Abstract
Reduced-intensity conditioning (RIC) and t-cell depletion (TCD) through CD34+ selection without the use of post-transplant immunosuppression are 2 strategies used to reduce non-relapse mortality (NRM) in older patients after allogeneic hematopoietic cell transplantation (allo-HCT). To compare the efficacy of both approaches (RIC and TCD), we evaluated the outcomes of AML and MDS patients > 50 years who underwent allo-HCT from an HLA-matched donor with one of these strategies. Baseline characteristics were comparable between patients receiving TCD (n=204) and RIC (n=151) except for more unrelated donors (68% vs. 40%, p<0.001) and higher comorbidity burden in the TCD cohort (HCT-CI ≥3: 51% vs. 38%, p<0.001). After analyzing outcomes at 3 years, patients with TCD showed higher chronic graft-versus-host disease (GVHD) / relapse-free survival (CRFS) (51% vs. 7%, p<0.001), lower incidence of grade 2–4 acute (18% vs. 46% at day +180) and chronic (6% vs. 55%, at 3 years) GVHD (p<0.001) and lower incidence of relapse (19% vs. 33%, at 3 years) (p = 0.001) compared with those receiving RIC allo-HCT. RFS, OS and NRM were similar between both groups. Combining transplant approach (RIC vs. TCD) and comorbidity burden (HCT-CI 0–2 vs. ≥ 3) patients with HCT-CI score of 0–2 seemed to benefit of receiving the TCD approach. In conclusion, in this retrospective study, the use of a CD34+ selected graft and a myeloablative conditioning was associated with higher CRFS and similar RFS and OS compared to unmodified allo-RIC in patients > 50 years with AML and MDS.
Keywords: RIC, t-cell depletion, allogeneic hematopoietic cell transplantation, GVHD
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is the only curative treatment for high risk acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Patients with standard and high risk AML and advanced MDS are usually considered candidates for allo-HCT due to the high risk of disease progression and relapse. However, this procedure has significant transplant-related mortality (TRM) and morbidity, especially in patients over 50 years of age. Several approaches have been considered to reduce TRM in older patients or those with comorbidities. The most widely used strategy is to lower the intensity of the preparative regimen to either a reduced-intensity (RIC) or a non-myeloablative (NMA) conditioning. While this approach can significantly reduce TRM, it also increases the risk of relapse1–4. Another approach is ex-vivo T-cell depletion of the allograft through CD34+ cell selection prior to transplant. This strategy has been shown to significantly decrease the incidence of acute and chronic graft-versus-host disease (GVHD) with acceptable long term relapse-free (RFS) and overall survival (OS) in patients with several diseases including AML and MDS.5–12 Moreover, the absence of a requirement for post-transplant immunosuppressive therapy makes this approach attractive for patients with comorbidities who could avoid drug-related toxicity, especially from calcineurin inhibitors. To compare the efficacy of both approaches (RIC and TCD) in the setting of a homogeneous population of patients > 50 years, we evaluated the outcomes of AML and MDS patients who underwent allo-HCT from an 8/8 HLA-matched donor with the TCD approach at Memorial Sloan Kettering Cancer Center (MSKCC) or with the RIC approach at a consortium of 4 University Hospitals in Spain.
Patients and Methods
Patients
Patients diagnosed with AML or MDS and undergoing allo-HCT from January 2005 through September 2014 at MSKCC and in the Spanish consortium of 4 large university centers were identified through institutional HCT registries. For inclusion in the study, patients had to meet all of the following: (1) being 50 years of age or older, (2) receiving a first allo-HCT, (3) being in CR or CR with incomplete hematological recovery (CRi) for AML or have < 5% blast in pre-transplant bone marrow evaluation for MDS, and (4) receiving G-CSF mobilized peripheral blood progenitors from an 8/8 HLA-matched related or unrelated donor. Patients transplanted at MSKCC received myeloablative conditioning followed by infusion of the graft with in vivo CD34+ positive selection (TCD approach). Patients transplanted in the Spanish consortium received RIC followed by unmodified graft infusion and immunosuppressive therapy (RIC approach). Written informed consent for treatment was obtained from all patients and donors. Approval for this retrospective review was obtained from the Institutional Review and Privacy Board of all participant institutions.
Transplant procedures and supportive care
The TCD approach consisted of myeloablative conditioning in all patients with either total body irradiation (TBI) or chemotherapy-based regimens. Either of two TBI-based regimens were used: TBI 1375 cGy given in 11 fractions followed by 2 daily doses of thiotepa (5 mg/kg/day) and, either 2 daily doses of cyclophosphamide (60 mg/kg/day) starting after thiotepa, or 5 daily doses of fludarabine (25 mg/m2/day) beginning on the first day of thiotepa.8,9 The chemotherapy-based preparative regimen consisted of intravenous busulfan (0.8 mg/kg/dose) every 6 hours for 10 doses, melphalan (70 mg/m2/day) for 2 doses and fludarabine (25 mg/m2/day) for 5 doses.10 TBI-based regimen was preferred in younger and fit patients while chemo-based regimen was designed for older patients or those not eligible for TBI. T cell depletion of granulocyte colony stimulating factor mobilized peripheral blood stem cells (PBSC) was performed as previously described.8,9,13 Positive selection of CD34+ cells was performed by using Isolex 300i Magnetic Cell Separator (Baxter, Deerfield, IL) and subsequent sheep RBC rosette depletion,9 or using the CliniMACS CD34 Reagent System (Miltenyi Biotech, Gladbach, Germany).14 Equine or rabbit anti-thymocyte globulin (ATG) (2.5mg/kg/day on days −3 and −2) were used to promote engraftment in the majority of cases. No other post-transplant immunosuppressive therapy was used for any of the patients. Preventive DLIs were not planned in either transplant protocols.
