Abstract
BACKGROUND
Indisulam possesses anticancer properties through down-regulation of various cell cycle checkpoint molecules, thereby blocking the phosphorylation of retinoblastoma protein and inducing p53 and p21. Indisulam exhibits synergy with nucleoside analogs and topoisomerase inhibitors.
METHODS
We designed a phase 2, study of indisulam in combination with idarubicin and cytarabine in relapsed/refractory AML and high-risk myelodysplastic syndrome. In stage 1, patients were treated with indisulam at 400 mg/m2 intravenously on days 1 and 8 in a 28-day cycle. If no response, patients received same dose-schedule of indisulam followed by idarubicin 8 mg/m2 IV daily x3 and cytarabine 1.0 g/m2 over 24 hours daily on days 9–12 (age <60 years) or days 9–11 (age>60 years) in a 28-day cycle. Primary endpoints included overall response rate and secondary objectives included overall survival.
RESULTS
Forty patients were enrolled. Of the 37 evaluable patients, 31 received indisulam with chemotherapy. Of them, 11 (35%) responded for a median duration of 5.3 months. The estimated 1-year overall survival was 51% for responders compared to 8 % for non-responders (p<0.001). The most common grade ≥3 non-hematological toxicities were electrolyte abnormalities (50%) and febrile neutropenia (28%).
CONCLUSION
The combination of indisulam with idarubicin and cytarabine yielded a 35% response rate in heavily pre-treated AML patients. With emerging data identifying expression of DCAF15, as a potential biomarker for activity, the combination of indisulam with idarubicin and cytarabine should be studied in a biomarker-driven trial or in patients with splicing factors mutations.
Keywords: AML, relapsed/refractory, indisulam, idarubicin, cytarabine, biomarker
Short abstract:
Indisulam, is a cell cycle checkpoint inhibitor which exhibits 35% response rate in heavily pre-treated AML patients.
This combination deserves evaluation in AML patients with splicing factors mutations following the dependence of indisulam’s antineoplastic activity on DCAF15 and RBM39.
BACKGROUND
The combination of an anthracycline (daunorubicin or idarubicin) and cytarabine remains a standard of care induction regimen for newly diagnosed patients with acute myeloid leukemia (AML), leading to remission rates approaching 80%.1, 2 Relapsed or refractory AML portend, in general, dire outcomes,3 and therapeutic options are limited with subsequent response rates being dismal for patients with short first remissions or primary refractory disease.4 Investigational agents alone or in combination with standard chemotherapy in clinical trials should be the most appropriate approach for these patients.
Indisulam (also known as E7070) is a sulfonamide derivative that has been reported to inhibit carbonic anhydrases and cytosolic malate dehydrogenase.5 This agent possesses antitumor properties due to its suppression of the expression of various cell cycle checkpoints molecules, including cyclins A, B1, H and CDK2, with subsequent reduction in Rb phosphorylation and induction of p53 and p21. In murine leukemia P388 cells, single-agent indisulam was found to induce cell-cycle arrest and delay at G1/S and G2/M transitions in parallel with profound down-regulation of redox and energy metabolism genes, followed by induction of apoptosis at higher doses.6, 7 Despite substantial research efforts over two decades, the precise molecular target of indisulam has yet to be elucidated until recently. Pre-clinical and clinical studies have established synergy of indisulam with nucleoside analogs as well as topoisomerase inhibitors.6, 8, 9 In a phase 2 study combining indisulam with chemotherapy in colorectal cancer, indisulam was used at the recommended dose of 400 mg/m2 on days 1 and 8 in a 21-day cycle.10 The combination was tolerated with acceptable toxicities including diarrhea, vomiting and myelosuppression. The combination of nucleoside analogs (e.g. cytarabine, fludarabine) with DNA-damaging agents such as idarubicin is considered standard salvage regimen for AML. We hypothesized that, with its ability to downregulate topoisomerase II, indisulam can potentiate the cytotoxic effect of an anthracycline and a nucleoside analog, when these 3 agents are used in combination.
We therefore designed a phase II study to evaluate the safety and efficacy of indisulam in combination with cytarabine and idarubicin in patients with relapsed refractory AML and high-risk MDS.
