Abstract
BACKGROUND
Immune-mediated destruction of hematopoietic stem cells is implicated in the pathophysiology of aplastic anemia (AA). Immunosuppressive therapy (IST) using antithymocyte globulin and cyclosporine is successful in this setting. Eltrombopag is active in refractory AA, presumably by increasing the marrow progenitors.
METHODS
This phase II trial was initially designed to evaluate standard IST in newly diagnosed severe AA and was later amended to add eltrombopag in order to simultaneously address immune destruction and stem cell depletion. The primary outcome was overall response rate (ORR) at 3 and 6 months.
RESULTS
Thirty-eight patients were enrolled; 17 (45%) received imunosuppressive therapy alone and 21 (55%) had additional eltrombopag. The ORR was 74%. Patients receiving IST+eltrombopag had similar ORR (76% vs 71%, p=0.72), complete remission rate (38% vs. 29%, p=0.73) and median time to response (84 vs. 57 days, p=0.30), compared to those receiving IST alone. The 2-year overall survival in IST group was 91% compared to 82% for IST+eltrombopag (p=0.82). No cumulative toxicities were noted after adding eltrombopag.
CONCLUSION
The addition of eltrombopag to standard IST was well-tolerated and resulted in similar responses.
Keywords: Aplastic anemia, horse ATG, cyclosporine, eltrombopag, stem cells
Short abstract
Immunosuppressive therapy using antithymocyte globulin and cyclosporine is successful in the treatment of severe aplastic anemia
The addition of eltrombopag to standard immunosuppressive therapy is well-tolerated but did not improve outcomes in our study.
BACKGROUND
Acquired aplastic anemia (AA) is a potentially fatal bone marrow (BM) failure syndrome characterized by paucity of hematopoietic stem (HSC) and progenitor cells with various degrees of cytopenias and fatty infiltration of the marrow space1. Underlying mechanisms include immune-mediated attack, telomeres defects,2 and inherent HSC compartment insufficiency. These events may occur individually or in concert, mostly involving effector T-cells3, 4.
Historical treatment included the use of high dose chemotherapy and allogeneic stem cell transplantation (SCT) as well as lymphotoxic immunosuppressive therapy (IST). The combination of horse anti-thymocyte globulin (hATG) with cyclosporine (CsA) produced 60–80% response rates and improved overall survival (OS) compared to either agent alone and to rabbit ATG5. Subsequently, the addition of CsA to steroids and hATG resulted in significantly higher overall response rate (ORR) at 3 months when compared to hATG with steroids alone (65% vs. 39%), without survival difference6, 7. These results may be hindered by early infectious mortality8. The upfront use of granulocyte colony-stimulating factors (GCSF) with IST decreased the early mortality rate to 8%, with 82% ORR and 43% complete remissions (CR)9.
Around 30% of patients remain refractory to conventional IST5,10. This refractoriness may illustrate incomplete ablation of auto-reactive T-cells or a profound HSC depletion in the presence of ongoing immune attack. Agents that engage HSC receptors and augment the pool of progenitors might therefore be particularly beneficial.
Thrombopoietin (TPO) regulates platelet production, maturation, and release through binding of c-mpl on megakaryocytes11. C-mpl is expressed on HSC and progenitor cells12 and the addition of recombinant TPO amplified primitive HSC in murine studies13. Eltrombopag (E-PAG) is an oral synthetic small-molecule non-competitive TPO agonist14 that was initially approved by the Food and Drug Administration (FDA) for treatment of chronic immune thrombocytopenic purpura.15 Single-agent activity of E-PAG was demonstrated in at least one lineage in 40–45% of AA patients refractory to IST16, leading to its FDA approval in this setting.
We herein report the results of a phase II trial evaluating the combination of hATG, CsA, steroids, and GCSF with and without E-PAG, in patients with newly diagnosed severe AA. E-PAG-based combination may increase response rates and expedite cell count recovery, while halting immune-mediated HSC depletion.
MATERIALS and METHODS
Study design
This is an open-label, prospective phase II trial conducted at MD Anderson Cancer Center (NCT01624805). It was originally designed to evaluate the combination of hATG, methylprednisolone, CsA and GCSF in severe AA. The first 17 patients received IST+GCSF alone. Following the encouraging results and approval of E-PAG in refractory AA, the protocol was amended to add E-PAG to IST+GCSF. The study was not sponsored and all patients were prescribed commercial E-PAG; therefore the availability and time of initiation relative to IST+GCSF depended on financial approval of the medication. The study was approved by the Institutional Review Board and conducted in concordance with the Declaration of Helsinki. All patients provided written, informed consent.
