Abstract
Purpose:
We assessed the feasibility and cancer detection rate of fluciclovine (18F) positron emission tomography-ultrasound fusion targeted biopsy vs standard template biopsy in the same patient with biochemical failure after nonsurgical therapy for prostate cancer.
Materials and Methods:
A total of 21 patients with a mean ± SD prostate specific antigen of 7.4 ± 6.8 ng/ml and biochemical failure after nonoperative prostate cancer treatment underwent fluciclovine (18F) positron emission tomography- computerized tomography (mean 364.1 ± 37.7 MBq) and planning transrectal prostate ultrasound with 3-dimensional image reconstruction. Focal prostatic activity on positron emission tomography was delineated and co-registered with planning ultrasound. During the subsequent biopsy session computer generated 12-core template biopsies were performed and then fluciclovine defined targets were revealed and biopsied. Histological analysis of template and targeted cores were completed.
Results:
Template biopsy was positive for malignancy in 6 of 21 patients (28.6%), including 10 of 124 regions and 11 of 246 cores, vs targeted biopsy in 10 of 21 (47.6%), including 17 of 50 regions and 40 of 125 cores. Five of 21 patients had positive findings on targeted biopsy only and 1 of 21 had positive findings on template biopsy only. An additional case was upgraded from Grade Group 2 to 3 on targeted biopsy. Extraprostatic disease was detected in 8 of 21 men (38.1%) with histological confirmation in all 3 who underwent lesion biopsy.
Conclusions:
Fluciclovine positron emission tomography real-time ultrasound fusion guidance for biopsy is feasible in patients with biochemical failure after nonsurgical therapy for prostate cancer. It identifies more recurrent prostate cancer using fewer cores compared with template biopsy in the same patient. Further study is required to determine in what manner targeted biopsy may augment template biopsy of recurrent prostate cancer.
Keywords: prostatic neoplasms, diagnostic imaging, image-guided biopsy, positron emission, tomography, ultrasonography
BIOCHEMICAL failure develops after definitive therapy of localized prostate cancer in approximately 20% to 60% of patients after external beam radiotherapy.1 TRUS guided prostate biopsy is the standard method of detecting recurrent prostate cancer. Yet TRUS biopsy is limited by sampling error, especially in recurrent disease since prostate distortion may occur. The reported detection rate ranges from 29% to 95% depending on study design and patient population, although in practice the detection rate is at the lower end of this range.2–5 Thus, a patient may have a negative biopsy despite harboring occult recurrent cancer. Alternatively cancer may be diagnosed but the case is under staged because the most aggressive histology has not been sampled.
Advanced techniques such as MRI have been proposed to increase the detection rate.2,6 However, to detect recurrent local disease in patients without prostatectomy MRI may be suboptimal due to distorted anatomy after therapy, therapy related sequelae and potential artifact resulting from metallic brachytherapy seed implants or fiduciary radiotherapy markers.7–10
PET with molecular imaging radiotracers, such as those based on choline, PSMA or (18F)-fluciclovine, have shown promising results to detect and localize prostate cancer.3,11,12 In patients with biochemical failure after prior therapy PET guided biopsy has an important treatment role with the added value of accurate whole body staging compared to conventional imaging.
We previously reported a novel technique of multimodality fusion of PET and ultrasound to guide targeted biopsy in real time.13,14 We set out to determine the feasibility of this technique using fluciclovine PET guided ultrasound fusion biopsy and to compare the cancer detection rate vs that of standard 12-core template biopsy using the patient as his own control.
MATERIALS AND METHODS
Patient Selection
Following Institutional Review Board approval and informed consent 21 consecutive patients with suspected recurrent prostate cancer who had earlier undergone nonprostatectomy definitive therapy were recruited in a prospective clinical trial between November 2015 and April 2017 (IRB No. IRB00080287). Inclusion criteria included suspicion of recurrent prostate cancer based on the ASTRO-RTOG (American Society for Radiation Oncology- Radiation Therapy Oncology Group) Phoenix criteria of elevated PSA greater than the nadir plus 2.0 ng/ml or the older ASTRO criteria of 3 consecutive PSA rises or earlier if clinically appropriate according to clinician discretion, more than 1 year since cryotherapy, external beam radiation or high intensity focused ultrasound, or 2 years for brachytherapy and more than 1 month since prior prostate biopsy to decrease false-positive rate due to inflammation. Patients younger than 18 years, those who could not provide written informed consent and those not otherwise eligible for prostate biopsy were excluded from study.