METHODS
Study design and participants
This is an open-label, phase 2 study of indisulam in combination with idarubicin and cytarabine conducted at MD Anderson Cancer Center. Patients aged 18 years or older with relapsed or refractory de-novo or secondary AML (excluding acute promyelocytic leukemia) per WHO criteria or high-risk MDS were enrolled.11 Primary refractory or resistant AML was defined by not achieving complete remission (CR) refractoriness to 2 cycles of conventional dose cytarabine or 1 cycle of high dose cytarabine.12 High-risk MDS was defined as Intermediate-2 or high risk by International Prognostic Scoring System (IPSS)13 or having >10% blasts in the bone marrow (BM). Other eligibility criteria included performance status of 0–2, normal cardiac ejection fraction and adequate renal and hepatic functions. Main exclusion criteria were a QTc interval > 480 milliseconds upon screening and recent (within 14 days) treatment for AML other than hydroxyurea which was permitted up to 48 hours prior to enrollment. Pregnant or lactating women and patients with any uncontrolled clinically significant illness were excluded. Infection prophylaxis was per institutional standards. The trial was approved by the Institutional Review Board. All patients provided written informed consent before enrollment according to the declaration of Helsinki. The trial was registered in ClinicalTrials.gov, number NCT01692197.
Bone marrow examination to assess response was done on day 28 (+/− 3 days) of cycle 1, and after that as indicated at the discretion of the treating physician until documentation of response. The entire coding sequences of 28 genes known to be frequently mutated in myeloid hematologic malignancies (ABL1, ASXL1, BRAF, DNMT3A, EGFR, EZH2, FLT3, GATA1, GATA2, HRAS, IDH1, IDH2, KIT, KRAS, MDM2, IKZF2, JAK2, MLL, MPL, MYD88, NOTCH1, NPM1, NRAS, PTPN11, RUNX1, TET2, TP53, and WT1) were sequenced using the Illumina MiSeq platform as previously described. Testing for splicing mutations was not included in the panel at the time of this study.14 Safety electrocardiograms were done on days 2 (+/− 1 day) and 8 (+/− 2 days) of cycle 1 at least 1 hour after completion of indisulam infusion.
Treatment plan
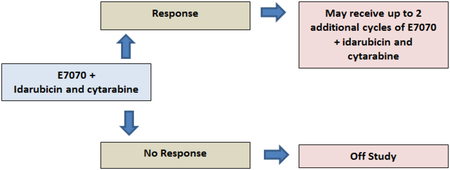
In order to assess single agent response to indisulam, patients were initially treated with one cycle of indisulam alone given at 400 mg/m2 intravenously (IV) on days 1 and 8 in a 28 day cycle. Responding patients were to receive post-remission therapy with indisulam at same dosing schedule, for up to five additional cycles as long as patient continued to have clinical benefit. Non responders were to receive cycle 2 with indisulam (at same dosing schedule as above), combined with idarubicin 8 mg/m2 IV daily x 3 (given on days 9–11) and cytarabine 1 g/m2 IV administered over 24 hours daily on days 9–12 (if age <60 years) or days 9–11 (age > 60 years). The administered doses of idarubicin and cytarabine, lower than the usual 12mg and 1.5 gram per meter squared that is used at our institution, were chosen to avoid undue toxicity when combined with indisulam. Responding patients with CR, CR with incomplete platelets recovery (CRp), partial response (PR) or marrow clearance of blasts received up to two additional cycles of indisulam in combination with chemotherapy at the above doses. Each cycle was given every 4 weeks (+/− 2 days). Dose modifications and delays were allowed for persistent cytopenias or non-hematological toxicities. Because of lack of single agent activity of indisulam, the protocol was modified after the first 20 patients to eliminate the cycle of single agent indisulam and follow the schema as below.
Outcomes
Primary endpoints were overall response rates (ORR), as well as safety and tolerability of the combination of indisulam with idarubicin and cytarabine. Definition of responses was based on criteria from the International Working Group for AML:15 complete remission (CR) was defined as BM sample showing <5% blasts with normal maturation of all cell lines and no dysplasia; a peripheral blood absolute neutrophil count (ANC) ≥1×109/L, hemoglobin ≥10 g/dL, and platelet count ≥100×109/L; partial remission (PR) as counts as above and at least a 50% decrease in the percentage of marrow aspirate blasts to 5–25%, or marrow blasts < 5% with persistent Auer rods. CRp and CRi indicate CR with <5% BM myeloblasts, normal maturation of all cell lines and no dysplasia, but with incomplete platelets recovery or incomplete blood counts recovery, respectively. Morphologic leukemia-free state (MLFS) was defined as <5% BM blasts, irrespective of cytopenias. Secondary endpoints included duration of response (DOR) and overall survival (OS).