Eligibility criteria
Patients of all ages with newly diagnosed severe AA were eligible. Severe AA was defined by BM cellularity of <30% and at least two of the following: absolute neutrophil count (ANC) <0–5×109/l, pre-transfusion platelet count <20×109/l, or pre-transfusion reticulocyte count <20×109/l. Patients were treatment-naive or previously treated with growth factors (excluding E-PAG). Other inclusion criteria comprised adequate hepatic and renal functions, and ECOG performance status of ≤2. Patients with any uncontrolled illness and those with documented hypersensitivity to any of the component medications were excluded.
Treatment plan
Prior to hATG, all patients received a subcutaneous test-dose for potential hypersensitivity. The following combination was given: hATG 40 mg/kg/day intravenous (IV) for 4 days; methylprednisone 1mg/kg/day IV daily for 4 days (prior to each dose of ATG) followed by oral (PO) prednisone tapered-off over 30 days; subcutaneous G-CSF starting day 5 as needed to keep the ANC ≥1.5, and CsA (5 mg/kg) PO daily starting day 6 to maintain serum concentrations around 200 mg/dL. CsA was continued for at least 6 months unless frequent dose reductions or interruptions were needed for major toxicity. E-PAG was introduced at 50 mg PO daily with escalation by 25–50 mg every 2 weeks to reach a maximum of 150 mg daily in the absence of platelet response. If platelet counts reached 120×109/L or toxicity was considered E-PAG-related, the drug was held until the count dropped to <100×109/L or there was resolution of the event. Patients were restarted at 50 mg daily and escalated as noted. For responders not meeting above criteria, E-PAG was continued indefinitely or discontinued at the discretion of treating physician. Enrolled patients received prophylactic antimicrobials and were transfused with blood products per institutional policy.
Clinical evaluation
Pretreatment evaluation included a complete history and physical examination, complete blood count with differential (CBCD) and serum chemistry; BM aspiration and biopsy within 1 month of enrollment; quantification of CD4/CD8 T-cell subsets; serum immunoglobulins level; polymerase chain reaction (PCR) testing of the BM for T-cell beta and gamma receptor (TCR) rearrangements; and peripheral blood flow cytometry (FC) for PNH antigens (CD55, CD59). Cytogenetic and mutational analyses were performed as previously described (supplementary table 1)17,18.
Patients were reassessed with CBCD and chemistry once or twice weekly during the first month and then every 2–4 weeks for up to 6 months. Serum CsA level was measured every 2–4 weeks while patients were on the drug. A repeat BM biopsy and/or aspiration for morphology and cytogenetics was performed at 3 and 6 months.
Response criteria
The primary objective of the study was to evaluate the efficacy of the combination of hATG/methylprednisolone/CsA/G-CSF, including complete response (CR) and partial response (PR). Secondary objectives were tolerability and toxicities of the combination, time to achieve a response, duration of response, and OS. CR was defined as BM sample showing <5% myeloblasts with normal maturation of all cell lines and no dysplasia; a peripheral blood ANC ≥1×109/L, hemoglobin ≥10 g/dL, and platelet count ≥100×109/L. PR was defined as transfusion independence with ANC ≥0.5×109/L, hemoglobin ≥8 g/dL, and platelets ≥20×109/L.
Responses were assessed at 3 months following initiation of therapy and monthly thereafter to document best response. The duration of response (DOR) was measured from the time of achieving CR or PR until disease recurrence or progression. OS was measured from the time of study enrollment until death from any cause or date of last follow-up.
Statistical analysis
Based on historical data, the anticipated response rate was 65%. OS and DOR were analyzed by Kaplan-Meier method. The time to response (TTR) was defined as the interval between treatment start and date of response. Log-rank and chi-square tests were applied to calculate the differences between risk groups with a level of significance of p<0.05. The Bayesian sequential method of Thall, Simon and Estey was used to perform safety monitoring.19 Adverse events (AEs) grading and reporting utilized the CTEP Version 4.0 of the NCI Common Terminology Criteria for Adverse Events. Independent prognostic factors for response were assessed by using logistic regression models and Cox proportional hazard models.
The effect of response on survival and disease progression was analyzed by modeling response as a time-varying covariate in the Cox model.