The RIC approach consisted of fludarabine based conditioning (150mg/m2) in combination with busulfan (8–10 mg/kg) or busulfan and thiotepa (5–10mg/kg). A few patients included in a specific protocol received fludarabine-melphalan (70–140mg/m2) conditioning, as previously reported2,15. Unmodified grafts from peripheral blood mobilized progenitors were infused on day 0. GHVD prophylaxis consisted of calcineurin inhibitors (tacrolimus or cyclosporine) in combination with either short course methotrexate (15mg/m2 on day +1 and 10mg/m2 on days +3,+6 and +11, followed by folinic acid rescue), mycophenolate mofetil started on day 0 (at least 10 hours after infusion of progenitors) at a dose of 15mg/kg tid, or sirolimus started on day −6 at 2–4 mg/day. ATG was used in this protocol only for patients receiving a graft from HLA mismatched unrelated donors. Thus, no patient included in this study received ATG.
HLA matching was established by DNA sequence-specific oligonucleotide typing for HLA-A, -B, C, DR-B1 and DQ-B1 loci. HLA matched patient-donor combination were defined if both alleles were matched at A, B, C and DRB1 locus (8/8). Patients receiving allo-HCT from HLA mismatched donors or haploidentical donors were excluded. All patients received supportive care and prophylaxis against opportunistic infections according to standard guidelines.
Cytogenetics, Disease Risk Index and Comorbidity assessment and scoring
Cytogenetics and Disease Risk Index were calculated and classified as recently refined by Armand et al.16 Since all patients had to be in remission in order to be included in the study, the stage risk category was low in all patients. Thus, there were no patients in the very high risk group. The HCT-CI was calculated as originally defined and following standard recommendations.17,18 Patients were classified in the same risk groups as the original publications of both models.
Endpoints, definitions and statistical analysis
Descriptive statistics were used to summarize patient characteristics. Comparisons of patient characteristics across transplant type (TCD vs RIC) were evaluated using Fisher-exact test for categorical variables and Wilcoxon-rank sum test for continuous variables.
The primary endpoint of the study was chronic-GVHD free/relapse free-survival (CRFS). Secondary end-points included overall survival (OS), relapse-free survival (RFS), non-relapse mortality (NRM) and relapse. All time-to-event outcomes started from the date of transplant (HCT date). CRFS considered moderate to severe chronic GVHD according to NIH consensus criteria global score,19 disease relapse or any-cause death as events; OS considered any-cause death as an event; RFS considered both disease relapse and any-cause death as events. The probabilities of OS, RFS and CRFS at select time point were estimated using Kaplan-Meier methods. Univariate comparisons of OS, RFS and CRFS across patient and transplant characteristics were evaluated using the log-rank test.
Competing risk analyses were used for NRM and relapse outcomes. NRM was defined as any-cause death treating relapse as a competing risk. Relapse was defined as disease relapse with death in the absence of relapse as a competing risk. The cumulative incidence failure (CIF) rates for NRM and relapse were estimated based on Gray20.Univariate comparisons for NRM and relapse were evaluated using Gray test21.
Multivariate models were developed for OS, RFS, and CRFS using the Cox proportional hazards model and NRM and relapse using the Fine and Gray model. The primary comparison was transplant type, and all models adjusted for age (>60 years) and donor (related vs. unrelated). Additional factors were considered based on having a univariate p value <0.1. Prognostic factors evaluated in the univariate analysis included: gender of the patient and donor, diagnosis (AML vs. MDS), DRI (low and intermediate vs. high), HCT-CI (low vs. intermediate vs. high) and HCT approach (TCD vs. RIC).
We also examined the association between a combination of transplant approach (TCD vs. RIC) and comorbidity burden (HCT-CI 0–2 vs. ≥ 3) with transplant outcomes. Another set of multivariate models were built using this combination variable [table 5].
Table 5.
Multivariate Analysis Model of Risk Factors for Transplantation Outcomes in All Patients, Including the Merged Category of HCT Approach and Comorbidity Burden
| Patient and Graft Characteristic | NRM | Relapse | OS | CRFS | RFS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (years) | .055 | .225 | .005 | .025 | .003 | |||||
| 50–59 | Reference | Reference | Reference | Reference | Reference | |||||
| >60 | 1.54 (.99–2.41) | 1.31 (.85–2.03) | 1.6 (1.16–2.22) | 1.34 (1.04–1.74) | 1.61 (1.18–2.19) | |||||
| Donor | .036 | .694 | .066 | .169 | .081 | |||||
| Related | Reference | Reference | Reference | Reference | Reference | |||||
| Nonrelated | 1.61 (1.03–2.52) | .95 (.59–1.41) | 1.37 (.98–1.9) | 1.2 (.92–1.57) | 1.32 (.97–1.81) | |||||
| Diagnosis | .023 | |||||||||
| AML | Reference | |||||||||
| MDS | 1.64 (1.07–2.5) | |||||||||
| DRI | - | - | <.001 | <.001 | <.001 | <.001 | ||||
| Low/intermediate | Reference | Reference | Reference | Reference | ||||||
| High | 3.33 (2.15–5.18) | 2.22 (1.58–3.14) | 1.94 (1.44–2.6) | 2.3 (1.65–3.19) | ||||||
| HCT approach and HCT-CI | .270 | .018 | .087 | <.001 | .047 | |||||
| TCD and HCT-CI 0–2 | Reference | Reference | Reference | Reference | Reference | |||||
| TCD and HCT-CI ≥ 3 | 1.57 (.9–2.75) | 1.32 (.75–2.34) | 1.49 (.96–2.29) | 1.53 (1.03–2.27) | 1.53 (1.02–2.3) | |||||
| RIC and HCT-CI 0–2 | .95 (.49–1.85) | 2.35 (1.31–4.2) | 1.66 (1.05–2.63) | 4.44 (3.03–6.53) | 1.71 (1.11–2.66) | |||||
| RIC and HCT-CI ≥ 3 | 1.21 (.59–2.47) | 2.13 (1.12–4.04) | 1.81 (1.09–2.99) | 5.05 (3.3–7.71) | 1.82 (1.13-2.94) | |||||
The patients were analyzed according to their status as of September 2015. All statistical analyses were performed by using R 3.2 and SAS 9.4.