Statistical Considerations
Summary statistics were used to describe the continuous variables of the study population. Categorical endpoints were summarized using frequencies and percentages. Fisher’s exact and Wilcoxon rank tests were used in univariate analyses of categorical and continuous variables, respectively. The primary objective of this study was to assess the efficacy and safety of indisulam with chemotherapy in leukemia patients. The efficacy was measured by the ORR (CR+CRp+PR + marrow clearance of blasts). Patients were considered evaluable for efficacy if they received indisulam alone and achieved response or received at least one treatment with indisulam and chemotherapy. The efficacy was assessed based on the Simon’s two-stage Minmax design. In particular, 27 patients were to be enrolled at the first stage. If there were two or fewer patients with response, the trial was to be terminated; otherwise another 13 patients were to be treated for a total of 40 patients. If, among 40 patients, there are 6 or fewer patients with response, the treatment was to be concluded ineffective. A maximum sample size of 40 patients on the study was chosen to differentiate between response rates of 10% and 25% with 90% power at a significance level (p-value) of 0.1. We used one-sample exact binomial test with a two-sided 0.05 significance level to evaluate the trial’s observed response rate (RR) in salvage 1 and 2 against expected RR previously recorded in historical cohorts. The parameters were chosen based on our historical expectation of 30–40% CR rate in patients receiving first salvage16 and 13% CR among patients receiving second salvage therapy for AML.17. A Bayesian sequential monitoring method was used to monitor the toxicity and the trial was to stop if clinically significant non-hematological toxicity was greater than 33% at any point.
OS was defined as the time between the date of starting treatment and the date of death or last follow-up. Patients alive at their last follow-up date were censored for OS. OS was estimated using Kaplan-Meier method. Statistical analysis was performed using Stata/SE version 14.1 (Stata Corp. LP, College Station, TX).
Adverse events (AEs) were classified according to the common terminology criteria for adverse events (CTCAE) version 4.0 and captured from time of enrollment until 30 days after the last dose of therapy.
Role of funding source
This study was funded by Eisai pharmaceuticals, the MD Anderson Cancer Center Support Grant CA016672, and the MD Anderson Cancer Center Leukemia SPORE CA100632 from the National Cancer Institute. The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to study data and the final responsibility to submit for publication.
RESULTS
Between February 2013 and June 2014, 40 patients were enrolled: 39 with AML and one with HRMDS. Baseline characteristics are summarized in table 1. The median age was 63 years (range, 25–75) and median number of prior therapies were 2 (range, 1–6). Seventeen (43%) patients had diploid karyotype and 16 (40%) had poor cytogenetics. Twenty-eight (70%) and 23 (58%) patients received prior intermediate to high dose cytarabine-based regimens and hypomethylating agents, respectively, while 9 (23%) patients progressed following SCT.
Table 1. Baseline patients’ characteristics.
Data are expressed as n (%) or median [range]
| Characteristics | Population (N=40) |
|---|---|
| Age (years) | 63 [25–75] |
| Male | 21 (53) |
| Diagnosis | |
| AML | 39 (98) |
| Secondary AML | 12 (31) |
| HRMDS | 1 (2) |
| Prior regimens | 2 [1–6] |
| Int/High dose cytarabine-based | 28 (70) |
| HMA | 23 (58) |
| Clofarabine-based | 11 (28) |
| Salvage | |
| 1 | 14 (35) |
| 2 | 14 (35) |
| ≥3 | 12 (30) |
| Duration of first remission (when achieved) (months) | 11 [1–52] |
| Duration of remission on therapy received closest to clinical trial (months) | 4.3 [1–21] |
| Prior SCT | 9 (23) |
| WBC(x106 /L) | 1.8 [0.4–56] |
| Hemoglobin (g/dL) | 9.5 [7.2–14.7] |
| Platelets (x109/L) | 27 [2–231] |
| Bone marrow blasts (%) | 34 [3–95] |
| Peripheral blood blasts (%) | 11 [0–90] |
| Cytogenetics | |
| Diploid | 17 (43) |
| Complex | 16 (40) |
| −5/5q- and/or −7/7q- | 13 (33) |
| Miscellaneous | 7 (17) |
| IDH1/2 mutations | 7/37 (19) |
| RUNX1 mutation | 6/37 (16) |
| NRAS mutation | 5/39 (13) |
| TP53 mutation | 4/37 (11) |
| FLT3 mutation | 3/39 (8) |
| NPM1 mutation | 3/38 (8) |
| TET2 mutation | 2/37 (5) |
| ASXL1 mutation | 2/37 (5) |
AML: acute myeloid leukemia; HRMDS: high-risk myelodysplastic syndrome; Int: Intermediate; HMA: hypomethylating agents; SCT: stem cell transplantation; WBC: white blood cell count.