RESULTS
Patient characteristics
Thirty-eight patients were enrolled; the first 17 patients received IST+GCSF alone and the next 21 received IST+GCSF+E-PAG. In the cohort receiving IST+GCSF+E-PAG, 13/21 (62%) patients started E-PAG within the first week (because of insurance coverage issues), with a median time to initiation of 5 days (range, 0–70). Patients treated with IST+GCSF+E-PAG had lower pretreatment ANC (median 0·34 vs. 0·73×109/L; p=0.002) and reticulocyte count (median 0·9 vs. 1.5×109/L; p=0.028). FC for PNH was performed in 36 (95%) patients and clones were numerically more prevalent in the IST+GCSF+E-PAG group compared to IST+GCSF (76% vs 67%; p=0.7). TCR gene rearrangement was detected in 10 patients (59%) treated with IST+GCSF and 7/19 (37%) patients on IST+GCSF+E-PAG (p=0.31). Median baseline BM cellularity was similar in both groups (Table 1).
TABLE 1.
Baseline Patient Characteristics by Treatment Cohorta
| Characteristics | Immunosuppressive Therapy N=17 | Immunosuppressive Therapy Plus Eltrombopag N=21 | P |
|---|---|---|---|
| Male sex | 8 (47) | 11 (52) | 1.00 |
| Age, y | 53 (24–80) | 60 (19–84) | .73 |
| White blood cell count, ×109/L | 2.2 (0.6–6.4) | 2.1 (0.2–4.8) | .62 |
| Platelet count, ×109/L | 30 (2–131) | 18 (5–64) | .20 |
| Hemoglobin, g/dL | 9.3 (7.5–10.8) | 8.8 (7.1–10.6) | .17 |
| Absolute neutrophil count, ×109/L | 0.73 (0–5.2) | 0.34 (0–3.5) | .002 |
| Reticulocyte count, % | 1.5 (0.3–7.1) | 0.9 (0.3–2.6) | .028 |
| Baseline percentage bone marrow cellularity | 5 (1–40) | 5 (1–40) | .97 |
| Cytogenetics | 0.37 | ||
| Diploid | 13 (76) | 19 (90) | |
| Insufficient metaphases | 3 (18) | 2 (10) | |
| Paroxysmal nocturnal hemoglobinuria clone | 10/15 (67) | 16 (76) | .70 |
| T-cell receptor clonality | 10 (59) | 7/19 (37) | .31 |
Data are expressed as the number (%) or median (range).
Efficacy
All 38 patients were evaluable for response (Table 2). The median follow-up was 21 months (range, 3–49). Among the 17 patients treated with IST+GCSF alone, the ORR was 71% with 5 (29%) CR and 7 (42%) PR. Among the 21 patients treated with IST+GCSF+E-PAG, 16 responded (76%) with 8 CR (38%) and 8 PR (38%). The difference in response rate between the two cohorts was not statistically significant, neither for ORR (p=0.72) nor CR (p=0.73); patients receiving IST+GCSF+E-PAG had a non-statistically significant longer median TTR (84 vs. 57 days; p=0.30).
TABLE 2.
Summary of Patient Responses According to Treatment Cohort
| Immunosuppressive Therapy N=17 | Immunosuppressive therapy Plus Eltrombopag N=21 | P | |
|---|---|---|---|
| Overall response rate (CR+PR), no. (%) | 12 (71) | 16 (76) | .72 |
| CR, no. (%) | 5 (29) | 8 (38) | .71 |
| Time to response (range), d | 57 (10–184) | 84 (21–184) | .30 |
| Percentage of bone marrow cellularity | 5 (1–40) | 5 (1–40) | .89 |
Abbreviations: CR, complete response; PR, partial response.
Non-responders had lower median ANC (0.1 vs 0.5×109/L; p=0.05) and were less likely than responders to have monoclonal TCR (67% vs 100%; p=0.02) (supplementaryTable 2). Among non-responders, males were slightly underrepresented (40%), although the difference in gender between both groups was statistically insignificant (54% vs 40%; p=0.71). Of the 30 patients whose samples were evaluated for genes mutations, 17 (57%) had detectable somatic/variant mutations with 11/17 (65%) having ≥1 mutation. The median number of mutations per patient was 1 (range, 1–6), and included ASXL1 (n=6), IDH1 and NOTCH1 (n=5, each) and KIT (n=4).