Results
Patients characteristics
A total of 356 patients (204 receiving TCD and 152 receiving RIC) fulfilled the inclusion criteria and constitute the study population. The main clinical characteristics are summarized in Table 1. In brief, the median age at HCT was 61 years (range 50–73). Underlying diseases included AML (n = 232, 65%) and MDS (n = 124, 35%). Patients with AML were mostly in first complete remission (n = 195, 84%). Fifty-two percent of the patients received a transplant from HLA identical sibling donors. Median HCT-CI score was 2 (range 0–13) and most patients had an intermediate DRI score. Patients in the TCD and RIC groups had similar cytogenetic risk for AML (low risk, n = 7 [5.4%] vs. n = 4 [4.1%]; intermediate risk, n = 102 [78.5%] vs. n = 83 [84.7%]; high risk, n = 21 [16.2%] vs. n = 11 [11.2%]) (p = 0.508) and MDS (low risk, n = 0 vs. n = 0; intermediate risk, n = 51 [72.9%] vs. n = 33 [63.5%]; high risk, n = 19 [27.1%] vs. n = 19 [36.5%]) (p = 0.324). Patients in the TCD group had more unrelated donors (n = 121/204 [60%] vs. n = 49/152 [32%], p < 0.001) and higher HCT-CI (HCT-CI ≥3: n = 104/204 [51%] vs. n = 58/152 [38%], p < 0.001) than patients in the RIC group. Median age for patients in the TCD group was 61 years while for the RIC group was 60 years (p = 0.030). All other baseline characteristics were balanced between both groups. Median time from diagnosis to transplant in the TCD and RIC groups were 5.5 months (range: 0.8 – 172.6 months) and 6.4 (range 2–103), respectively. The median follow-up for survivors was 3.8 years (range 0.4–10.6).
Table 1.
Patient Characteristics
| Characteristic | All (n = 356) | TCD Group (n = 204) | RIC Group (n = 152) | P Value |
|---|---|---|---|---|
| Age, yr, median (range) | 61 (50–73) | 61 (50–73) | 60 (50–71) | .03 |
| Male sex, n (%) | 213 (60) | 114 (56) | 99 (65) | .08 |
| Female donor to male recipient, n (%) | 66 (19) | 35 (17) | 31 (20) | .50 |
| Underlying disease, n (%) | ||||
| AML | 232 (65) | 133 (65) | 99 (65) | 10 |
| MDS | 124 (35) | 71 (35) | 53 (35) | |
| Disease status for acute leukemia*, n (%) | ||||
| CR1 | 195 (84) | 109 (82) | 86 (87) | .40 |
| CR2–3 | 37 (16) | 24 (18) | 13 (13) | |
| Donor type, n (%) | ||||
| Related HLA-identical | 184 (52) | 81 (40) | 103 (68) | <.001 |
| Matched unrelated | 170 (48) | 121 (60) | 49 (32) | |
| Conditioning regimen†, n (%) | NA | |||
| Busulfan-melphalan-fludarabine | 177 (86) | 177 (86) | - | |
| TBI-based | 27 (13) | 27 (13) | - | |
| Fludarabine-busulfan 2 | 124 (82) | - | 124 (82) | |
| Fludarabine-busulfan 2-thiotepa | 18 (12) | - | 18 (12) | |
| Fludarabine-melphalan | 10 (7) | - | 10 (7) | |
| T-cell depletion method, n (%) | NA | |||
| CliniMACS | 126 (62) | 126 (62) | - | |
| Isolex | 78 (38) | 78 (38) | - | |
| GVHD prophylaxis, n (%) | NA | |||
| Tacrolimus-sirolimus | 70 (46) | - | 70 (46) | |
| CNI-mycophenolate mofetil | 38 (25) | - | 38 (25) | |
| CNI-methotrexate | 44 (29) | - | 44 (29) | |
| HCT-CI, n (%) | ||||
| 0 | 97 (27) | 38 (19) | 59 (39) | <.001 |
| 1–2 | 97 (27) | 62 (30) | 35 (23) | |
| ≥3 | 162 (46) | 104 (51) | 58 (38) | |
| DRI‡, n (%) | ||||
| Low | 11 (3) | 7 (4) | 4 (3) | .925 |
| Intermediate | 269 (77) | 153 (77) | 116 (77) | |
| High | 70 (20) | 40 (20) | 30 (20) | |
| Follow-up for survivors, yr, median (range) | 3.78 (.4–10.6) | 3.75 (1–10.6) | 3.88 (.4–8.9) | - |
NA indicates not applicable.
Percentage over the AML cases, only.
One patient in the TCD group received clofarabine-thiotepa-melphalan.
Because only patients in remission or untreated were included in this study, no patient was in DRI high-risk stage. Thus, no patient could meet the criteria for classification in the very high risk DRI category. Cytogenetics were not available for 4 patients in the TCD group (2%) and in 2 patients in the RIC group (1%). DRI could not be calculated for these patients.
Engraftment, OS, RFS and CRFS
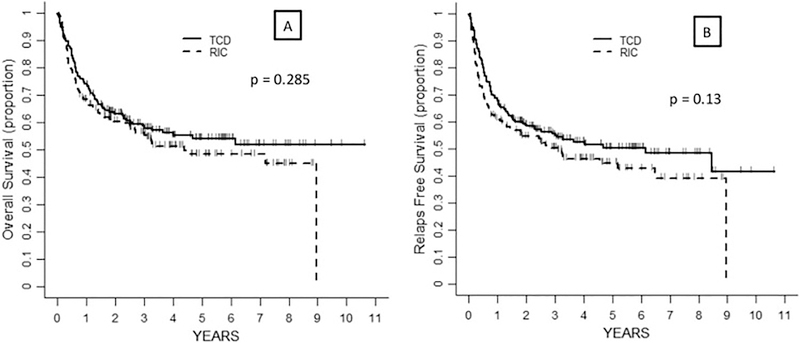
Three-hundred and fifty-four patients were evaluable for engraftment and all of them engrafted. Two patients receiving TCD died due to organ toxicity on days +1 and +10 and were not evaluable for this outcome. One hundred and ninety-six patients were alive at last follow-up. The probability of OS at 3 years in the TCD and RIC groups of 58% (95%CI 51–65) and 56% (95%CI 48–64), respectively (p = 0.285) (Figure 1A, Table 2). In the univariate analysis, risk factors for lower OS included age > 60 years (p = 0.002) and DRI High (compared to reference Low-Intermediate, p < 0.001) (Table 3). After multivariate analysis both variables retained statistical significance whereas patients receiving RIC (compared to TCD) showed a trend for increased risk of death (p = 0.056) (Table 4).
