Abstract
Single stranded DNA (ssDNA) oligonucleotides are useful as aptamers, hybridization probes and for emerging applications in DNA nanotechnology. Current methods to purify ssDNA require both strand separation and a separate size separation step and may still leave double stranded DNA impurities in the sample. We use commercially-available acrydite DNA primers to immobilize one strand of a PCR product within a polyacrylamide matrix. Electrophoresis moves the non-crosslinked DNA into the gel where the single stranded, size-appropriate product can be recovered. We show that this produces a high yield of pure ssDNA.
Introduction
Single stranded DNA (ssDNA) is useful for aptamers(1), self-assembly(2), DNA DNA circuits(4), hybridization probes(5), and DNA robotics(6). Chemically synthesized DNA is single stranded by default.
We present a method to produce single-stranded DNA from a PCR product. The use of a PCR product may be advantageous over synthetic DNA because PCR introduces fewer mutations than chemical synthesis and is not limited to ~120 bases. Starting from a clonal sample, PCR products can have significantly higher sequence purity than chemically synthesized DNA. High sequence purity is critical for high performance in DNA circuit technology(7).
Our single-strand generation (SSG) method is scalable and requires only one purification step. This removes the unwanted complementary strand and performs size-based purification from residual primer and unwanted PCR products. SSG by co-polymerization and electrophoresis may be applied to the product of advanced PCR protocols such as Gibson assembly(8) to create long, single stranded products of arbitrary sequence. This may be of use for DNA origami and other self-assembly applications.
SSG by co-polymerization and electrophoresis uses a commercially available DNA modification known as an acrydite. This modification adds a polymerizable vinyl group which incorporates into a growing acrylamide polymer chain(9). Acrydite modified DNA has been used for a number of applications including a switchable hydrogel (10), capture of mercury (11) or PDGF (12), and for modifying the rate of electrophoresis of specific sequences (13). Our technique is superficially similar to the method by which the Church group immobilized polymerase colonies within thin polyacrylamide gels. The acrydite is critical for the formation of clonal PCR products (‘polonies’) in some forms of DNA sequencing(14).
In cases when significant quantities of pure, single-stranded DNA are required, our approach using acrydite modified primers and polyacrylamide immobilization is a worthwhile option. We also show that this technique can integrate SSG, size separation and electro-elution into a single step.
Method Summary
We use commercially-available 5’-acrydite modified DNA primers to purify ssDNA by immobilizing one strand of a PCR product within a polyacrylamide matrix.
Materials and methods:
Materials:
Unless otherwise stated, materials were acquired from Sigma Aldrich (St. Louis, MO) and used without further purification. DNA was acquired from IDT, Integrated DNA Technologies (Coralville, IA), and used without further purification. This included the following sequences (see Table 1. List of DNA Sequences Used)