Survival Endpoints
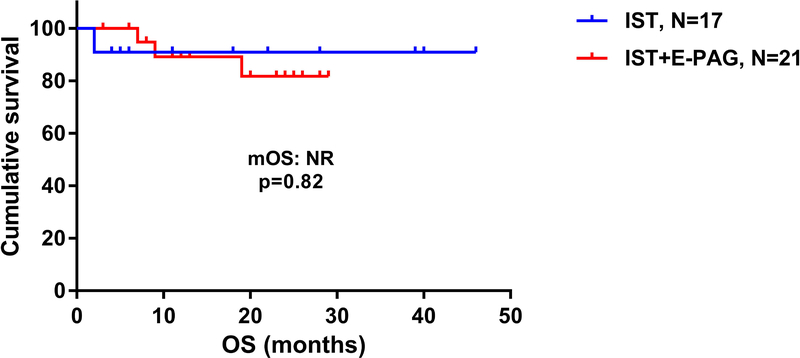
The median OS was not reached in both cohorts (p=0.82; HR 0.78, 95% CI [0.09–6.69]) (Figure 1). The estimated 2-year OS was 91% in IST+GCSF cohort vs 82% in IST+GCSF+E-PAG. In each cohort, achieving a clinical response (CR or PR) predicted for improved survival at 2 years (p=0.002).
Figure 1:
Overall survival of patients with aplastic anemia treated with immunosuppressive therapy (IST) alone, compared to those treated with IST with eltrombopag (IST+E-PAG). OS: overall survival; mOS: median overall survival; NR: not reached
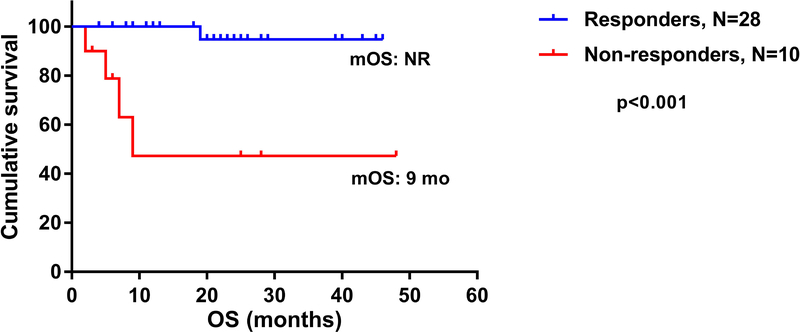
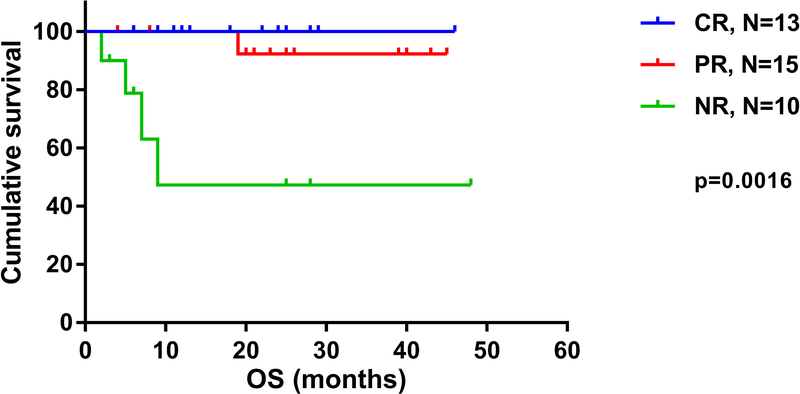
The median OS for responders was not reached, compared to 9 months for non-responders (p<0.001 HR 0.05, 95% CI [0.001–0.15]) (Figure 2). The estimated 3-year OS was 47% for non-responders compared to 95% for responders (p<0.001). For patients who achieved CR, the estimated 4-year OS was 100%, compared to 92% in patients with PR and 47% in those with NR (p=0.0016) (Figure 3). Although higher baseline ANC and TCR monoclonality were associated with response (p=0.05 vs p=0.02, respectively), none of these factors and others including the presence of PNH clone, reticulocyte count and cytogenetics predicted for improved response on multivariate analysis. When analyzing factors predicting for OS, including segregation by treatment cohort (IST+GCSF vs IST+GCSF+E-PAG), PNH clone, cytogenetics, baseline WBC, reticulocyte counts and clinical response, only achieving CR or PR predicted for improved survival on multivariate analysis (p=0.02).
Figure 2:
Overall survival of patients with aplastic anemia who have achieved clinical response in general in both cohorts (complete response [CR] or partial response [PR]) compared to those who had no response. OS: overall survival; mOS: median overall survival; NR: not reached
Figure 3:
Overall survival of patients with aplastic anemia per response in both cohorts.. CR: complete response; PR: partial response; NR: no response; OS: overall survival; mOS: median overall survival; NR: not reached.