Response to therapy
The median duration of treatment was 7 weeks (range, 1–21 weeks) for all patients. Three patients (8%) were inevaluable for response; 1 was taken off study after 2 days as patient was found to have targetable FLT3 mutation, one because of chest pain and grade 1 increase in cardiac troponin level while receiving indisulam alone, and the third had rapid disease progression on day 2 of therapy.
Indisulam had no single agent activity. Of the 37 evaluable patients, 31 received indisulam along with idarubicin and cytarabine and 6 received indisulam alone without proceeding to receive the combination (within the first 20 patients who received indisulam alone in cycle 1 before protocol amendment); of the 31 patients, 8 (26%) achieved complete remission (CR) including 1 patient who had prior SCT while 2 (6%) achieved CR with incomplete counts recovery (CRi) and 1 (3%) CRp. Responders completed a median of 3 cycles of therapy (range, 1–4) with a median time to respond (TTR) of 8 weeks (range, 3–12) and median DOR of 5.3 months (range, 0.4–13). Six responders later proceeded to SCT. Nine of the eleven responders had prior exposure to cytarabine or clofarabine or both. The median number of regimens received by the responders prior to enrolling in this trial was 2 (range, 1–6). Comparison of characteristics showed that non-responders had lower median platelets count (25 vs 34×109/L; p=0.03), higher median BM blasts (37.5 vs 16%; p=0.009) and peripheral blasts percentages (23 vs 0; p=0.01) (Table 2) compared to responders. Additionally, none of the 11 responders had poor cytogenetics (0 vs 12%; p=0.006) when compared to non-responders.
Table 2:
Baseline characteristics of responding patients compared to non-responders.
| Characteristics | Responders (N=11) | Non-Responders (N=26) | P Value |
|---|---|---|---|
| Age (years) | 65 [50–75] | 63 [25–75] | 0.47 |
| Male | 6 (55) | 13 (50) | >0.99 |
| Secondary AML | 2 (18) | 7 (27) | 0.69 |
| AML status | 0.27 | ||
| Primary refractory | 2 (18) | 10 (38) | |
| Relapsed | 9 (82) | 16 (62) | |
| Prior regimens | 2 [1–3] | 2 [1–6] | 0.11 |
| Int/High dose cytarabine-based | 7 (64) | 19 (73) | 0.69 |
| HMA | 5 (45) | 16 (62) | 0.47 |
| Clofarabine-based | 3 (27) | 7 (27) | >0.99 |
| Prior SCT | 1 (9) | 8 (31) | 0.22 |
| WBC (x106 /L) | 1.5 [1–5.6] | 1.8 [0.4–7.7] | 0.76 |
| Hemoglobin (g/dL) | 9.7 [7.4–13.6] | 9.5 [7.7–14.6] | 0.85 |
| Platelets (x109/L) | 34 [6–231] | 25 [2–148] | 0.03 |
| Bone marrow blasts (%) | 16 [7–68] | 37.5 [3–95] | 0.009 |
| Peripheral blasts (%) | 0 [0–41] | 23 [0–84] | 0.01 |
| Cytogenetics | |||
| Diploid | 10 (91) | 8 (31) | 0.001 |
| Complex | 0 | 12 (46) | 0.006 |
| −5/5q- and/or −7/7q- | 0 | 12 (46) | 0.006 |
AML: acute myeloid leukemia; Int: Intermediate; HMA: hypomethylating agents; SCT: stem cell transplantation; WBC: white blood cell count.
Considering salvage status, 5 of the 13 (39%) evaluable patients in salvage 1 responded. Based on an expected response rate of 30–40%16, the observed RR was not significantly different in our trial (p-values of 0.69 and 1). For the 13 evaluable patients in salvage 2, 5 (39%) also responded and this observed RR was significantly higher (p=0.039) than the expected 13% RR seen with historical cohort receiving second salvage therapy at MDACC17.