Table 1.
List of DNA Sequences Used
| Name | Description | Sequence |
|---|---|---|
| Test template | Test template for demonstrating PCR (75nt) | CACGAATTAC ATGTTGCTCT TACCATACCT TTATATAGAC AATTCTCTGA ATATCCCCAC AAGGAACACGCAAAT |
| Test Template Primer 1 | 5’-Fluorescein modified 20nt | 5’ Fluorescein-ATTTGCGTGTTCCTTGTGGG |
| Test Template Primer 2 | 5’-Fluorescein modified 23nt | 5’Acrydite-CACGAATTACATGTTGCTCTTAC |
| Cy5 modified Primer 2 | 5’-acrydite modified & internal Cy-5 modified 23nt | 5’Acrydite-internal Cy5-CACGAATTACATGTTGCTCTTAC |
| Quench Probe | 18nt 3’-modified | CACAAGGAACACGCAAAT-3’Iowa Black Quencher |
| Aptamer | Clone 1 anti-lysozyme aptamer (80nt) | GGGAATGGAT CCACATCTAC GAATTCATCA GGGCTAAAGA GTGCAGAGTT ACTTAGTTCA CTGCAGACTT GACGAAGCTT |
| F-Aptamer | Clone 1 anti-lysozyme aptamer-F (80nt) | 5’Fluorescein- GGGAATGGAT CCACATCTAC GAATTCATCA GGGCTAAAGA GTGCAGAGTT ACTTAGTTCA CTGCAGACTT GACGAAGCTT |
| Clone 1 Primer 1 | 23nt 5’-Fluorescein modified primer for clone 1 | 5’Fluorescein-AAATACGGGAATGGATCCACATC |
| AC-clone-1-P2 | 23nt 5’-acrydite modified primer for clone 1 | 5’Acrydite-ATAAGCTTCGTCAAGTCTGCAGT |
| Clone 1 Biotinylated Primer 2 | 23nt 5’-biotin modified clone 1 primer | 5’biotin-ATAAGCTTCGTCAAGTCTGCAGT |
| F-DNA* | 19nt 3’-FAM modified DNA | GTCTCTGTGCCGCTATAAT-/FAM/ |
| AC-DNA | 19nt 5’-acrydite modified DNA | /5Acryd /ATTATAGCGGCACAGAGAC |
| AC-DNA-F | 19nt 5’-acrydite and 3’-FAM modified DNA | /5Acryd /ATTATAGCGGCACAGAGAC/36-FAM/ |
Demonstration of capture of acrydite strand:
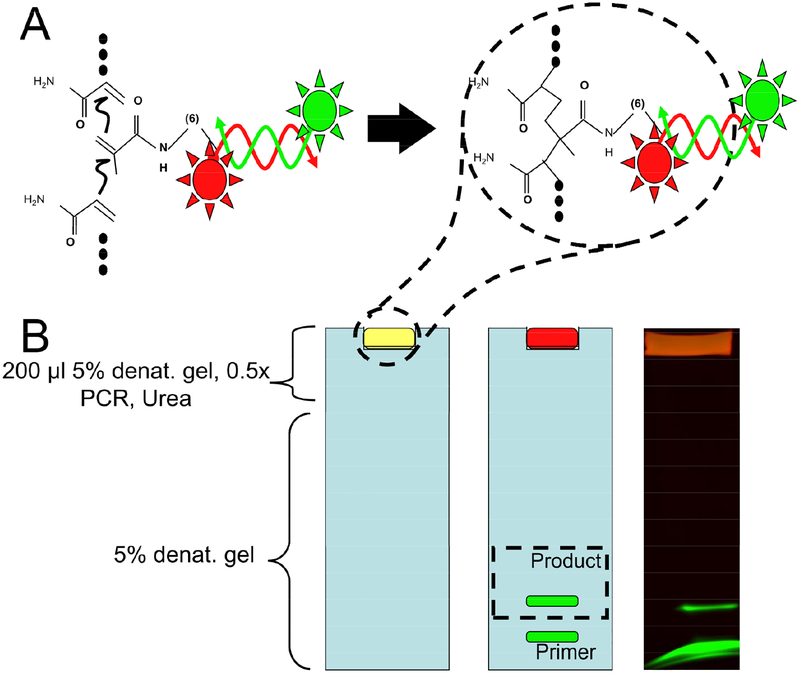
In the initial demonstration of single strand generation by Co-polymerization with Acrylamide and Electrophoresis, we modified one primer with acrydite and Cy5 red-fluorescent dye. The other primer was purchased with a green fluorescent 5’ fluorescein modification. Figure 1 shows how the DNA sample was prepared by mixing 100μl of 10% denaturing gel (prepolymer before initiation of polymerization) with 100μl PCR product + appropriate mass of dry urea (5% final acrylamide concentration, 7M final urea concentration). The DNA/prepolymer was loaded into the dry well of 5% denaturing PAGE gel. The gel was heated as described below and electrophoresis was performed to separate the green-fluorescent strand from the immobilized red-fluorescent strand. The result was imaged using a standard gel imaging system with LED illumination (FluorChem Imaging System, ProteinSimple, San Jose, CA).
Figure 1: Outline of the technique.
(A) A schematic shows how double-stranded DNA is produced from a primer modified with the acrydite and Cy5 at its 5’ terminus and a reverse primer modified with fluorescein. These primers produce a double-stranded PCR product. When co-polymerized with acrylamide, this product is immobilized within the polymer matrix. (B) Fluorescence gel images show that the Cy5-modified, copolymerized strand remains at the top of the gel. Single-stranded product and primer migrate separately.