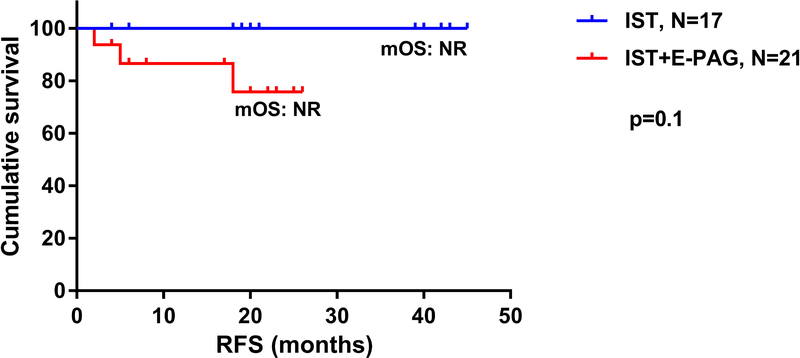
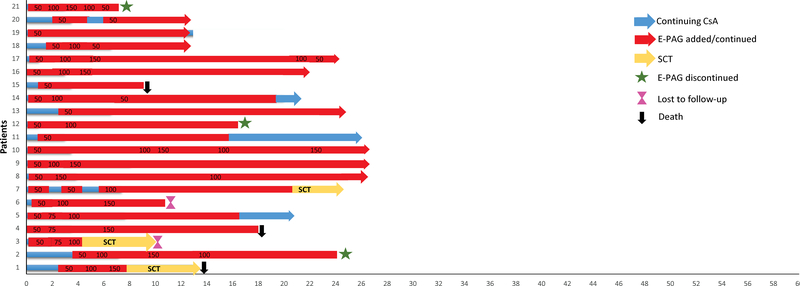
The median relapse-free survival was not reached in both groups (Figure 4) and remained statistically insignificant (p=0.10; 95% CI [0.015–1.432]). All responders from IST+GCSF arm maintained their responses at 2 years, compared to 76% in IST+GCSF+E-PAG group. Among the 28 responders in both cohorts, a total of 16 (57%) have continued some form of maintenance treatment: 10 (36%) are receiving CsA+E-PAG and 6 (21%) are on CsA alone, with stable counts at a median follow-up of 15 months (range 1–33). Among non-responders (n=10), counts are maintained on CsA in 2 patients (20%) and CsA + E-PAG in another 2 (20%). Indeed, 16 of the 28 (57%) responders from both cohorts were kept on either CsA alone (21%) or CsA+E-PAG (36%), and had their counts maintained with no relapses (Figure 5).
Figure 4:
Relapse-free survival of patients with aplastic anemia treated with immunosuppressive therapy (IST) alone, compared to those treated with IST with eltrombopag (IST+E-PAG); OS: overall survival; mOS: median overall survival; NR: not reached.
Figure 5:
This figure summarizes the dose distribution of eltrombopag (E-PAG). The X-axis represents time in months. Time 0 represents the initiation of immunosuppressive therapy. The numbers in red bars represent the dose of E-PAG in milligrams. CsA: cyclosporine A; SCT : stem cell transplantation.
Five patients underwent SCT for refractoriness (1 from IST+GCSF group and 4 from IST+GCSF+E-PAG group).
At a median follow-up of 21 months (range, 3–49), cytogenetic abnormalities were detected in 5 patients (2 treated with IST+GCSF and 3 with IST+GCSF+E-PAG): 3 involving partial deletion of chromosome 7, 1 deletion of chromosome Y and 1 addition of chromosome Y.
Safety and tolerability
All patients were eligible for toxicity evaluation (Table 3). Overall, both treatment arms had acceptable toxicities. Seven patients from both cohorts (18%) experienced infusion reactions despite extensive premedication. The reactions were mostly of low-grade (except one grade 3 event in IST+GCSF cohort for which the patient has been taken off-study) with no events of anaphylaxis; these reactions included chills, dyspnea, fever, and rash, and were managed with increased doses of steroids, antihistamines and meperidine as needed.
TABLE 3.