Positron Emission Tomography-Computerized Tomography
Imaging Protocol.
Fluciclovine preparation was completed as earlier reported under IND (Investigational New Drug Application) 72,437.15 Patients ingested oral contrast medium after at least 4 hours of fasting. An initial cT with a 3.75 mm slice thickness and 3.25 mm spacing was completed to correct attenuation (approximately 100 mA). Next a mean ± SD of 364.1 ± 37.7 MBq (9.84 ± 1.02 mCi) intravenous fluciclovine was administered. Five minutes after injection the patient underwent dual time point PET from pelvis to diaphragm at 2.5 minutes per bed position for 4 table positions. Scanning was completed on a Discovery MV690 PET-CT scanner (GE Healthcare, Wauwatosa, Wisconsin). Images were reconstructed with an iterative technique using a VUE Point Fx (GE Healthcare) with 3 iterations, 24 subsets and a filter cutoff of 6.4 mm. Images were transferred to a MIMVista work station (MIM Software, Cleveland, Ohio) for interpretation.
Interpretation and Lesion Delineation.
Images were interpreted according to previously reported dual time point criteria by a board certified nuclear medicine physician with 25 years of experience.12 The nuclear medicine physician was blinded to the patient clinical history and other imaging results to avoid interpretation bias. The prostate and individual lesions were manually delineated on the work station and exported to the biopsy planning system. Fluciclovine uptake measured by the SUV and target-to-background ratios were recorded. Abnormal focal uptake over the prostate background with at least moderate activity (greater than the mean SUV of marrow at L3) between early and delayed sequences was considered suspicious for malignancy and targeted for biopsy.
Positron Emission Tomography-Ultrasound Fusion Targeted Biopsy System
The PET-ultrasound fusion targeted biopsy system allows for real-time tracking and recording of biopsy sites as a physician manipulates the ultrasound transducer. PET directed biopsy was done using an Artemis platform (Eigen, Grass Valley, California) with customized software to fuse PET-CT and TRUS images as we previously described.13,14,16,17 Prebiopsy serial 2-D ultrasound images were acquired in the same patient several days before targeted biopsy and reconstructed into a 3-D image to determine prostate volume. On an offline work station suspicious prostate lesions which were previously delineated on PET were fused to the prebiopsy ultrasound using our PET-ultrasound guidance approach and the BK Flex Focus 400 (BK Ultrasound North America, Peabody, Massachusetts) (fig. 1). This was done to incorporate lesion information from the PET-CT to the ultrasound guided biopsy. Deformable image registration was used to combine PET-CT and ultrasound images in 3 di-mensions.13,14 The 3-D registration was achieved by minimizing the distance between the corresponding prostate which had been segmented from the CT and ultrasound images. As described previously17,18 we used CT images as the bridge to register PET with TRUS because PET and CT images were acquired from a combined PET-CT system.
Figure 1.
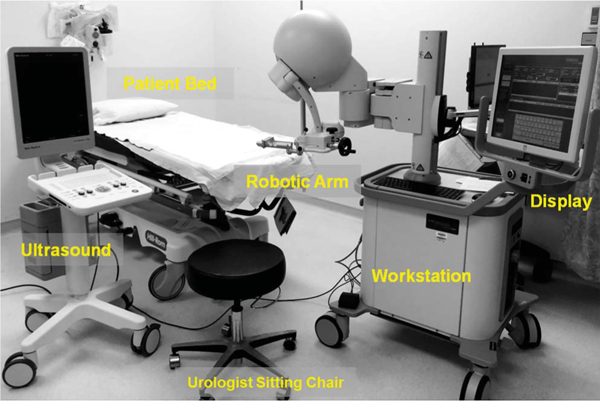
PET-ultrasound fusion targeted biopsy system includes robotic arm, clinical ultrasound scanner and computer work station. Ultrasound probe was attached to robotic arm for 3-D ultrasound image acquisition. Prostate boundaries on 2-D ultrasound were segmented and used to generate 3-D prostate model. Computer work station was used to register PET-CT with 3-D ultrasound data. Lesion target seen on PET was mapped to 3-D model. PET-ultrasound fusion images were used to guide targeted biopsy in patients.