Single Strand Generation and Purification (Vertical Gel Electrophoresis):
PCR was performed in all cases with the Accuprime Pfx reaction kit (Life Technologies, Grand Island, NY) using 50 nM template, 10 μM concentration of each primer. and eight thermal cycles. The PCR product was encouraged to denature by adding solid urea to a final concentration of 7M in a 100 μl reaction. Polyacrylamide was prepared from a commercially prepared stock solution of 40% acrylamide in water with a 19:1 ratio of acrylamide:bis-acrylamide (Biorad, Hercules, CA). Denaturing polyacrylamide was prepared with final concentrations of 7M urea, 20% acrylamide, and 0.25x TBE buffer. Polymerization was initiated by adding 1 μl of TEMED (N,N,N’,N’-Tetramethylethylenediamine) and 10 μl 10% ammonium persulfate to an aliquot of 1 mL of denaturing acrylamide prepolymer. A portion of this mixture (prepolymer before initiation of polymerization) was rapidly mixed 1:1 with 0.1 ml of PCR product with 7M urea (10% acrylamide final concentration). The PCR/polymerizing acrylamide mixture was added to a dry well in a standard vertical denaturing PAGE gel. Full polymerization of the PCR/denaturing acrylamide was ensured by flushing the space above the well with a gentle stream of argon or 99.97% nitrogen (to displace any oxygen that inhibits the polymerization). After about 30 minutes of polymerization, we set up the electrophoresis rig. Crushing the polyacrylamide and loading the macerated pieces into the well produced a very broad, irregularly shaped band and this approach was discarded (data not shown). Polymerization within the well was a superior approach. We heated running buffer in a microwave to boiling and added the hot (~80–90 °C) buffer to the cathode reservoir (by pouring the hot buffer over the filled wells) in order to encourage release of the DNA from the well. The addition of the hot buffer was continued until the gel rig was full to the necessary volume for electrophoresis. The DNA-containing gel was in direct contact with hot buffer as voltage was applied. The hot buffer should be applied promptly and not allowed to cool. We then electrophoresed according to the diagram shown in Figure 1.
SB and TBE buffers can be used as running buffers. SB and TBE were prepared at RT at pH 8.42 and 8.36 respectively. At 80 °C, these pH values change to 8.21 and 7.47 respectively. This pH change is transient. The gel cools to 30–40 °C during electrophoresis. This change in pH did not noticeably cause degradation of the DNA.
In order to visualize the separation of the two strands, we used primers modified with two different fluorophores. The mobile strand was generated from a fluorescein-modified primer. The immobile (acrydite) primer was prepared with an internal Cy5 modification. Using these two dyes, we could visualize the progress of the separation. We scanned this gel with a gel imager (FluorChem Q, ProteinSimple, Santa Clara, CA) after 45 min of electrophoresis at 500 v.
As an alternative approach, the PCR product can be pre-concentrated by standard ethanol precipitation. The pellet can then be dissolved in a smaller volume of acrylamide prepolymer. In our hands, the increased concentration in the well was offset by losses during precipitation. In some cases (e.g. for a faint product band) this approach may be preferred.
The fluorescent band bearing the single-stranded, fluorescein-modified product was cut from the gel under blue LED illumination at ~475nm (Bulldog Bio, Portsmouth, NH). The DNA was eluted by the standard crush and soak method(15). When the eluted (recovered) DNA was too dilute for efficient ethanol precipitation, we concentrated by extracting with butanol. The product was then precipitated with ethanol and analyzed further as per below.
PAGE analysis
The single-stranded results (single-stranded DNA release from denaturing PAGE analysis) were analyzed for purity with native PAGE. We resuspended the single-stranded product in 10 μl. We quantified with UV-vis spectroscopy. We made equal molar concentration of primer and single stranded product. Then PAGE analysis was performed. We electrophoresed on a 15% native PAGE gel and visualized with the Storm scanner (using the covalently attached fluorescein unstained) and a 25 base pair green fluorescent ladder (Jena Bioscience, Jena, Germany).
Quencher analysis:
To further establish that the product was in fact single-stranded, we applied a complementary oligonucleotide (sequence ”Quench Probe”) with a 3’ quencher modification. When properly hybridized, the quench probe positions an Iowa Black quencher within a few nanometers of the 5’ fluorescein modification on the complementary strand. This causes a sharp decrease in fluorescence intensity. The quench probe will only hybridize to single-stranded DNA. If the product is double-stranded, then hybridization will be blocked and fluorescence will remain. Triplicate samples of 10 μl of 100 nM fluorescein-modified product (PCR product, single-strand generation product and primer control) were prepared in PCR tubes. These were measured with a QuantiFluor fluorometer (Promega, Madison, WI) for their initial fluorescence values.1.1 equivalents of quench probe DNA were then added, vortexed to mix, and centrifuged and allowed to incubate for approximately 1 minute. The quenched fluorescence was then recorded.