Figure 1:
Probability of OS (A) and RFS (B) according to transplant approach
Table 2.
Comparison of Outcomes in the CD34+ Cell-Selected and Unmodified Grafts
| Outcome | CD34+ Cell-Selected MAC, % (95% CI) | Unmodified RIC, % (95% CI) | P Value |
|---|---|---|---|
| RFS | |||
| 1 year | 68.1 (61.3–74) | 61 (52.8–68.3) | .13 |
| 3 years | 54.9 (47.6–61.7) | 50.4 (42–58.3) | |
| OS | |||
| 1 year | 74 (67.4–79.5) | 68.3 (60.2–75.1) | .285 |
| 3 years | 57.9 (50.5–64.6) | 56.2 (47.7–63.9) | |
| Relapse incidence | |||
| 1 year | 13.7 (9–18.5) | 27.7 (20.6–34.9) | .001 |
| 3 years | 19.1 (13.6–24.6) | 33.1 (25.4–40.8) | |
| NRM | |||
| 100 days | 9.3 (5.3–13.3) | 3.9 (.8–7.1) | .106 |
| 1 year | 18.1 (12.8–23.4) | 11.3 (6.2–16.3) | |
| 3 years | 25.9 (19.7–32.1) | 16.5 (10.4–22.6) | |
| Acute GVHD grade II-IV | |||
| 180 days | 17.6 (12.4–22.9) | 46.1 (38.1–54.1) | <.001 |
| Chronic GVHD | |||
| 3 years | 6 (2.7–9.4) | 55.3 (47.1–63.4) | <.001 |
| CRFS | |||
| 1 year | 64.7 (57.7–70.8) | 21.3 (15.1–28.1) | <.001 |
| 3 years | 51.1 (43.8–57.9) | 7.4 (3.8–12.6) |
Table 3.
Univariate Analysis of Risk Factors for Transplantation Outcomes, All Patients
| Patient and Graft Characteristic | NRM | Relapse | OS | CRFS | RFS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | .627 | .189 | .177 | |||||||
| Male | Reference | Reference | Reference | .293 | Reference | .071 | Reference | |||
| Female | .89 (.57–1.39) | .75 (.48–1.15) | .84 (.61–1.16) | .79 (.6–1.02) | .81 (.59–1.1) | |||||
| Donor/recipient sex match | .977 | .757 | .925 | .852 | .868 | |||||
| Female to male | 1 (.6–1.7) | 1.08 (.65–1.8) | 1.02 (.69–1.52) | .97 (.7–1.34) | 1.03 (.71–1.5) | |||||
| Other | Reference | Reference | Reference | Reference | Reference | |||||
| Age (years) | .014 | .287 | .002 | .158 | .002 | |||||
| 50–59 | Reference | Reference | Reference | Reference | Reference | |||||
| >60 | 1.72 (1.1–2.67) | 1.24 (.82–1.88) | 1.65 (1.19–2.28) | 1.2 (.93–1.55) | 1.6 (1.18–2.18) | |||||
| Donor | .004 | .355 | .057 | .526 | .092 | |||||
| Related | Reference | Reference | Reference | Reference | Reference | |||||
| Nonrelated | 1.81 (1.19–2.77) | .81 (.54–1.22) | 1.35 (.99–1.85) | .92 (.72–1.19) | 1.29 (.96–1.73) | |||||
| Diagnosis | .009 | .104 | .184 | .225 | .421 | |||||
| AML | Reference | Reference | Reference | Reference | Reference | |||||
| MDS | 1.75 (1.15–2.67) | .68 (.43–1.08) | 1.24 (.9–1.71) | 1.18 (.9–1.53) | 1.13 (.83–1.54) | |||||
| HCT-CI | .038 | .168 | .121 | .332 | .11 | |||||
| 0 | Reference | Reference | Reference | Reference | Reference | |||||
| 1–2 | 1.67 (.87–3.2) | .58 (.33–1.02) | 1.01 (.65–1.57) | .83 (.59–1.18) | .91 (.6–1.39) | |||||
| ≥3 | 2.07 (1.25–3.43) | .83 (.58–1.19) | 1.39 (.95–2.03) | 1.05 (.78–1.42) | 1.3 (.91–1.86) | |||||
| DRI | .872 | <.001 | <.001 | <.001 | <.001 | |||||
| Low/intermediate | Reference | Reference | Reference | Reference | Reference | |||||
| High | .94 (.54–1.63) | 3.27 (2.15–4.96) | 2.31 (1.64–3.24) | 1.86 (1.39–2.5) | 2.35 (1.7–3.26) | |||||
| HCT approach | .106 | .001 | .285 | <.001 | .13 | |||||
| CD34+ cell selection MAC | Reference | Reference | Reference | Reference | Reference | |||||
| Unmodified RIC | .69 (.45–1.07) | 1.96 (1.3–2.94) | 1.18 (.87–1.62) | 3.4 (2.61–4.43) | 1.26 (.93–1.69) | |||||
| HCT approach and HCT-CI | .089 | .009 | .084 | <.001 | .041 | |||||
| TCD and HCT-CI 0–2 | Reference | Reference | Reference | Reference | Reference | |||||
| TCD and HCT-CI ≥ 3 | 1.62 (.93–2.81) | 1.42 (.78–2.6) | 1.65 (1.07–2.53) | 1.56 (1.06–2.31) | 1.67 (1.11–2.51) | |||||
| RIC and HCT-CI 0–2 | .81 (.52–1.26) | 2.4 (1.53–3.77) | 1.46 (.93–2.28) | 4.01 (2.76–5.83) | 1.57 (1.03–2.4) | |||||
| RIC and HCT-CI ≥ 3 | 1.07 (.69–1.65) | 2.33 (1.48–3.66) | 1.74 (1.06–2.87) | 4.92 (3.26–7.43) | 1.81 (1.13–2.9) |
Abbreviations: NRM, non-relapse mortality; OS, overall survival, CRFS Chronic GVHD-Free, Relapse-Free Survival; D/R, donor/recipient; HCT-CI, Hematopoietic Cell Transplantation Comorbidity Index; DRI, Disease-risk index; AML, acute myeloid leukemia.