Survival Endpoints
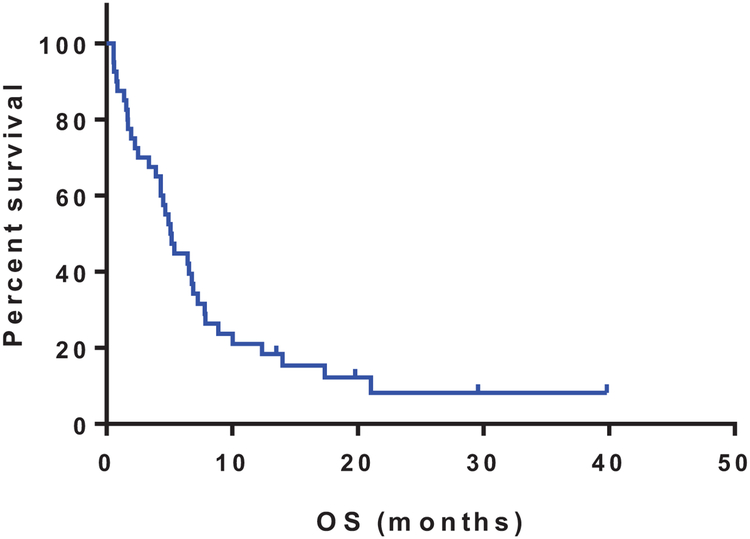
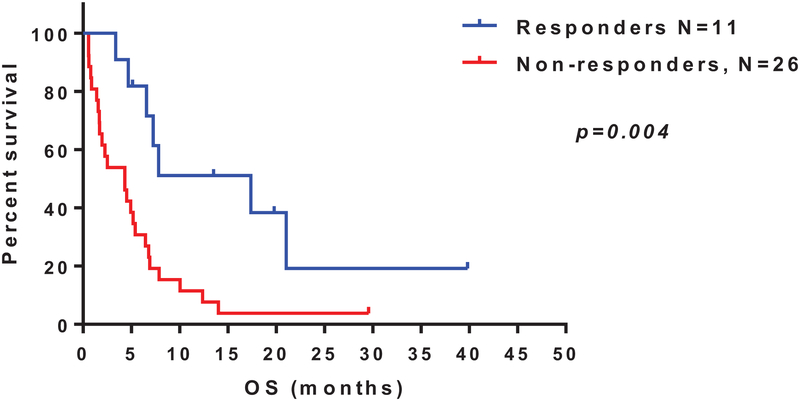
At a median follow-up of 6 months (range, 1–40), 5 patients remain alive. The median OS was 5.2 months (range, 0.6–40; 95% CI [4.96–10.21]) (Figure 1). The median OS for responders (n=11) was 17.4 months, compared to 4.3 months for non-responders (p=0.004; HR 0.32, 95% CI [0.16–0.65]) (Figure 2). Six of the responding patients were successfully bridged to SCT.
Figure 1:
Overall survival of patients treated on the trial, plotted using Kaplan-Meier method
Figure 2.
Overall survival of patients who achieved clinical response (complete response [CR] or partial response [PR]) compared to those who had no response; plotted using Kaplan-Meier method.
Toxicities
All 40 patients who received the study therapy were eligible for toxicity evaluation (Table 3). The most common grade 1/2 toxicities were electrolyte imbalances (n=30; 75%), nausea (n=26, 65%), transaminitis (n=25, 63%), and hyperbilirubinemia (n=21, 53%). These were generally transient and manageable with supportive measures. The most common grade 3/4 non-hematological toxicities irrespective of attribution were transient electrolyte abnormalities (n=20, 50%). Febrile neutropenia without a clear source of infection was encountered in 11 patients (28%). Pneumonia and soft tissue infections requiring hospital admission (grade ≥3) occurred in 7 (18%) patients, each and colitis and/or diarrhea (grade ≥3) in 4 (10%) patients. Four patients (10%) died within the first 4 weeks from progressive disease and associated complications.
Table 3.