Most Common Adverse Events Irrespective Of Attribution
| Immunosuppressive Therapy N=17 | Immunosuppressive Therapy Plus E-PAG N=21 | |||||
|---|---|---|---|---|---|---|
| Adverse Event | All Grades No. (%) | Grade 1/2 No. (%) | Grade 3/4 No. (%) | All Grades No. (%) | Grade 1/2 No. (%) | Grade 3/4 No. (%) |
| Fatigue | 4 (24) | 2 (12) | 2 (12) | 3 (14) | 3 (14) | 0 |
| Infusion reaction | 6 (35) | 5 (29) | 1 (6) | 1 (5) | 1 (5) | 0 |
| Headache | 2 (12) | 2 (12) | 0 | 1 (5) | 0 | 1 (5) |
| Nausea | 1 (6) | 1 (6) | 0 | 3 (14) | 3 (14) | 0 |
| Muscle cramps | 2 (12) | 1 (6) | 1 (6) | 1 (5) | 1 (5) | 0 |
| Abdominal bloating/pain | 4 (24) | 3 (18) | 1 (6) | 1 (5) | 1 (5) | 0 |
| Peripheral neuropathy | 1 (6) | 1 (6) | 0 | 2 (10) | 1 (5) | 1 (5) |
| Tremor | 1 (6) | 1 (6) | 0 | 1 (5) | 1 (5) | 0 |
| Gingival hyperplasia | 1 (6) | 1 (6) | 0 | 1 (5) | 1 (5) | 0 |
| Facial hair growth | 1 (6) | 1 (6) | 0 | 0 | 0 | 0 |
| Rash | 5 (29) | 4 (24) | 0 | 0 | 0 | 0 |
| Joint pain | 1 (6) | 1 (6) | 0 | 3 (14) | 2 (10) | 1 (5) |
| Chest pain | 1 (6) | 0 | 1 (6) | 2 (10) | 1 (5) | 1 (5) |
| Serum sickness | 1 (6) | 0 | 1 (6) | 0 | 0 | 0 |
| Fever | 3 (18) | 3 (18) | 0 | 1 (5) | 0 | 0 |
| Febrile neutropenia | 2 (12) | 0 | 2 (12) | 1 (5) | 0 | 1 (5) |
| Infections, other | 2 (12) | 0 | 2 (12) | 0 | 0 | 0 |
| Renal failure | 2 (12) | 2 (12) | 0 | 0 | 0 | 0 |
| Hypertension | 5 (29) | 5 (29) | 0 | 0 | 0 | 0 |
| Hepatotoxicity | 2 (12) | 0 | 2 (12) | 0 | 0 | 0 |
| Visual problems | 1 (6) | 1 (6) | 0 | 1 (5) | 1 (5) | 0 |
Abbreviation: E-PAG, eltrombopag.
In both cohorts, infectious complications requiring hospital admission (grade ≥3) were infrequent, occurring in 5/38 (13%) patients. These included one case of urinary tract infection and one case of CMV viremia (both in IST+GCSF cohort), as well as 2 cases of febrile neutropenia of undetermined source.
The most common non-hematological AEs included hypertension (n=5 (29%) in IST+GCSF cohort), rash (11%; n=4 in IST+GCSF cohort), nausea (11%), and non-specific joint pain (11%; n=4). Seven (18%) patients reported fatigue including 2 grade 3–4 cases in IST+GCSF group. Two patients from IST+GCSF cohort had grade 3–4 transaminitis and hyperbilirubinemia that were reversible upon drug adjustment. Other grade 3 events included subarachnoid hemorrhage (n=1) and serum sickness (n=1), both in IST+GCSF cohort. A 70 year-old patient treated with IST+GCSF died of bacterial sepsis on day 63. The 100-day mortality on the study is therefore 3%.
DISCUSSION
In severe AA patients, responses to conventional IST have been stagnant around 60% for three decades.20 Our study was initially designed to evaluate the combination of IST+GCSF. Following the encouraging results of E-PAG in refractory AA, we allowed the addition E-PAG to IST+GCSF and study its tolerability and efficacy.
The primary endpoint was ORR as historical data correlated CR with improved survival and reduced clonal evolution (CE). The 71% ORR obtained in IST+GCSF arm matches that of historical reports, while the 29% CR rate observed here is higher than the 10% historical rate.21 When E-PAG was concurrently added to IST+GCSF, the ORR increased from 71 to 76% and CR from 29% to 38%, albeit statistically insignificant and with longer median TTR. No specific baseline or laboratory marker predicted for better response.
While patients in both groups were treated sequentially and not in a comparative randomized fashion, we still expected better responses with the addition of E-PAG to IST+GCSF, but instead observed similar outcomes. This contrasts with the phase II trial by Townsley et al22 which enrolled 88 treatment-naive severe AA patients to receive standard IST+E-PAG. In their study, E-PAG was administered at an upfront dose of 150 mg daily to three cohorts: starting from day 14 until 6 months (cohort 1) or 3 months (cohort 2), or concurrently with hATG on day 1 and up to 6 months (cohort 3). The highest ORR and CR rates were recorded among those who received concurrent IST+E-PAG (92% and 54% at 6 months, respectively).