Table 4.
Multivariate Analysis of Risk Factors for Transplantation Outcomes, All Patients
| Patient and Graft Characteristic | NRM | Relapse | OS | CRFS | RFS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (years) | .047 | .222 | .004 | .025 | .002 | |||||
| 50–59 | Reference | Reference | Reference | Reference | Reference | |||||
| >60 | 1.57 (1.01–2.45) | 1.31 (.85–2.03) | 1.61 (1.17–2.24) | 1.34 (1.04–1.74) | 1.62 (1.19–2.2) | |||||
| Donor | .035 | .749 | .056 | .163 | .071 | |||||
| Related | Reference | Reference | Reference | Reference | Reference | |||||
| Nonrelated | 1.61 (1.03–2.51) | .93 (.61–1.43) | 1.38 (.99–1.92) | 1.21 (.93–1.57) | 1.33 (.98–1.82) | |||||
| Diagnosis | .025 | - | - | |||||||
| AML | Reference | |||||||||
| MDS | 1.62 (1.06–2.48) | |||||||||
| HCT-CI | .084 | - | - | - | - | - | - | - | - | |
| 0 | Reference | |||||||||
| 1–2 | 1.6 (.83–3.09) | |||||||||
| ≥3 | 1.89 (1.06–3.38) | |||||||||
| DRI | - | - | <.001 | <.001 | <.001 | <.001 | ||||
| Low/intermediate | Reference | Reference | Reference | Reference | ||||||
| High | 3.3 (2.16–5.15) | 2.28 (1.62–3.21) | 1.98 (1.47–2.66) | 2.34 (1.69–3.24) | ||||||
| HCT approach | .62 | .002 | .056 | <.001 | .038 | |||||
| CD34+ cell-selected MAC | Reference | Reference | Reference | Reference | Reference | |||||
| Unmodified RIC | .89 (.56–1.41) | 1.95 (1.27–2.98) | 1.38 (.99–1.92) | 3.7 (2.8–4.88) | 1.39 (1.02–1.9) |
Probability of 3-year RFS was 55% (95%CI 48–62) in the TCD group vs. 50% (95%CI 42–58) in the RIC group (p = 0.130) (Figure 1B, Table 2). In the univariate analysis, the risk of relapse or death was higher for age > 60 years (p = 0.002) and DRI High (compared to reference Low-Intermediate, p < 0.001) (Table 3). In the multivariate analysis, variables associated with lower RFS were age (p = 0.002) and DRI (p < 0.001). There was no univariate association of transplant type (TCD vs. RIC) and RFS risk (p = 0.130); however, upon multivariate adjustment, a statistically significant association towards higher RFS in the TCD group was observed (p = 0.038). (Table 4).
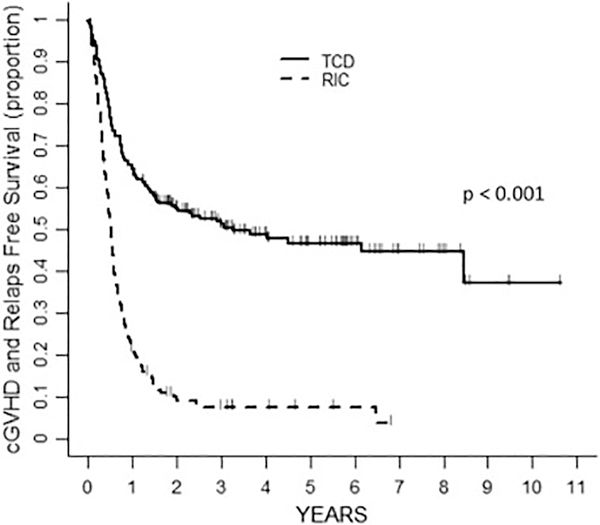
The 3-year probability of CRFS was higher in the TCD group 51% (95%CI 44–58) than in the RIC group 7% (95%CI 4–13) (p < 0.001) (Figure 2, Table 2). In the univariate analysis, the factors associated with an increased risk of the composite CRFS endpoint were DRI Intermediate and High (p < 0.001) and RIC approach (p<0.001), whereas patient age showed a trend (p= 0.071) (Table 3). In the multivariate analysis all variables remained significant (DRI: p< 0.001; RIC approach: p< 0.001 and age: p=0.025) (Table 4).
Figure 2:
Probability of CRFS according to transplant approach
Non-relapse mortality
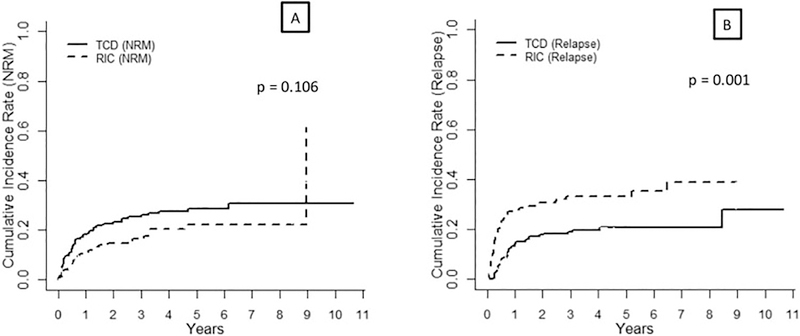
Eighty-five patients had experienced NRM in our cohort, 55 in the TCD group and 30 in the RIC group. Most frequent causes of NRM in the TCD group were infection (n = 23, 42%), organ failure (n = 16, 29%), GVHD (n = 7, 13%), other malignancy (n = 3, 5%), other cause (n = 5, 9%) and unknown cause (n = 1, 2%). In the RIC group, patients experienced NRM due to GVHD (n = 10, 33%), infection (n = 8, 27%), organ failure (n = 3, 10%) other malignancy (n = 2, 7%), other cause (n = 4, 13%) and unknown cause (n = 3, 10%). Cumulative incidence of NRM at +100 days, 1 year and 3 years in the TCD group were 9% (95% CI 5–13), 18% (95%CI 13–23) and 26% (95%CI 20–32), respectively, whereas in the RIC group were 4% (95% CI 1–7), 11% (95%CI 6–16) and 17% (95%CI 10–23), respectively (p = 0.106) (Table 2, Figure 3A).
