from 36 subjects. Mean age was 58 years, with a standard deviation of 10 years. Baseline characteristics are shown in Table 1. Most hemorrhages were in a deep location (91.7%). All had IVH and median ICH volume was 3.7 mL (IQR 0.8–12.6).
Table 1.
Baseline Characteristics in Studies One and Two
| Variable | Study One | Study Two |
|---|---|---|
| Age, y | 57.0 (10.2) | 69.2 (10.9) |
| Male sex | 25 (69.4%) | 247 (61.9%) |
| Systolic BP, mmlHg | 192.7 (42.5) | 176.1 (28.8) |
| Diastolic BP, mmlHg | 110 (24.9) | 93.6 (17.6) |
| Serum creatinine, μmol/L* | N/A | 77.6 (27.4) |
| Baseline NIHSS, median (IQR) | 23.0 (1.0–37.0) | 15.0 (11.0–19.0) |
| Smoker | N/A | 57 (14.3%) |
| DM | 10 (28.7%) | 65 (16.3%) |
| AF | 3 (8.3%) | 36 (9.0%) |
| Hypertension | 29 (80.6%) | 293 (73.4%) |
| Ml | 4 (11.1%) | 17 (4.3%) |
| Previous stroke | 3 (8.3%) | 90 (22.6%) |
| ↑cholesterol | N/A | 50 (12.5%) |
| ICH location | ||
| Deep | 33 (91.7%) | 343 (86.0%) |
| Lobar | 2 (5.6%) | 54 (13.5%) |
| Posterior Fossa | 0 (0.0%) | 1 (0.0%) |
| Primary IVH | 1 (2.8%) | 1 (0.0%) |
| ICH Volume, cm3 | 3.7 (0.8–12.6) | 16.4 (9.9–41.2) |
| Graeb Score, median (IQR) | 20 (16–32) | 6 (3–11) |
For continuous variables, values are expressed as mean (standard deviation) unless stated. For dichotomous variables, values are n (%). AF indicates atrial fibrillation; BP, blood pressure; DM, diabetes mellitus; ICH, intracerebral hemorrhage; IQR, interquartile range; IVH, intraventricular hemorrhage; MI, previous myocardial infarction; and NIHSS, National Institutes of Health Stroke Scale. N/A = not available in CLEAR B dataset. P values are for t test, Mann–Whitney tests or χ2 comparison.
Not available for 73 patients in VISTA analysis.
Correlation Between oGS, mGS, and Measured IVH Volume
The oGS and mGS were highly correlated with IVH volume (Figure 2, R=0.77, P<0.0001, R2 0.60 for oGS and R=0.80, P<0.0001, R2 0.65 for mGS [n=318 scans with IVH included]). This difference was not statistically significant (z=0.37, P=0.71). The correlation between mGS score and IVH volume appears strongest in smaller IVH cases (< 40 mL; R = 0.90, versus R = 0.71 in cases of IVH > 40 mL). The change in oGS and mGS were also correlated with change in IVH volume (R=0.46, P=0.005 and R=0.57, P<0.001 respectively, n=36). This difference was not statistically significant (z=0.63, P=0.52). However, the relationship between change in oGS and IVH volume appeared more variable than that for change in mGS (r2 0.21 for oGS compared with r2 0.33 for mGS).
Figure 2.
Scatter plot of measured intraventricular hemorrhage volume in milliliters vs the original and modified Graeb scores.
Prediction of Day-180 Outcome Using oGS and mGS
Baseline mGS and IVH volume were predictive of outcome. The area under the curve (AUC) for the prediction of poor 180-day outcome was 0.74, 95% CI 0.58 to 0.91 for IVH volume, 0.74 (95% CI 0.57–0.90) for the mGS and 0.63 (95% CI 0.45–0.82) for the oGS (Figure 3).
Figure 3.
Receiver operating characteristics curves for baseline intraventricular hemorrhage (IVH), original Graeb score (oGS), and modified Graeb score (mGS) for prediction of poor day-180 outcome. Area under the curve for baseline IVH 0.74 (95% CI, 0.58–0.91). AUC for mGS 0.74 (95% CI, 0.57–0.90). AUC for oGS 0.63 (95% CI, 0.45–0.82).
Reliability and Reproducibility of the mGS
The ICC between the 2 readers using the free-hand tracing technique to assess IVH volume was 0.98 (95% confidence interval [CI], 0.98–0.98). The consistency between readers for the mGS was also high (ICC > 0.94; 95% CI, 0.93–0.95). Intrareader reproducibility for the mGS score was also good (ICC 0.90; 95% CI, 0.85–0.95).