Biopsy Protocol
At the start of the biopsy session real-time 2-D ultrasound images were freshly acquired to detect motion during biopsy. Computer generated template biopsies were first completed while blinded to PET targets using 6 standard regions, including the left and right apex, mid and base, with 2 cores per region when possible. After the template biopsies were completed fluciclovine defined targets were revealed and biopsied using the 3-D visualization and navigation platform to guide the biopsy needle and record its path. Transrectal biopsies with the patient under local anesthesia using standard techniques were completed by 1 board certified urologist with 30 years of experience. The duration of the biopsy procedure (template and PET guided) was approximately 20 to 40 minutes per patient. Figure 2 shows an example of this workflow.
Figure 2.
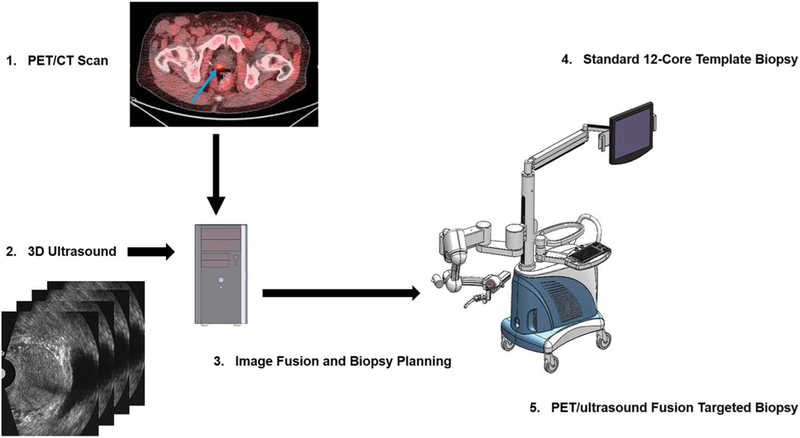
Work flow and comparison of standard 12-core template biopsy with PET-ultrasound fusion targeted biopsy. 1, PET-CT images with fluciclovine acquired from patient show focal lesion in prostate (arrow). 2, prebiopsy 2-D ultrasound image was acquired in same patient using mechanically assisted navigation device and reconstructed into 3-D image. 3, 3-D ultrasound image was registered with PET-CT data to plan targeted biopsy. 4, at biopsy same mechanical device was used to acquire 2-D ultrasound images of patient. Computer generated 12-core template biopsy was performed under TRUS image guidance. 5, fused PET-ultrasound images were used to guide targeted biopsy while patient was on same platform.
Biopsy tissue was processed for standard histological examination. Gleason scores (Grade Groups) were reported in malignant cores in which there was no artifact of therapy related changes.
Statistical Analysis
All study data were collected prospectively. The frequency, mean ± SD, median and range are reported. On patient, region/lesion and core based analyses the cancer detection rate was calculated for standard TRUS guided biopsy and for PET-ultrasound fusion targeted biopsy. The test of significance was performed with the chi-square test. Statistical significance was considered at p <0.05. Analysis was done with SAS®, version 9.4.
RESULTS
Demographics
A total of 21 patients who met study inclusion criteria completed standard TRUS guided biopsy and PET-ultrasound fusion targeted biopsy. Table 1 lists demographic information. A total of 124 regions (an average of 5.9 per patient) with a total of 246 cores (average 1.98 per region) were sampled on template biopsy. Also, 50 lesions (average 2.38 lesions per patient) with a total of 125 cores (average 2.5 per lesion) were sampled on targeted biopsy. Mean ± SD time between fluciclovine PET-CT and biopsy was 50.1 ± 22.4 days.
Table 1.