Figure 3:
Cumulative Incidence of NRM (A) and relapse (B) according to transplant approach
In the univariate analysis, risk factors for an increased NRM risk (Table 3) included age > 60 years (p = 0.014), unrelated donor (p = 0.004), diagnosis of MDS (p = 0.009) and HCT-CI 1–2 and ≥ 3 (compared to reference HCT-CI = 0; overall p = 0.038). In the multivariate analysis, all these variables retained statistical significance or border line significance (age: p = 0.047; unrelated donor: p = 0.035, diagnosis of MDS: p = 0.025, HCT-CI 1–2 and ≥ 3: p = 0.084 (Table 4). The transplant approach (TCD vs. RIC) was not associated with NRM risk in the multivariate model (p = 0.620).
Relapse
A total of 87 patients experienced disease relapse at 3 years, 38 in the TCD group and 49 in the RIC group. The 3-year cumulative incidence of relapse in the TCD and RIC groups were 19% (95%CI 14–25) and 33% (95%CI 25–41, p = 0.001), respectively Figure 3B). In the univariate analysis, high DRI (compared to reference Intermediate-Low, p < 0.001) and RIC approach (compared to TCD, p = 0.001) were associated with increased relapse risk (Table 2–3). In multivariate analysis, both variables remained statistically significant (RIC approach: p = 0.002; DRI: p < 0.001) (Table 4).
Acute and chronic GVHD
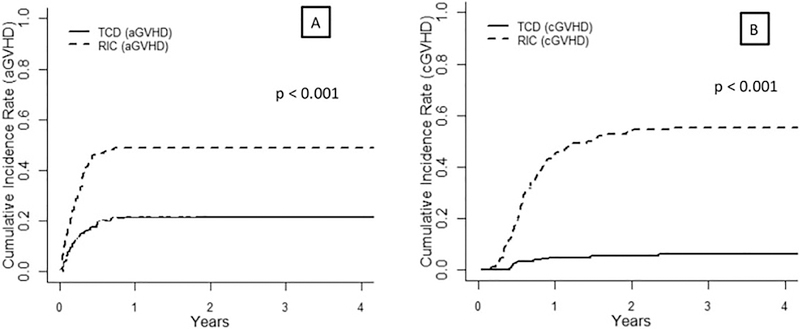
Cumulative incidence of grade 2–4 acute GVHD at day +180 in TCD and the RIC groups were 18% (95%CI 12–23%) and 46% (95%CI 38–54%), respectively (p < 0.001), whereas cumulative incidence of 3-year chronic GVHD in TCD and the RIC groups were 6% (2.7–9.4%) and 55.3% (47.1–63.4%), respectively (p < 0.001, Figure 4). Among the 12 patients with chronic GVHD features at 3 years in the TCD group, disease severity was mild in 6 patients, moderate in 2 and severe in 4 patients. In the RIC group, 82 patients experienced chronic GVHD: 28 mild, 28 moderate and 26 severe.
Figure 4:
Cumulative Incidence of acute (A) and chronic (B) GVHD
Subgroup analysis of comorbidity burden and HCT approach
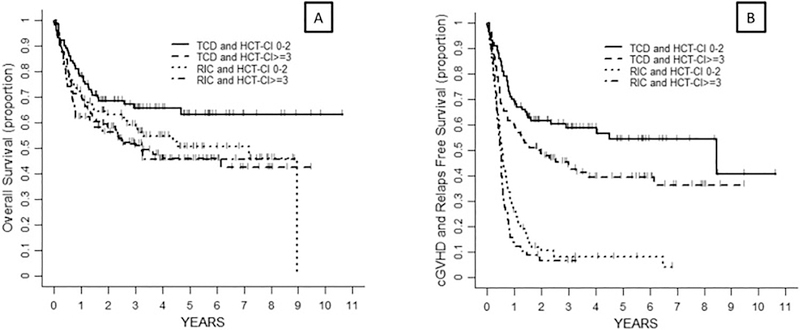
After combining the comorbidity burden and HCT approach, patients were classified into 4 groups: TCD and HCT-CI = 0–2 (n = 100, 28%), TCD and HCT-CI ≥ 3 (n = 104, 29%), RIC and HCT-CI= 0–2 (n = 94, 26%) and RIC and HCT-CI ≥ 3 (n = 58, 16%). In the univariate analysis, there were overall differences in relapse risk (p = 0.009), CRFS (p < 0.001) and RFS (p = 0.04), with the lowest risk among patients in the TCD group and HCT-CI 0–2 (compared to TCD and HCT-CI ≥ 3, RIC and HCT-CI= 0–2 and RIC and HCT-CI ≥ 3). No differences in NRM were observed (p = 0.089) (table 3). In the multivariate analysis, these variables retained statistical significance except for the NRM, which was similar in patients receiving TCD irrespective of their HCT-CI score (p = 0.270) In addition, patients in the TCD group and HCT-CI 0–2 (compared to RIC and HCT-CI= 0–2 and RIC and HCT-CI ≥ 3) showed a trend towards higher OS (p = 0.087; HR = 1.66 [95%CI: 1.05–2.63] and 1.81[1.09–2.99]) (Table 5, Figure 5A).
Figure 5:
Probability of OS (A) and CRFS (B) according to transplant approach and comorbidity burden
Footnote Figure 5. Patients were categorized into 4 subgroups: TCD and HCT-CI = 0–2 (n = 100), TCD and HCT-CI ≥ 3 (n = 104), RIC and HCT-CI = 0–2 (n = 94) and RIC and HCT-CI ≥ 3 (n = 58)
Discussion
Patients > 50 years are not usually considered for allo-HCT with myeloablative conditioning and unmodified grafts due to a high risk of NRM, especially those with comorbidities. The most common option used for these patients is proceeding with a RIC allo-HCT with an unmodified graft. An alternative option is to proceed with a myeloablative conditioning and a CD34+ selected graft. This approach bypasses the need for post-transplant immunosuppression, which may lead to less organ damage and result in lower NRM. However, these approaches have not been compared.