Study Two (VISTA Analysis)
Of the 1250 patients reviewed, 31.9% (399) had IVH and were included in subsequent analyses. Baseline characteristics are shown in Table 1. Briefly, the majority of hemorrhages were in a deep location (86.0%), median ICH volume was 16.4 cm3 (IQR 9.9–41.2 cm3), and median mGS was 6, IQR 3 to 11. Poor outcome occurred in 272 (69.2%), and 117 (29.3%) died. In 6 cases (1.5%), a primary outcome could not be assigned because the participants were known to be alive but had no recorded mRS score. Sensitivity analysis, where the missing outcomes of these cases were assumed to all be poor versus assumed to all be good, did not change results given below (data not shown).
mGS and 90-Day Outcome
On univariate analysis, greater age, increasing baseline National Institutes of Health Stroke Scale score, ICH volume, lobar hemorrhage location, and mGS score were predictive of poor outcome. When these variables were included in a multivariable model, the mGS remained predictive of poor outcome. Each unit increase in score led to a 12% increase in the odds of a poor outcome (OR, 1.12; 95% CI, 1.05–1.19; Table 3). When previous antithrombotic drug use (n=336) and Glasgow Coma Scale (GCS; n=185) were added to the model, the mGS remained similarly predictive of outcome. GCS was not independently predictive. When blood glucose level was added to the model (n=169), the 95% CI for the mGS crossed unity (95% CI for OR 0.99–1.14). Neither lobar hemorrhage or blood glucose predicted of outcome in this analysis.
Reliability of the mGS Score
The agreement rate between the 2 observers (n=1250 scans) was 73.7% (95% CI, 71.0–76.2) with corresponding intraclass correlation coefficient of 0.94 (95% CI, 0.93–0.95).
Discussion
Our studies show that the mGS, a simple semiquantitative score that takes only a few minutes to administer, is a valid and reproducible measure of IVH volume. In our CLEAR B investigation, we have shown that the mGS has good inter- and intrareader reliability, is closely related to IVH volume and is similarly predictive of outcome to actual IVH volume. Further, it gives greater flexibility in terms of its ability to detect IVH in differing ventricular compartments and gives a more accurate measure of change in IVH volume over time. We then explored the predictive ability of mGS in a wider population of IVH patients using VISTA dataset, the largest dataset of its kind, with a sufficient sample size for adjusted analyses to be performed. Here we found a 12% increased odds of poor outcome for each incremental increase in mGS independent of other measures such as ICH volume and baseline stroke severity. Our data come from 2 separate studies, conducted in different institutions by similarly trained but different observers and in different populations of IVH patients. Not only do our data show that the mGS is a useful means of quantifying IVH extent, it highlights the crucial importance of quantifying IVH in both clinical research and clinical practice.
There are other methods available for the semiquantitative assessment of IVH volume.28 This IVH score is a quick and easy assessment tool in which the user assesses the scan, and then uses a simple exponential equation to approximate the IVH volume in mL. It correlates well with measured IVH volume (R = 0.8),28 but has not been validated using multiple longitudinal assessments nor has it been reduced to a form that is used in prospective clinical trials. Another assessment method, semiautomatic image segmentation, has been proposed for IVH measurement. This technique uses minimal user input to identify IVH, and can give a very accurate measure of the total volume of the segmented structure.29 However, this method requires sophisticated software that is not validated, nor is it readily available in most clinical settings. Using our proposed methods, the CT scan is all that is required to assign the mGS. Furthermore, the ease of use and excellent reproducibility means that the mGS can be used by highly trained neuroradiologists and less sophisticated CT readers alike.
It is already known that presence of IVH and IVH volume are strong predictors of mortality and outcome.5,30 We found that mGS score adds to the predictive information provided by other key variables such as baseline National Institutes of Health Stroke Scale (NIHSS) score and ICH volume in our VISTA analysis. We were unable to adjust for potentially important variables such as blood glucose level, GCS, score and previous antithrombotic drug use as they were measured in only a small number of the eligible participants (n=169, 185, and 336, respectively). However we explored sensitivity of our results to this in these smaller sample sizes and results were broadly similar. It is important to note that patients with suppressed GCS were typically excluded from clinical trials contained in the VISTA archive meaning this analysis cannot confirm utility of the mGS with regard to outcome prediction in those with profoundly suppressed consciousness on admission. However, mGS is related to outcome independent of NIHSS score (which was more strongly predictive of outcome than GCS in our analysis) and many participants in CLEAR B had suppressed consciousness meaning it is likely similarly predictive in this setting. Our data reaffirm the need for clinical trials of strategies designed to limit the extent of or remove IVH, and also raises the importance of real time measuring IVH presence and extent in clinical trials of ICH. Imbalances in baseline IVH rates and size may have confounded results of other recent and important trials.31 We believe randomization strategies should ensure that presence of IVH is balanced between treatment arms in clinical trials for ICH, or that mGS could be adjusted for in statistical analysis.