Adverse events recorded on the trial regardless of attribution
| Adverse Event | Grade 1–2 | Grade 3–4 |
|---|---|---|
| Nausea | 26 (65) | 0 |
| Vomiting | 12 (30) | 0 |
| Diarrhea | 14 (35) | 2 (5) |
| Constipation | 16 (40) | 0 |
| Fatigue | 16 (40) | 0 |
| Anorexia | 11 (28) | 0 |
| Abdominal pain | 6 (15) | 2 (5) |
| Musculoskeletal pain | 12 (30) | 2 (5) |
| Chest pain | 4 (10) | 1 (3) |
| Electrolyte Imbalance | 30 (75) | 20 (50) |
| Hyperglycemia | 20 (50) | 9 (23) |
| Hypoglycemia | 3 (8) | 2 (5) |
| Hyperbilirubinemia | 21 (53) | 5 (13) |
| Transaminitis | 25 (63) | 1 (3) |
| Mucositis | 2 (5) | |
| Enterocolitis | 0 | 2 (5) |
| Acute kidney Injury | 13 (33) | 1 (3) |
| Hypertension | 2 (5) | 4 (10) |
| Atrial fibrillation | 3 (8) | 2 (5) |
| Capillary leak syndrome | 0 | 1 (3) |
| Edema | 17 (43) | 0 |
| Rash | 1 (3) | 1 (3) |
| Febrile neutropenia | 5 (13) | 11 (28) |
| Pneumonia | 1 (3) | 7 (18) |
| Skin/soft tissue infections | 5 (13) | 7 (18) |
DISCUSSION
In this phase 2 study, we evaluated the efficacy and safety of idarubicin and cytarabine with indisulam, a sulfonamide drug, in relapsed and refractory AML patients. In such a heavily pretreated population who have failed a median of 2 prior treatments (range, 1–6), including cytarabine (70%), HMA (58%) and SCT (23%), the combination in the current study led to a composite complete remission rate (CR+CRi+CRp) of 35%. Importantly, the median duration of responses was 5.3 months (range, 0.4–13) and that allowed 6 responders to be bridged to receive allogeneic SCT. In our cohort of patients, the median duration of their first CR was 11 months (range, 1–52) and the median duration of remission immediately prior to enrollment in this clinical trial was 4.3 months (range, 1–21). To put this response in perspective, the RR achieved in this trial appears to be mostly significant in patients receiving second salvage, as compared to a historical analysis of our institutional data, where13% RR was reported with a median OS of 1.5 months and median CR duration of 7 months.17 The cohort size precludes generalization of this observation.
For the combination of indisulam with chemotherapy, responses were observed in patients who have diploid cytogenetics and none of patients with adverse cytogenetics responded (p=0.001 and p=0.006, respectively). Responses seem to be clinically relevant as responders had a significantly longer survival (Figure 2).
Overall, the combination showed a clinically manageable safety profile. As cytopenia was dose limiting toxicity in phase 1 study in solid tumors,7, 18 there was a concern about extended hematological toxicities with its combination with chemotherapy. The time interval between cycles was between 4 to 5 weeks, reflecting the absence of profound myelosuppression and prolonged hematologic recovery, except in one patient who had delayed myelosuppression beyond 42 days, following the second course of treatment. With regard to non-hematologic toxicity, there was a high rate of electrolyte abnormalities and hypoglycemia, likely related to the intrinsic pharmacological activity of indisulam as sulfonamide diuretic and insulin releasing agent.19 Other non-hematological AEs included fatigue, nausea, hyperbilirubinemia and transaminitis. All these AEs were mostly of grade1/2, transient and manageable with supportive care. Mucositis was infrequently seen on this trial, with 4 grade 2/3 events, unlike in phase 1 trials7 and as expected from the cell cycle inhibitory action of indisulam and the combination with idarubicin and cytarabine. Grade 3 and 4 events were mostly reversible electrolyte abnormalities. Hand foot syndrome and stomatitis were reported in phase trial combining indisulam and capecitabine8 which was not encountered in our trial. The likely explanation of this better toxicity profile is that the doses of idarubicin and cytarabine administered in this study are lower than the usual 12 mg and 1.5 gram per meter squared, respectively, used as induction regimen at our institution. Additionally, indisulam was given at 400 mg/m2 on days 1 and 8 every 4 weeks to allow for the addition of chemotherapy with limited scope of cumulative adverse events.
Of note, 2 patients died within the first 30 days on therapy of disease progression, leading to an early mortality rate of 5% and indicating that the aforementioned AEs did not result in increased treatment-related mortality.