When comparing response rates from the current study to those in Townsley’s study,22 we delineate some important differences. Patients treated on our study were significantly older, with a median age of 60 years, compared to 32 years. Age is an important predictor for response to IST23 and likely had a major contribution to differences in overall outcomes. Second, selection bias, non-congruent baseline covariates, and differences in supportive care measures might represent confounding factors when current results are to be compared to historical controls and across studies. For instance, all patients enrolled on the current study received GCSF to shorten the duration of neutropenia, and presumably mobilize hematopoietic progenitors to peripheral blood.9 While earlier studies using GCSF in combination with IST9 have reported higher ORR (82%) compared to those without GCSF (65%)6, other reports failed to show any significantly better outcomes with growth factors24. The latter finding has been attributed, in part, to possible absence of GCSF receptors on the surface of HSC and progenitor cells24. E-PAG does not compete with TPO for c-MPL binding and may therefore activate hematopoiesis independently, through JAK-STAT and MAPK pathways25. The lack of significantly better ORR when E-PAG was used may be explained by the slower intrinsic impact on stem and progenitor cells (as reflected by longer TTR even in those who responded), compared to faster effect on maturing megakaryocytes affected in ITP. This could also reflect the possibility of profound and variably reversible HSC compartment depletion with heterogeneous repopulation capacities, even in the absence of an ongoing immune attack. Hence, using higher earlier doses of E-PAG (as in Townsley’s study or even above 150 mg) may be necessary to generate a critical mass of HSCs capable of sustaining hematopoiesis. In our study, only 13 (62%) of 21 patients were able to start E-PAG within the first week of IST (median 5 days, range 0–70) because of logistical delays (Figure 4) and the median dose per patient was 75 mg (range, 50–150) during the 6- months study period. The cohort with the best response in the NIH study started a high dose (150 mg) on the first day of IST. The lack of uniformity in time of E-PAG initiation as well as the gradual dose-escalation scheme applied from the previous study in refractory AA26, coupled with a lower dose-intensity may have contributed to the lack of improved responses with the addition of E-PAG. Finally, we have not assessed the impact of telomeres length on responses in both groups and therefore the effect of this variable cannot be excluded.
Most IST protocols have advised a 6-month course of CsA. Using this approach, hematologic relapses were documented in 30–40% of patients over 5 years following ATG therapy8. In our study, we chronically continuied lower doses of CsA following a slow taper from the initial protocol27. When feasible, we also continued E-PAG in an attempt to delay or prevent relapses. Indeed, 16 (57%) of the 28 responders from both cohorts were kept on CsA alone (21%) or CsA+E-PAG (36%) with no relapses. Longer follow-ups are however needed especially in patients continuing CsA+E-PAG.
At a median follow-up of 21 months (range, 3–49), 5 patients acquired cytogenetic abnormalities (2 in IST+GCSF and 3 with IST+GCSF+E-PAG): 3 involving partial deletion of chromosome 7 (2 in IST+GCSF cohort with one evolving to AML), 1 deletion of chromosome Y and 1 addition of chromosome Y. The estimated CE rate in the study is therefore 13% and 14% in patients receiving E-PAG. The risk of CE is variable, ranging from 1.7%−57% over 5–11 years follow-up6, 28 with a common scenario of evolution to MDS with isolated monosomy 728. Trisomy 8, del 13q, and del Y are far less common abnormalities28.
Both CE and acquisition of PNH clones may reflect mechanisms of immunologic escape8, 29. CE is of more theoretical concern in patients treated with E-PAG as 8 (17%) of the 43 refractory AA patients studied by Olnes et al acquired cytogenetic lesions with dysplastic changes over a period of few months26. The follow-up on our study remains relatively short (3–49 months) especially in patients continuing E-PAG beyond 6 months, and longer surveillance is being pursued.
Overall, both combinations were safe, with expected and manageable AEs with no anaphylactic reactions or infusion-related deaths on this study. A generally low rate of serious infections was also noticed, mostly including neutropenic fevers (overall 8%; grade 3 events in 3 patients) with100-day mortality of 3%. When compared to previous studies of hATG+CsA 6, 9, 21, 30, this reduction in early infection-related mortality is clearly favorable and may be at least partly explained by the routine use of GCSF and prophylactic antimicrobials at our institution.
In conclusion, our study may reflect a real-life experience of patients with severe AA where commercial E-PAG supply may be limited by insurance authorization, pending regulatory approval of the medication in the frontline setting. The combination of IST+GCSF+E-PAG resulted in similar response rates compared to IST+GCSF, and no cumulative toxicities were seen when E-PAG was added as adjunct therapy. Maximizing dose-intensity and earlier initiation of E-PAG may be important factors associated with higher responses. Longer follow-up is also needed to determine the effect of E-PAG, if any, on OS in patients with AA.