Patient population characteristics
| Overall | Recurrence | No Recurrence | p Value | ||||
|---|---|---|---|---|---|---|---|
| No. pts | 21 | 10 | 11 | – | |||
| Mean ± SD age (IQR)/median (range) | 69.6 ± 7.4 | (67–75)/69.0 (55–83) | 69.7 ± 5.3 | (66–74)/68.5 (63–79) | 69.6 ± 9.1 | (61–761/72.0 (55.0–83.0) | 0.96 |
| Mean ± SD MBq fluciclovine dose (IQR)/median (range) | 364.1 ± 37.7 | (366.3–377.41/370 (203.5–392.2) | 372.1 ±4.8 | (367.7–377.41/371.5 (366.3–379.6) | 357.1 ± 52.3 | (358.9−378.11/370.0 (203.5–392.2) | 0.36 |
| Mean ± SD ng/ml prePET PSA (IQR)/median (range) | 7.4 ± 6.8 | (3.4–7.61/4.5(1.0–26.7) | 7.4 ± 5.1 | (3.7–12.21/4.6 (3.4–17.3) | 7.4 ± 8.3 | (1.9–7.31/4.0 (1.1–26.7) | 0.90 |
| Mean ± SD mos PSA doubling time (IQR)/median (range)* | 23.1 ± 21.7 | (9.9–27.31/16.6 (3.2–84.2) | 23.5 ± 19.6 | (11.6–27.41/18.0 (7.4–69.1) | 22.7 ± 24.3 | (5.8–29.81/13.8 (3.2–84.2) | 0.94 |
| No. initial Grade Group (%):† | 0.37 | ||||||
| 1 | 5 | (29.4) | 1 | (11.1) | 4 | (50.0) | |
| 2 | 2 | (11.8) | 2 | (22.2) | 0 | ||
| 3 | 6 | (35.2) | 4 | (44.5) | 2 | (25.0) | |
| 4 | 2 | (11.8) | 1 | (11.1) | 1 | (12.5) | |
| 5 | 2 | (11.8) | 1 | (11.1) | 1 | (12.5) | |
| No. initial therapy (%):‡ | 0.50 | ||||||
| Radiotherapy | 9 | (42.9) | 5 | (50) | 4 | (36.4) | |
| Nonradiotherapy | 3 | (14.2) | 2 | (20) | 1 | (9.1) | |
| Mixed | 9 | (42.9) | 3 | (30) | 6 | (54.5) | |
Insufficient data to calculate in 3 patients.
Initial Grade Group reported in 17 patients and mean maximum SUV of positive lesions was significantly higher than in false-positive lesions at early time point (mean ± SD 6.62 ± 1.70 vs 4.92 ± 1.27, p <0.01) and at delayed time point (5.19 ± 1.22 vs 4.10 ± 1.33, p = 0.02) with SUV reported per detected lesion.
Radiotherapy includes external beam radiation, intensity modulated radiation therapy, proton beam radiation and brachytherapy, nonradiotherapy includes cryotherapy only and mixed therapy includes combination of radiotherapy and/or cryotherapy, and/or hormonal therapy.
Cancer Detection Rate
Template biopsy detected malignancy in 6 of 21 patients (28.6%) involving 10 of 124 regions (8.1%) with 11 of 246 cores (4.5%). Targeted biopsy detected malignancy in 10 of 21 patients (47.6%) involving 17 of 50 targeted lesions (34%) with 40 of 125 cores (32.0%). While there was a statistically significant difference in the cancer detection rate on the region and core levels (p <0.01), there was a nonsignificant trend on the patient level (p = 0.204, table 2).
Table 2.
Biopsy cancer detection rate
| No. Biopsy Ca Detection/Total No. (%) |
|||
|---|---|---|---|
| Standard | Targeted | p Value | |
| Per region | 6/21 (28.6) | 10/21 (47.6) | 0.204 |
| Per pt | 10/124 (8.1) | 17/50 (34.0) | <0.01 |
| Per core | 11/246 (4.5) | 40/125 (32.0) | <0.01 |
Targeted biopsy detected malignancy in an additional 5 of the 15 patients with negative template biopsy (table 3 and fig. 3). Of the 11 patients with negative targeted biopsy malignancy was detected by template biopsy in 1 additional patient. The supplementary table (https://www.jurology.com ) lists all patients, the number of cores, the template and the targeted regions obtained.
Table 3.
Biopsy cancer detection rate per patient
| No. Targeted Biopsy (%) |
|||
|---|---|---|---|
| Standard Biopsy | No. Pts | Pos | Neg |
| Overall | 21 | 10 | 11 |
| Pos | 6 | 5 (50) | 1 (9.1) |
| Neg | 15 | 5 (50) | 10 (90.9) |
Figure 3.