This is the first study comparing the use of CD34+ selection of the graft and myeloablative conditioning with the use of RIC and unmodified grafts for patients undergoing allo-HCT. Despite a higher comorbidity burden and more unrelated donors in the TCD group, these patients showed higher CRFS and lower incidence of acute and chronic GVHD compared with those receiving RIC allo-HCT. RFS, OS and NRM were similar between both groups. When combining transplant approach and HCT-CI, the patients with TCD and low HCT-CI also had improved OS compared to patients undergoing RIC regardless of their HCT-CI.
The lower incidences of chronic GVHD and CRFS observed in the TCD group may lead to a better quality of life and an improvement in self-reported outcomes in patients treated with this procedure. Although no specific data on these topics was available in our study, several investigations have shown an association between a lower incidence of chronic GVHD and a better psychological well-being and a higher likelihood of returning to work in the patients receiving allo-HCT.22,23
Regarding the causes of these differences in chronic GVHD and CRFS, one is, obviously, the lower number of T-cells in the CD34+ selected graft. However, we cannot exclude that the ATG used to promote engraftment in the TCD group may have also contributed to the lower incidence of chronic GVHD in these patients. Since ATG is an essential part of this transplant platform we cannot evaluate the individual impact of each of these two factors, but we can conclude that the risk of acute and chronic GVHD in the setting of matched related or unrelated donors is lowered by using the TCD platform when compared with the RIC approach with unmodified grafts.
The use of ATG in the setting of matched related donor RIC allo-HCT remains controversial, due to a potential higher incidence of relapse in patients receiving ATG reported in some24 but not all studies25–28. Other unsolved questions on the use of ATG in RIC allo-HCT include type and dose of ATG and timing of the administration. Thus, we consider that the allo-RIC with CNI-based GVHD prophylaxis without ATG is a valid comparator with CD34+ selection, at least until prospective studies clarify the optimal usage of ATG in this population of patients. Comparing MAC vs. RIC in patients around 50 years of age could be debatable. While the BMT-CTN 0901 trial showed that MAC was superior to RIC in patients with AML (but not those with MDS),29 a similarly designed randomized trial by the German group (AML)30 and the EBMT (MDS and secondary AML)31 failed to demonstrate this advantage. In light of these contradictory results and while awaiting further trials, we consider that the RIC vs. MAC debate in patients > 50 years is not closed and the comparison remains valid.
Other studies have compared the use of CD34+ selection of the graft as the sole immunosuppressive therapy with the use of unmodified grafts and post-HCT immunosuppression in patients with AML, MDS and acute lymphoblastic leukemia (ALL), all of them in the myeloablative conditioning setting. Pasquini et al. compared the outcomes of patients with AML and MDS treated on the BMT CTN 0303 TCD trial to a matched cohort of patients receiving unmodified grafts on BMT CTN 0101 protocol and showed similar survival and relapse rates, but significantly lower chronic GVHD and higher CRFS in the TCD group.12 More recently, two studies have retrospectively compared patients receiving unmodified grafts and CD34+ selected grafts in the setting of AML7 and ALL5 in two different institutions, showing similar results. All these studies, including ours, revealed consistent results with similar OS and NRM between groups but with lower incidence of acute and chronic GVHD in the TCD setting.
Our findings on disease relapse require further considerations. Studies from the 1990s and early 2000s evaluating the use of TCD seemed to show a higher relapse risk for patients treated with this approach32. This observation was explained by the lower number of T cells in the graft, which would lead to a less profound graft-versus-leukemia effect. However, these studies were performed in an era when chronic myelogenous leukemia (CML) was the main indication for allo-HCT and bone marrow was the preferred stem cell source. CML has been recognized as especially sensitive disease to the GVL effect32. Despite the fact that more contemporary studies in acute leukemia and MDS with new CD34+ selection strategies and conditioning regimens have not confirmed the higher relapse risk in the TCD setting5,7,12 compared with unmodified grafts, there are still some concerns in the transplant community about the risk of relapse after the TCD procedure. In the study reported herein, the relapse risk in the TCD group was actually lower than in the RIC group. This finding might be driven by the lower intensity of the preparative regimen in the RIC group as shown in a recent prospective trial comparing MAC and RIC in T-cell replete transplants (BMT CTN 0901, NCT01339910)29 and several retrospective studies1,3. Thus, in acute leukemia and MDS the regimen intensity may in fact be more critical than the GVL effect in controlling disease relapse. This contrasts with results of allo-HCT in CML, where the use of TCD results in higher relapse and the role of GVL is clearly established. Since the DRI were well matched between the two groups we consider that this finding might not be attributed to the different inclusion criteria for allo-HCT in both institutions. Also, calcineurin inhibitors, widely used in the RIC approach (as opposed to TCD), are known to promote the reconstitution of regulatory T-cells, which have been associated with disease relapse after allo-HCT.33,34
We recently reported that lower HCT-CI and HCT-CI/age were associated with lower NRM and higher OS in CD34-selected alloHCT.35 In the present study, the results of our analysis of subgroups combining the HCT-CI and the transplant approach showed that, in terms of CRFS, patients benefit from TCD allo-HCT (as compared to RIC) irrespective of their comorbidity burden. Noteworthy, patients with low comorbidity scores seemed to benefit the most from this strategy and had also an advantage in RFS. Patients with more severe comorbidities had higher NRM with the TCD approach than with the RIC (mainly due to organ toxicity in as recently reported in TCD allo-HCT36–38), although the lower risk of relapse counterbalanced the deleterious effect on NRM leading to similar RFS but with higher CRFS with the TCD approach than with the RIC.