The strengths of our program include the longitudinal aspect of the CLEAR analysis, the large sample size in the VISTA analysis, and the use of a simple tool and its training program already used in clinical trials. This is combined with the fact that our methods were validated using 2 datasets in differing environments. Data from the VISTA archive come from rigorously conducted and monitored clinical trials; we were unable to assign end point status in only 6 cases (0.5%). We could adjust for ICH volume, location, and baseline NIHSS but were unable to include all cases of ICH contained within VISTA since CT scans were not available for review for all clinical trials. As mentioned, a further potential weakness is selection bias in medical trials of ICH where patients with significantly reduced conscious level have often been excluded. This may explain why in the CLEAR B study the proportion with deep ICH was higher and case fatality lower than that seen in previous population cohorts.32
In conclusion, we have shown that the mGS is a suitable tool to assess the extent of IVH. It is reliable and valid, and more closely related to change in IVH volume and outcome than the oGS. The mGS could readily be used to assess outcomes in clinical trials of ICH and IVH and to monitor progress of thrombolytic therapy for IVH.
Table 2.
Multivariable Logistic Regression Analysis in Study Two.
| Variable | OR | 95% CI for OR | P Value |
|---|---|---|---|
| Age, y | 1.10 | 1.06–1.13 | <0.0001 |
| Baseline NIHSS | 1.20 | 1.12–1.27 | <0.0001 |
| ICH volume, cm3 | 1.04 | 1.02–1.06 | <0.0001 |
| Lobar hemorrhage | 2.42 | 0.83–7.00 | 0.104 |
| mGS | 1.12 | 1.05–1.19 | <0.0001 |
CI indicates confidence interval; ICH, intracerebral hemorrhage; mGS, modified Graeb score; NIHSS, National Institutes of Health Stroke Scale; and OR, odds ratio for poor outcome (mRS>3) and refers to change in odds ratio per unit increase in variable for continuous variables.
Acknowledgments
VISTA-Acute members: K.R. Lees (Chair), A. Alexandrov, P.M. Bath, E. Bluhmki, L. Claesson, S.M Davis, G. Donnan, H. C. Diener, M. Fisher, B. Gregson, J. Grotta, W. Hacke, M.G. Hennerici, M. Hommel, M. Kaste, P. Lyden, J. Marler, K. Muir, R. Sacco, A. Shuaib, P. Teal, N.G. Wahlgren, S. Warach, and C. Weimar. CLEAR Executive Committee: D. F. Hanley (Chair), I.A. Awad (Co-Chair), M. Diener-West, M.E. Griswold, P. Keyl, K.R. Lees, A. Marmarou, B. Gregson, J. Mighty, D.H. Rhoney, K. Dickersin, C. Kase, and I. Barofsky.
Sources of Funding
The CLEAR B study was funded by the Food and Drug Administration, Division of Orphan Products, and National Institutes of Health (ClinicalTrials.gov Identifier: ). The VISTA study was funded by the Stroke Association (grant 2007/08) and approved by the VISTA steering committee. N.K.M. was supported by an Overseas Research Student Award from the University of Glasgow. D.H. is funded by CLEAR III 5U01-NS062851–03 and MISTIE II 5R01-NS046309–07.
Footnotes
Disclosures
None.
References
- 1.Adams HP, Torner JC, Kassell NF. Intraventricular hemorrhage among patients with recently ruptured aneurysms: a report of the Cooperative Aneurysm Study. Stroke.. 1992;23:140. [Google Scholar]
- 2.Conway JE, Oshiro EM, Piantadosi S. Ventricular blood is an admission CT variable which predicts poor clinical outcome after aneurysmal subarachnoid hemorrhage. American Association of Neurological Surgeons Annual Meeting, Philadelphia, Pennsylvania. J Neurosurg. 1998;88:398A. [Google Scholar]
- 3.Daverat P, Castel JP, Dartigues JF, Orgogozo JM. Death and functional outcome after spontaneous intracerebral hemorrhage. A prospective study of 166 cases using multivariate analysis. Stroke. 1991;22:1–6. [DOI] [PubMed] [Google Scholar]
- 4.Lisk DR, Pasteur W, Rhoades H, Putnam RD, Grotta JC. Early presentation of hemispheric intracerebral hemorrhage: prediction of outcome and guidelines for treatment allocation. Neurology. 1994;44:133–139. [DOI] [PubMed] [Google Scholar]
- 5.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–621. [DOI] [PubMed] [Google Scholar]
- 6.Matchett SC, Castaldo J, Wasser TE, Baker K, Mathiesen C, Rodgers J. Predicting mortality after intracerebral hemorrhage: comparison of scoring systems and influence of withdrawal of care. J Stroke.Cerebrovasc Dis. 2006;15:144–150. [DOI] [PubMed] [Google Scholar]
- 7.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Validation and comparison of models predicting survival following intracerebral hemorrhage. Crit Care Med. 1995;23:950–954. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. [DOI] [PubMed] [Google Scholar]