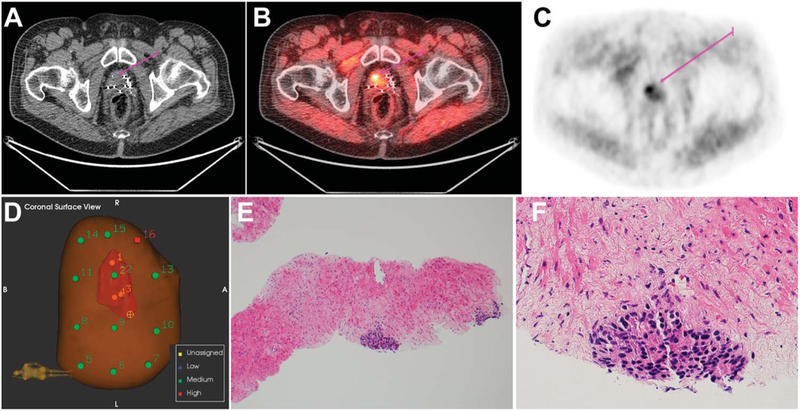
Recurrent prostate cancer 9 years after brachytherapy and external beam radiation in 76-year-old patient with PSA 3.35 ng/ml. Template biopsy was negative while targeted biopsy revealed malignant disease in the right base. A, CT. B, PET. C, fused PET-CT. D, prostate needle core biopsy. E, minute foci of prostatic adenocarcinoma, Gleason score 4 + 4 = 8 (Grade Group 4). H&E, reduced from ×100. F, minute prostatic adenocarcinoma focus, Gleason score 4 + 4 = 8 (Grade Group 4). H&E, reduced from ×400.
Positive Targeted Lesion Locations and Uptake
Two of the 10 patients with recurrent cancer had central lesions only, 5 had peripheral lesions only and 3 had central and peripheral lesions. The mean maximum SUV of positive targeted lesions was significantly higher than that of false-positive lesions at each time point, including 6.62 ± 1.70 vs 4.92 ± 1.27 for the early time point (OR 2.15, 95% CI 1.33–3.50, p <0.01) and 5.19 ± 1.22 vs 4.10 ± 1.33 for the delayed time point (OR 2.01, 95% CI 1.12–3.62, p = 0.02).
Gleason Score (Grade Group) Upgrading
Only 3 of the 5 patients with positive lesions on template and targeted biopsies had reportable Gleason scores (Grade Groups). In 1 of these 3 patients the Gleason score was upgraded from 3 + 4 (Grade Group 2) on template to 4 + 3 (Grade Group 3) on targeted biopsy (fig. 4).
Figure 4.
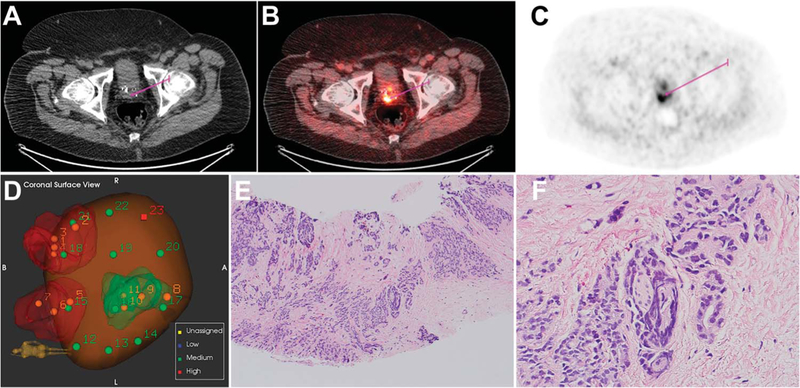
Recurrent prostate cancer 6 years after brachytherapy and external beam radiation in 63-year-old patient with PSA 11.44 ng/ml. Recurrent prostate cancer identified in right posterior base was upgraded from Gleason score 3 + 4 on template biopsy to Gleason 4 + 3 on targeted biopsy. A, CT. B, PET. C, fused PET-CT. D, prostate needle core biopsy. E, prostatic adenocarcinoma, Gleason score 4 + 3 = 7 (Grade Group 3). H&E, reduced from ×100. F, note perineural invasion focus. H&E, reduced from ×400.
Extraprostatic Disease
Extraprostatic disease was detected on fluciclovine PET-CT in 8 of 21 patients (38.1%), including 1 bone and 7 pelvic nodes. Malignancy was histologically confirmed in 3 of 8 patients in whom extraprostatic lesions were biopsied, including 2 lymph nodes and 1 bone.
DISCUSSION
Although TRUS guided prostate biopsy is currently the standard of care, it has limited diagnostic performance to detect recurrent disease in the treated prostate.2–5 To our knowledge molecular image directed, 3-D reconstructed, ultrasound guided biopsy represents a novel approach to diagnose recurrent disease, facilitating salvage therapy.