While awaiting for the results of the ongoing randomized phase III trial evaluating the role of CD34+ selection as a calcineurin-free GVHD prophylaxis strategy in the myeloablative setting (BMT CTN 1301, NCT02345850), our current study shows that the use of CD34+ selected graft and a myeloablative conditioning is associated with higher CRFS and at least similar RFS and OS compared to unmodified allo-RIC in patients > 50 years with AML and MDS.
Highlights.
MAC with CD34+ selection results in similar DFS and OS than RIC with unmanipulated grafts in AML and MDS
MAC and CD34+ selection showed lower risk of GVHD and higher CRFS than RIC with unmanipulated grafts
Patients with MAC and CD34+ selection showed lower risk of relapse than RIC with unmanipulated grafts
For patients with low HCT-CI, MAC with CD34+ selection resulted in higher OS than unmanipulated RIC
Acknowledgments
Financial disclosures: This research was supported in part by National Institutes of Health award numbers P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. PB was supported by the Instituto de Salud Carlos III FIS16/01433 and PERIS 2018-2020 from Generalitat de Catalunya (BDNS357800) grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108(3):836–846. [DOI] [PubMed] [Google Scholar]
- 2.Valcárcel D, Martino R, Caballero D, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26(4):577–584. [DOI] [PubMed] [Google Scholar]
- 3.Mohty M, Labopin M, Volin L, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116(22):4439–4443. [DOI] [PubMed] [Google Scholar]
- 4.Martino R, Henseler A, van Lint M, et al. Long-term follow-up of a retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic transplantation from matched related donors in myelodysplastic syndromes. Bone Marrow Transplant. 2017. [DOI] [PubMed] [Google Scholar]
- 5.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 2015;50(4):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg JD, Linker A, Kuk D, et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(2):208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19(6):898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91(3):1083–1090. [PubMed] [Google Scholar]
- 9.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110(13):4552–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(4):458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(9):1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kernan NA, Flomenberg N, Collins NH, O’Reilly RJ, Dupont B. Quantitation of T lymphocytes in human bone marrow by a limiting dilution assay. Transplantation. 1985;40(3):317–322. [DOI] [PubMed] [Google Scholar]
- 14.Keever-Taylor CA, Devine SM, Soiffer RJ, et al. Characteristics of CliniMACS(R) System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(5):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barba P, Martino R, Pérez-Simón JA, et al. Combination of the Hematopoietic Cell Transplantation Comorbidity Index and the European Group for Blood and Marrow Transplantation score allows a better stratification of high-risk patients undergoing reduced-toxicity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(1):66–72. [DOI] [PubMed] [Google Scholar]
- 16.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121(15):2854–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(3):389–401.e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray R A class of K-sample tests for comparing the cumulative incidence of a competing risk. In. Vol 16 Annals of Statistics:1141–1154. [Google Scholar]
- 21.Fine J, RJ G. A proportional hazards model for the subdistribution of competing risk. In. Vol 94: JASA:496–509. [Google Scholar]
- 22.Lee SJ, Logan B, Westervelt P, et al. Comparison of Patient-Reported Outcomes in 5-Year Survivors Who Received Bone Marrow vs Peripheral Blood Unrelated Donor Transplantation: Long-term Follow-up of a Randomized Clinical Trial. JAMA Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallua S, Giesinger J, Oberguggenberger A, et al. Impact of GvHD on quality of life in long-term survivors of haematopoietic transplantation. Bone Marrow Transplant. 2010;45(10):1534–1539. [DOI] [PubMed] [Google Scholar]
- 24.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devillier R, Crocchiolo R, Castagna L, et al. The increase from 2.5 to 5 mg/kg of rabbit anti-thymocyte-globulin dose in reduced intensity conditioning reduces acute and chronic GVHD for patients with myeloid malignancies undergoing allo-SCT. Bone Marrow Transplant. 2012;47(5):639–645. [DOI] [PubMed] [Google Scholar]
- 26.Walker I, Panzarella T, Couban S, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17(2):164–173. [DOI] [PubMed] [Google Scholar]
- 27.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–864. [DOI] [PubMed] [Google Scholar]
- 28.Kröger N, Solano C, Wolschke C, et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N Engl J Med. 2016;374(1):43–53. [DOI] [PubMed] [Google Scholar]
- 29.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol. 2017;35(11):1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bornhäuser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13(10):1035–1044. [DOI] [PubMed] [Google Scholar]
- 31.Kröger N, Iacobelli S, Franke GN, et al. Dose-Reduced Versus Standard Conditioning Followed by Allogeneic Stem-Cell Transplantation for Patients With Myelodysplastic Syndrome: A Prospective Randomized Phase III Study of the EBMT (RICMAC Trial). J Clin Oncol. 2017;35(19):2157–2164. [DOI] [PubMed] [Google Scholar]
- 32.Sehn LH, Alyea EP, Weller E, et al. Comparative outcomes of T-cell-depleted and non-T-cell-depleted allogeneic bone marrow transplantation for chronic myelogenous leukemia: impact of donor lymphocyte infusion. J Clin Oncol. 1999;17(2):561–568. [DOI] [PubMed] [Google Scholar]
- 33.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao XS, Wang XH, Zhao XY, et al. Non-traditional CD4+CD25-CD69+ regulatory T cells are correlated to leukemia relapse after allogeneic hematopoietic stem cell transplantation. J Transl Med. 2014;12:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barba P, Ratan R, Cho C, et al. Hematopoietic Cell Transplantation-Specific Comorbidity Index Predicts Outcomes in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndromes Receiving CD34(+) Selected Grafts for Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosuri S, Adrianzen Herrera D, Scordo M, et al. The Impact of Toxicities on First-Year Outcomes after Ex Vivo CD34+−Selected Allogeneic Hematopoietic Cell Transplantation in Adults with Hematologic Malignancies. Biol Blood Marrow Transplant. 2017;23(11):2004–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scordo M, Shah GL, Kosuri S, et al. Effects of Late Toxicities on Outcomes in Long-Term Survivors of Ex-Vivo CD34+−Selected Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah GL, Scordo M, Kosuri S, et al. Impact of Toxicity on Survival for Older Adult Patients after CD34+ Selected Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]