In recent years, several attempts at optimizing salvage regimens in AML patients have failed to improve upon remission rates, survival and overall outcomes, especially in heavily pretreated patients who received prior standard treatments including allogeneic SCT. Mutational studies identified new targets in AML, with these mostly representing driver mutations. Nevertheless, considering the complex clonal and molecular dynamics of this disease, there is increasing understanding that rational combinatorial approaches are needed to address the redundant survival pathways in leukemic cells. Cyclin-dependent kinases (CDKs) are crucial components of the cell cycle apparatus that regulate proper transition between different phases of this cycle.20 These enzymes are sequentially controlled by phosphorylation and activated by cyclins.20 Deregulated CDK function with subsequent overexpression of cyclins or low or absent levels of CDK inhibitors is a universal occurrence in human cancers that is facilitated through various genetic and epigenetic abnormalities.21 These events induce Rb phosphorylation and malignant cell cycle progression thereby granting neoplastic cells a selective proliferation advantage.21
Indisulam is a synthetic aryl sulfonamide that halts the progression of G1/S phase.22 When compared to other CDK inhibitors, indisulam does not competitively inhibit the ATP binding site of the CDK enzymes,19 thus making it an attractive agent to combine with cell cycle active agents and potentially with ATP competitive CDK inhibitors. The cell cycle events modulated by indisulam include depletion of cyclin E and transcriptional repression of cyclin H with subsequent reduced phosphorylation of the Rb protein, a CDK2 substrate during G1/S phase transition.6 Additionally, indisulam may up-regulate p53 and p21 leading to further decrease in Rb phosphorylation and enhanced apoptosis.23 The anticipated effects of indisulam on leukemic proliferation would therefore be both cytostatic and cytotoxic. In our study, however, we did not observe any single-agent indisulam activity with the dose and schedule used.
Cytosolic malate dehydrogenase (cMDH), a metabolic enzyme essential for gluconeogenesis and glycolysis, was identified as indisulam-specific binding protein.23, 24 However, cMDH-indisulam binding property has never been demonstrated as conditional for the antineoplastic activity of the drug. Very recent studies have revealed that indisulam stimulates the recruitment and bridging of the splicing factor RNA binding motif protein 39 (RBM39), also designated as CAPERα, to the CUL4-DCAF15 E3 ubiquitin ligase, leading to indisulam-induced RBM39 polyubiquitination and proteasomal degradation.25–27 This results in aberrant pre-mRNA splicing, along with transcriptional down-regulation of redox and energy metabolism genes as well as cell-cycle checkpoint genes. This process is somewhat analogous to the means by which immunomodulatory drugs (IMiDs) such as lenalidomide bridge cereblon to degrade IKZF1, IKZF3, and CK1.28 The same studies have found that the sensitivity of hematopoietic cancer cell lines to indisulam correlates with the level of DCAF15 (DDB1 CUL4 Associated Factor 15) expression, while mutations in RBM39 that prevent CUL4-DCAF15 recruitment increase RBM39 stability and confer resistance to indisulam.26, 27 Nevertheless, the process by which indisulam binds these proteins remains to be established. Given the occurrence of spliceosome mutations in myeloid malignancies, the sulfonamide class of anti-neoplastic agents may prove to be effective in subgroups of patients.26, 27
In conclusion, the combination of indisulam with idarubicin and cytarabine in patients with relapsed refractory AML is effective and largely well-tolerated. With the current knowledge of dependence of indisulam’s antineoplastic activity on DCAF15 and RBM39, we propose that the combination should be studied in a more homogeneous group of AML or high risk MDS patients and whose leukemic cells express mutant splicing factors e.g. U2AF1, SF3B1, SRSF2, and ZRSR2 or express high levels of DCAF15. Pre-clinical studies to establish rationale are underway.
Acknowledgements:
None
Funding source: Eisai pharmaceuticals, the MD Anderson Cancer Center Support Grant CA016672, and the MD Anderson Cancer Center Leukemia SPORE CA100632 from the National Cancer Institute.