Therefore, we set out to determine whether molecular targeted TRUS guided biopsy with fluciclovine PET is feasible and whether it has the potential to improve the histological cancer detection rate over that of standard template biopsy alone.
The strengths of our study include the prospective design and intrapatient controls on a platform in which computer generated 12-core template biopsy was completed before imaging targets were revealed to the urologist for biopsy. This eliminated a potential source of bias when comparing template to targeting techniques.
In this relatively small study we found that molecular targeted biopsy is feasible and can be performed with a functional work flow. Moreover, we found that targeted biopsy detected malignancy in 47.6% of patients vs 28.6% by template biopsy. This higher detection rate was achieved when sampling fewer regions and cores with a targeted vs a template technique (50 vs 124 regions and 125 vs 246 cores, respectively). Malignancy was detected in 5 of the 15 patients with negative results on standard template biopsy and yet in 1 patient malignancy was detected on template biopsy alone.
Our findings are important since standard TRUS guided biopsy is in effect a blind procedure with the well recognized potential for sampling error in diagnosing and under grading histological recurrence in the primary and recurrent disease settings. Thus, MRI guided biopsy has become the developing standard for primary disease interrogation, detecting a greater number of significant cancers.6,19 In the nonprostatectomy setting the post-therapy effects of radiotherapy, cryotherapy and brachytherapy may significantly distort the prostate, resulting in technical limitations in TRUS and MRI when differentiating scar from recurrence.7–10
Most work based on image guided prostate biopsy has been done in primary disease cases. There is less literature in the recurrence setting in regard to advanced molecular imaging guided biopsy. For example, Piert et al reported a prospective study of 36 patients with suspected primary prostate cancer who underwent FCH PET-CT and mpMR.20 PET was registered to MRI and then MRI was registered to TRUS. After the targeted biopsy 12-core template biopsies were obtained which also covered the targeted regions. Overall, cancer was detected in 14 of 36 cases (38.9%) by each technique. However, FCH PET targeting improved the detection of significant cancer, defined as Gleason score 3 + 4 or greater, or Grade Group 2 or greater.
In a study of 46 patients who underwent 11C-choline PET-CT and subsequent TRUS biopsy after radiotherapy which showed local recurrence, cancer was detected on nontargeted biopsy in 18 (39.1%).21 Others similarly reported performing FCH PET to identify primary disease after a negative initial prostate biopsy with focal prostate uptake in 13 of 20 patients and a positive guided biopsy in 5 of 13 (38.5%). These studies were retrospective and without intrapatient controls. In another retrospective study of 32 patients after radiotherapy or brachytherapy in which they underwent FCH PET and mpMR followed by reference (nontargeted) systematic transperineal ultrasound biopsy, 23 and 22 of 31 patients showed positive local recurrence on FCH and mpMR, respectively, with a combined 68% positive predictive value, demonstrating the potential promise of combined PET-MRI techniques.22
One limitation of our study is the relatively small number of patients. On the patient level the non-statistically significant trend of the greater detection rate which we report for targeted biopsy must be confirmed in a larger study. Another limitation is the PET radiotracer itself. Although fluciclovine is approved by the Food and Drug Administration to detect recurrent prostate cancer, its greatest utility is in whole body staging and the detection of extraprostatic disease.23 Relatively lower specificity has been reported in the prostate due to nonspecific activity in hypertrophic tissue and possibly inflammation.24,25 Also, Gleason scores (Grade Groups) could not be assigned to some sampled lesions due to significant therapy related architectural changes.
Furthermore, PET-ultrasound fusion guidance is a labor intensive method due to the current version of the technology. Although this procedure required an additional TRUS, we expect that as we develop the technology we will eliminate the need for prebiopsy ultrasound, such that PET could be fused with ultrasound during the biopsy procedure.
At the current state of technology the prostate had to be outlined based on the CT appearance, which is less than ideal. In addition, the entire suspected lesion was manually contoured based on subjective visual analysis of uptake when ideally the isocenter of highest radiotracer activity should be specifically targeted. Thus, there may have been misregistration of the prostate from CT to ultrasound as well as sampling error in the lesion, which may have intermixed activity on PET with a combination of tumor and inflammation. Future versions of the device will use automatic prostate anatomical segmentation and lesion isocontouring, and co-registration with MRI may also be done.