- 9.Young WB, Lee KP, Pessin MS, Kwan ES, Rand WM, Caplan LR. Prognostic significance of ventricular blood in supratentorial hemorrhage: a volumetric study. Neurology. 1990;40:616–619. [DOI] [PubMed] [Google Scholar]
- 10.Brott T, Thalinger K, Hertzberg V. Hypertension as a risk factor for spontaneous intracerebral hemorrhage. Stroke. 1986;17:1078–1083. [DOI] [PubMed] [Google Scholar]
- 11.Mohr G, Ferguson G, Khan M, Malloy D, Watts R, Benoit B, et al. Intraventricular hemorrhage from ruptured aneurysm. Retrospective analysis of 91 cases. J Neurosurg. 1983;58:482–487. [DOI] [PubMed] [Google Scholar]
- 12.Adams RE, Diringer MN. Response to external ventricular drainage in spontaneous intracerebral hemorrhage with hydrocephalus. Neurology. 1998;50:519–523. [DOI] [PubMed] [Google Scholar]
- 13.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 2. In vivo safety study of intraventricular urokinase. Neurosurgery. 1986;19:547–552. [DOI] [PubMed] [Google Scholar]
- 14.Wagner KR, Xi G, Hua Y, Zuccarello M, de Courten-Myers GM, Broderick JP, et al. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: edema reduction and blood-brain barrier protection. J Neurosurg. 1999;90:491–498. [DOI] [PubMed] [Google Scholar]
- 15.Mayfrank L, Kissler J, Raoofi R, Delsing P, Weis J, Küker W, et al. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28:141–148. [DOI] [PubMed] [Google Scholar]
- 16.Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000;247:117–121. [DOI] [PubMed] [Google Scholar]
- 17.Morgan T, Awad I, Keyl P, Lane K, Hanley D. Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl. 2008;105:217–220. [DOI] [PubMed] [Google Scholar]
- 18.CLEAR III Investigators Study Group. Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III (CLEAR III). 2009; Available at: http://clinicaltrials.gov/. Accessed 02/20, 2011.
- 19.Keir SL, Wardlaw JM, Sandercock PA, Chen Z. Antithrombotic therapy in patients with any form of intracranial haemorrhage: a systematic review of the available controlled studies. Cerebrovasc Dis. 2002;14: 197–206. [DOI] [PubMed] [Google Scholar]
- 20.Naff N, Williams MA, Keyl PM, Tuhrim S, Bullock MR, Mayer SA, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42:3009–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. [DOI] [PubMed] [Google Scholar]
- 22.Huttner HB, Steiner T, Hartmann M, Köhrmann M, Juettler E, Mueller S, et al. Comparison of ABC/2 estimation technique to computer-assisted planimetric analysis in warfarin-related intracerebral parenchymal hemorrhage. Stroke. 2006;37:404–408. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman RD, Maldjian JA, Brun NC, Horvath B, Skolnick BE. Radiologic estimation of hematoma volume in intracerebral hemorrhage trial by CT scan. AJNR Am J Neuroradiol. 2006;27:666–670. [PMC free article] [PubMed] [Google Scholar]
- 24.Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143:91–96. [DOI] [PubMed] [Google Scholar]
- 25.Hinson HE, Hanley DF, Ziai WC. Management of intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2010;10:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali M, Bath P, Brady M, Davis S, Diener HC, Donnan G, et al. ; VISTA Steering Committees. Development, expansion, and use of a stroke clinical trials resource for novel exploratory analyses. Int J Stroke. 2012;7: 133–138. [DOI] [PubMed] [Google Scholar]
- 27.Morgan TC, Hanley DF, Mayo S. CLEAR III CT Assessment Training Materials -- Online Posting. 2009; Available at: http://braininjuryoutcomes.com/community/documents/viewcategory/4,2011.
- 28.Hallevi H, Dar NS, Barreto AD, Morales MM, Martin-Schild S, Abraham AT, et al. The IVH score: a novel tool for estimating intraventricular hemorrhage volume: clinical and research implications. Crit Care Med. 2009;37:969–74, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardera A, Boada I, Feixas M, Remollo S, Blasco G, Silva Y, et al. Semi-automated method for brain hematoma and edema quantification using computed tomography. Comput Med Imaging Graph. 2009;33:304–311. [DOI] [PubMed] [Google Scholar]
- 30.Naff NJ, Hanley DF, Keyl PM, Tuhrim S, Kraut M, Bederson J, et al. Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery. 2004;54:577–83; discussion 583. [DOI] [PubMed] [Google Scholar]
- 31.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. ; FAST Trial Investigators. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. [DOI] [PubMed] [Google Scholar]
- 32.Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40:394–399. [DOI] [PubMed] [Google Scholar]