Footnotes
Trial Registration ID: Clinicaltrials.gov identifier:
Conflicts of Interest: None
REFERENCES
- 1.Stein EM, Tallman MS. Emerging therapeutic drugs for AML. Blood. 2016;127: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter RB, Othus M, Burnett AK, et al. Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia. 2015;29: 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23: 1969–1978. [DOI] [PubMed] [Google Scholar]
- 4.Pemmaraju N, Kantarjian H, Garcia-Manero G, et al. Improving outcomes for patients with acute myeloid leukemia in first relapse: a single center experience. Am J Hematol. 2015;90: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owa T, Yoshino H, Okauchi T, et al. Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J Med Chem. 1999;42: 3789–3799. [DOI] [PubMed] [Google Scholar]
- 6.Ozawa Y, Sugi NH, Nagasu T, et al. E7070, a novel sulphonamide agent with potent antitumour activity in vitro and in vivo. Eur J Cancer. 2001;37: 2275–2282. [DOI] [PubMed] [Google Scholar]
- 7.Van Kesteren C, Mathôt RA, Raymond E, et al. Population pharmacokinetics of the novel anticancer agent E7070 during four phase I studies: model building and validation. J Clin Oncol. 2002;20: 4065–4073. [DOI] [PubMed] [Google Scholar]
- 8.Siegel-Lakhai WS, Zandvliet AS, Huitema AD, et al. A dose-escalation study of indisulam in combination with capecitabine (Xeloda) in patients with solid tumours. Br J Cancer. 2008;98: 1320–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozawa Y, Kusano K, Owa T, Yokoi A, Asada M, Yoshimatsu K. Therapeutic potential and molecular mechanism of a novel sulfonamide anticancer drug, indisulam (E7070) in combination with CPT-11 for cancer treatment. Cancer Chemother Pharmacol. 2012;69: 1353–1362. [DOI] [PubMed] [Google Scholar]
- 10.Mainwaring PN, Van Cutsem E, Van Laethem JL, et al. A multicentre randomised phase II study of E7070 in patients with colorectal cancer who have failed 5-fluorouracil-based chemotherapy. Proc Am Soc Clin Oncol. 2002; 21: 153a. [Google Scholar]
- 11.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127: 2391–2405. [DOI] [PubMed] [Google Scholar]
- 12.Rowe JM, Kim HT, Cassileth PA, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116: 5012–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120: 2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luthra R, Patel KP, Reddy NG, et al. Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica. 2014;99: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21: 4642–4649. [DOI] [PubMed] [Google Scholar]
- 16.Estey E, Kornblau S, Pierce S, Kantarjian H, Beran M, Keating M. A stratification system for evaluating and selecting therapies in patients with relapsed or primary refractory acute myelogenous leukemia. Blood. 1996;88: 756. [PubMed] [Google Scholar]
- 17.Giles F, O’Brien S, Cortes J, et al. Outcome of patients with acute myelogenous leukemia after second salvage therapy. Cancer. 2005;104: 547–554. [DOI] [PubMed] [Google Scholar]
- 18.Raymond E, ten Bokkel Huinink WW, Taïeb J, et al. Phase I and pharmacokinetic study of E7070, a novel chloroindolyl sulfonamide cell-cycle inhibitor, administered as a one-hour infusion every three weeks in patients with advanced cancer. J Clin Oncol. 2002;20: 3508–3521. [DOI] [PubMed] [Google Scholar]
- 19.Yokoi A, Kuromitsu J, Kawai T, et al. Profiling novel sulfonamide antitumor agents with cell-based phenotypic screens and array-based gene expression analysis. Mol Cancer Ther. 2002;1: 275–286. [PubMed] [Google Scholar]
- 20.Sherr CJ. Cancer cell cycles. Science. 1996;274: 1672–1677. [DOI] [PubMed] [Google Scholar]
- 21.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13: 1501–1512. [DOI] [PubMed] [Google Scholar]
- 22.Van Kesteren C, Beijnen JH, Schellens JH. E7070: a novel synthetic sulfonamide targeting the cell cycle progression for the treatment of cancer. Anticancer Drugs. 2002;13: 989–997. [DOI] [PubMed] [Google Scholar]
- 23.Fukuoka K, Usuda J, Iwamoto Y, et al. Mechanisms of action of the novel sulfonamide anticancer agent E7070 on cell cycle progression in human non-small cell lung cancer cells. Invest New Drugs. 2001;19: 219–227. [DOI] [PubMed] [Google Scholar]
- 24.Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg Med Chem Lett. 2004;14: 217–223. [DOI] [PubMed] [Google Scholar]
- 25.Bulatov E, Ciulli A. Targeting Cullin-RING E3 ubiquitin ligases for drug discovery: structure, assembly and small-molecule modulation. Biochem J. 2015;467: 365–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han T, Goralski M, Gaskill N, et al. Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science. 2017;356. [DOI] [PubMed] [Google Scholar]
- 27.Uehara T, Minoshima Y, Sagane K, et al. Selective degradation of splicing factor CAPERα by anticancer sulfonamides. Nat Chem Biol. 2017;13: 675–680. [DOI] [PubMed] [Google Scholar]
- 28.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]