In addition to the promise of the higher recurrence detection rate for PET guidance vs standard template biopsy demonstrated in this pilot study, we have established the feasibility of a platform in which molecular guided 3-D ultrasound techniques can be tested in a controlled and rigorous manner, and adapted to other radiotracers. For example, PSMA radiotracers have shown utility for recurrence after radiotherapy.26 Zettinig et al reported a multimodal, image guided prostate fusion biopsy system using PSMA PET in primary disease.27 Finally, the whole body staging made possible with molecular techniques can help inform salvage therapy decisions. Our group and others have found the added value of PET techniques over conventional imaging alone for staging and restaging prostate cancer.24,28,29
CONCLUSIONS
Fluciclovine PET images and real-time ultrasound images were combined to guide fusion targeted biopsy of the prostate in patients with biochemical failure after nonsurgical therapy of prostate cancer. In this feasibility prospective study PET-ultrasound fusion targeted biopsy had a higher cancer detection rate than standard TRUS guided biopsy in patients with recurrent disease and it did so with fewer regions and cores sampled. Further work is required to improve technical automation. Since 1 case was detected by template biopsy alone and microscopic or small volume disease detection may be limited when using PET, we do not suggest that template biopsy should be bypassed at this time. However, there is value in supplementing template biopsy with targeted biopsy in recurrent disease.
Supplementary Material
ACKNOWLEDGMENT
Blue Earth Diagnostics Ltd. provided Emory University with fluciclovine synthesis cassettes.
Supported by NIH (National Institutes of Health) Grants R21CA176684 (BF, VAM), R01CA156775 (BF) and R01CA204254 (BF).
Abbreviations and Acronyms
- 2-D
2-dimensional
- 3-D
3-dimensional
- CT
computerized tomography
- FCH
18F-fluorocholine
- mpMR
multiparametric MRI
- MRI
magnetic resonance imaging
- PCa
prostate cancer
- PET
positron emission tomography
- PSA
prostate specific antigen
- PSMA
prostate specific membrane antigen
- SUV
standard update value
- TRUS
transrectal ultrasound
Footnotes
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
Mark M. Goodman and Emory University are entitled to a royalty derived from the sale of products related to the research described in this manuscript.
Registered at ClinicalTrials.gov (NCT02744534).
Financial interest and/or other relationship with Emory University.
Financial interest and/or other relationship with Syncona and AIM Specialty Health.
The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
REFERENCES
- 1.D’Amico AV, Whittington R, Malkowicz SB et al. : Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer 2002; 95: 281. [DOI] [PubMed] [Google Scholar]
- 2.Pondman KM, Futterer JJ, ten Haken B et al. : MR-guided biopsy of the prostate: an overview of techniques and a systematic review. Eur Urol 2008; 54: 517. [DOI] [PubMed] [Google Scholar]
- 3.Evangelista L, Zattoni F, Rossi E et al. : Early detection of prostate cancer relapse by biochemistry and diagnostic imaging. Q J Nucl Med Mol Imaging 2015; 59: 359. [PubMed] [Google Scholar]
- 4.Martino P, Scattoni V, Galosi AB et al. : Role of imaging and biopsy to assess local recurrence after definitive treatment for prostate carcinoma (surgery, radiotherapy, cryotherapy, HIFU). World J Urol 2011; 29: 595. [DOI] [PubMed] [Google Scholar]
- 5.Crook J, Robertson S, Collin G et al. : Clinical relevance of trans-rectal ultrasound, biopsy, and serum prostate-specific antigen following external beam radiotherapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys 1993; 27: 31. [DOI] [PubMed] [Google Scholar]
- 6.Verma S, Choyke PL, Eberhardt SC et al. : The current state of MR imaging-targeted biopsy techniques for detection of prostate cancer. Radiology 2017; 285: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas HA, Wassberg C, Akin O et al. : MR imaging of treated prostate cancer. Radiology 2012; 262: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel P, Mathew MS, Trilisky I et al. : Multi- parametric MR imaging of the prostate after treatment of prostate cancer. Radiographics 2018; 38: 437. [DOI] [PubMed] [Google Scholar]
- 9.Coakley FV, Hricak H, Wefer AE et al. : Brachy- therapy for prostate cancer: endorectal MR imaging of local treatment-related changes. Radiology 2001; 219: 817. [DOI] [PubMed] [Google Scholar]