Supplementary Material
Acknowledgments
Financial Support: This research is supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2013;2013: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheinberg P, Cooper JN, Sloand EM, Wu CO, Calado RT, Young NS. Association of telomere length of peripheral blood leukocytes with hematopoietic relapse, malignant transformation, and survival in severe aplastic anemia. JAMA. 2010;304: 1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young NS, Maciejewski J. The pathophysiology of acquired aplastic anemia. N Engl J Med. 1997;336: 1365–1372. [DOI] [PubMed] [Google Scholar]
- 4.Risitano AM, Maciejewski JP, Green S, Plasilova M, Zeng W, Young NS. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta- CDR3 sequencing. Lancet. 2004;364: 355–364. [DOI] [PubMed] [Google Scholar]
- 5.Scheinberg P, Nunez O, Weinstein B, Biancotto A, Wu CO, Young NS. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365: 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. The German Aplastic Anemia Study Group. N Engl J Med. 1991;324: 1297–1304. [DOI] [PubMed] [Google Scholar]
- 7.Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H, Group GAAS. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003;101: 1236–1242. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289: 1130–1135. [DOI] [PubMed] [Google Scholar]
- 9.Bacigalupo A, Broccia G, Corda G, et al. Antilymphocyte globulin, cyclosporin, and granulocyte colony-stimulating factor in patients with acquired severe aplastic anemia (SAA): a pilot study of the EBMT SAA Working Party. Blood. 1995;85: 1348–1353. [PubMed] [Google Scholar]
- 10.Schrezenmeier H, Marin P, Raghavachar A, et al. Relapse of aplastic anaemia after immunosuppressive treatment: a report from the European Bone Marrow Transplantation Group SAA Working Party. Br J Haematol. 1993;85: 371–377. [DOI] [PubMed] [Google Scholar]
- 11.Kaushansky K Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111: 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeigler FC, de Sauvage F, Widmer HR, et al. In vitro megakaryocytopoietic and thrombopoietic activity of c-mpl ligand (TPO) on purified murine hematopoietic stem cells. Blood. 1994;84: 4045–4052. [PubMed] [Google Scholar]
- 13.Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med. 2009;60: 193–206. [DOI] [PubMed] [Google Scholar]
- 14.Sun H, Tsai Y, Nowak I, Liesveld J, Chen Y. Eltrombopag, a thrombopoietin receptor agonist, enhances human umbilical cord blood hematopoietic stem/primitive progenitor cell expansion and promotes multi-lineage hematopoiesis. Stem Cell Res. 2012;9: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357: 2237–2247. [DOI] [PubMed] [Google Scholar]
- 16.Desmond R, Townsley DM, Dumitriu B, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123: 1818–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury JD, Sen F, Abruzzo LV, Hayes K, Glassman A, Medeiros LJ. Cytogenetic findings in blastoid mantle cell lymphoma. Hum Pathol. 2003;34: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Singh RR, Patel KP, et al. BRAF kinase domain mutations are present in a subset of chronic myelomonocytic leukemia with wild-type RAS. Am J Hematol. 2014;89: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med. 1995;14: 357–379. [DOI] [PubMed] [Google Scholar]
- 20.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120: 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y, Liu Y, Chu Y. Immunosuppressive therapy for acquired severe aplastic anemia (SAA): a prospective comparison of four different regimens. Exp Hematol. 2006;34: 826–831. [DOI] [PubMed] [Google Scholar]
- 22.Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med. 2017;376: 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tichelli A, Marsh JC. Treatment of aplastic anaemia in elderly patients aged >60 years. Bone Marrow Transplant. 2013;48: 180–182. [DOI] [PubMed] [Google Scholar]
- 24.Gurion R, Gafter-Gvili A, Paul M, et al. Hematopoietic growth factors in aplastic anemia patients treated with immunosuppressive therapy-systematic review and meta-analysis. Haematologica. 2009;94: 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erickson-Miller CL, Delorme E, Tian SS, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheinberg P, Rios O, Weinstein B, Wu CO, Young NS. Prolonged cyclosporine administration after antithymocyte globulin delays but does not prevent relapse in severe aplastic anemia. Am J Hematol. 2014;89: 571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Li X, Ge M, et al. Long-term follow-up of clonal evolutions in 802 aplastic anemia patients: a single-center experience. Ann Hematol. 2011;90: 529–537. [DOI] [PubMed] [Google Scholar]
- 29.Maciejewski JP, Risitano A, Sloand EM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99: 3129–3135. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995;85: 3058–3065. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.