- 10.Oppenheimer DC, Weinberg EP, Hollenberg GM et al. : Multiparametric magnetic resonance imaging of recurrent prostate cancer. J Clin Imaging Sci 2016; 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calais J, Fendler WP, Eiber M et al. : Impact of (68)Ga-PSMA-11 PET/CT on the management of prostate cancer patients with biochemical recurrence. J Nucl Med 2018; 59: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster DM, Nieh PT, Jani AB et al. : Anti-3-[(18) F]FACBC positron emission tomography-computerized tomography and (111 )In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol 2014; 191: 1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fei B, Nieh PT, Schuster DM et al. : PET-directed, 3D ultrasound-guided prostate biopsy. Diagn Imaging Eur 2013; 29: 12. [PMC free article] [PubMed] [Google Scholar]
- 14.Fei B, Nieh PT, Master VA et al. : Molecular imaging and fusion targeted biopsy of the prostate. Clin Translational Imaging 2017; 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McConathy J, Voll RJ, Yu W et al. : Improved synthesis of anti-[18F]FACBC: improved preparation of labeling precursor and automated radiosynthesis. Appl Radiat Isot 2003; 58: 657. [DOI] [PubMed] [Google Scholar]
- 16.Fei B, Schuster DM, Master V et al. : A molecular image-directed, 3D ultrasound-guided biopsy system for the prostate. Proc SPIE Int Soc Opt Eng 2012; 2012: 831613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fei B, Master V, Nieh P et al. : A PET/CT directed, 3D ultrasound-guided biopsy system for prostate cancer. Prostate Cancer Imaging (2011) 2011; 6363: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fei B, Schuster D, Master V et al. : Incorporating PET/CT images into 3D ultrasound-guided biopsy of the prostate (abstract TU-A-BRA-02). Med Phys, suppl., 2012; 39: 3888. [Google Scholar]
- 19.Kasivisvanathan V, Rannikko AS, Borghi M et al. : MRI-targeted or standard biopsy for prostate- cancer diagnosis. N Engl J Med 2018; 378: 1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piert M, Montgomery J, Kunju LP et al. : 18F-Choline PET/MRI: the additional value of PET for MRI-guided transrectal prostate biopsies. J Nucl Med 2016; 57: 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceci F, Castellucci P, Graziani T et al. : 11C-choline PET/CT detects the site of relapse in the majority of prostate cancer patients showing biochemical recurrence after EBRT. Eur J Nucl Med Mol Imaging 2014; 41: 878. [DOI] [PubMed] [Google Scholar]
- 22.Kanoun S, Walker P, Vrigneaud JM et al. : (18) F-Choline positron emission tomography/ computed tomography and multiparametric magnetic resonance imaging for the detection of early local recurrence of prostate cancer initially treated by radiation therapy: comparison with systematic 3-dimensional transperineal mapping biopsy. Int J Radiat Oncol Biol Phys 2017; 97:986. [DOI] [PubMed] [Google Scholar]
- 23.Parent EE and Schuster DM: Update on (18)F-fluciclovine PET for prostate cancer imaging. J Nucl Med 2018; 59: 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akin-Akintayo O, Tade F, Mittal P et al. : Prospective evaluation of fluciclovine ((18)F) PET-CT and MRI in detection of recurrent prostate cancer in non-prostatectomy patients. Eur J Radiol 2018; 102: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkbey B, Mena E, Shih J et al. : Localized prostate cancer detection with 18F FACBC PET/ CT: comparison with MR imaging and histopathologic analysis. Radiology 2014; 270: 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Einspieler I, Rauscher I, Duwel C et al. : Detection efficacy of hybrid (68)Ga-PSMA ligand PET/CT in prostate cancer patients with biochemical recurrence after primary radiation therapy defined by Phoenix criteria. J Nucl Med 2017; 58: 1081. [DOI] [PubMed] [Google Scholar]
- 27.Zettinig O, Shah A, Hennersperger C et al. : Multimodal image-guided prostate fusion biopsy based on automatic deformable registration. Int J Comput Assist Radiol Surg 1997; 10: 2015. [DOI] [PubMed] [Google Scholar]
- 28.Kitajima K, Murphy RC, Nathan MA et al. : Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C- choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med 2014; 55: 223. [DOI] [PubMed] [Google Scholar]
- 29.Maurer T, Gschwend JE, Rauscher I et al. : Diagnostic efficacy of (68)gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol 2016; 195: 1436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