Comparison of single strand generation techniques for aptamer production:
In order to ascertain the effectiveness of this method for generating functional aptamers, we purchased a template for PCR amplification of a known aptamer sequence for lysozyme. We amplified this template with a fluorescein modified primer and a acrydite modified reverse primer. We performed the single-stranded generation protocol as described above. We conjugated lysozyme to 5.8 ym carboxylate modified beads (Bangs Labs, Fishers, IN) by the standard EDC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide) coupling protocol. These peas were incubated with the single strand generation product, randomized fluorescent DNA (no sequence similarity to the aptamer), and a chemically synthesized fluorescent aptamer of the same sequence. We also incubated on coated beads with the chemically synthesized fluorescent aptamer as a negative control. Incubation’s are carried out for 30 minutes and then the beads were washed three times. Flow cytometry was performed on these particles with a FACScalibur (BD Bioscience San Jose, CA). The distribution of fluorescence intensities with exultation 488 nm was recorded.
Single Strand Generation and Purification (Horizontal Gel Electrophoresis):
A 7 M urea denaturing polyacrylamide gel was cast horizontally as described in a previous work(16). Briefly, the 25 ml of the prepolymer solution (7M urea, 6% acrylamide/bis, SB buffer) was initiated with 84 μl of APS and 20 μl TEMED. The gel prepolymer was then poured into a horizontal mold. The mold was then placed in a plastic bag which was purged with nitrogen. This was allowed to polymerize for 30 minutes. A solution of fluorescein-labeled and acrylamide-labeled DNA were assembled in 1 M sodium phosphate at pH 8 and annealed by raising the temperature to 80 °C and cooling slowly at 0.1 °C per second to room temperature. The mixture was cooled slowly to promote intermolecular hybridization between two strands. 2.5 mL of the prepolymer mixture were initiated by adding 8.4 μL of APS and 2 μL of TEMED. Once mixed, 20 μL of this solution were added to the 5 μL of DNA solution. This was then transferred to a (dry) well in the polyacrylamide gel. In order to avoid distortions in the electric field lines, the remaining wells were also filled with (non-DNA) acrylamide. Once again, the loaded gel was placed in a plastic bag and purged with nitrogen. The gel was run at 10 V/cm for 10 minutes. It was then imaged using a blue LED transilluminator and a digital camera. In order to release the ssDNA from the cross-linked gel, 25 mL of SB buffer for heated to boiling in a microwave. This hot water was then poured over the gel. Electrophoresis was continued at 10 the/CM for 10 minutes. The gel was imaged again as above for comparison.
Initial association between the fluorescein and acrydite labeled DNA was encouraged using 1M buffer. If the DNA were only held together by hybridization, 7M urea and the exchange of buffer into 5mM SB during electrophoresis would be adequate to release the DNA into the gel. Instead, heat was required. This demonstrated that the DNA is associated or entangled with the gel rather than with its complementary DNA
Results and Discussion:
Acrydite modified DNA is immobile under electrophoresis
Of the two strands of the dsDNA PCR product, the acrydite strand is trapped in the co-polymer while the complementary strand is mobile. To demonstrate this, PCR was performed with one primer modified with both acrydite and red fluorescent dye (Cy5). The reverse primer was modified with fluorescein. The PCR product was polymerized into a denaturing polyacrylamide gel electrophoresis (PAGE) gel (5%). For a schematic of the process, see Figure 1. Initially, both the green-fluorescent and red-fluorescent strands were co-localized (yellow-colored gel). Upon application of voltage, only the fluorescein-labeled product (green) migrates into the gel. The acrydite and Cy5 co-labeled strand (red) is fixed. This is shown in the final gel image in Figure 1B. Additionally, residual primer from the PCR reaction is clearly visible as a second green band. After electrophoresis, the pure, single-stranded product can be cut and eluted per standard gel purification protocols.
Verification that the product was single-stranded
We isolated single-stranded, fluorescein-modified DNA by cutting and eluting the band of interest. To show that the product recovered by this method is single-stranded, we used two orthogonal methods. The first method was native gel analysis. Figure 2A shows an image of a native gel of the primer, PCR product (dsDNA), and ssDNA purified by this method. The native gel shows clear separation between the dsDNA and ssDNA. After the single strand generation with the acrydite primer (followed by cutting the gel and eluting the product) most of the result is single-stranded.
Figure 2: Verification of single-stranded DNA.
(A) Native gel image shows clear resolution of SSG product as compared to dsDNA PCR product. (B) Fluorescence intensity shows the result of FRET-based quenching using a hybridization probe to bind single-stranded DNA.
We corroborated with a quencher-modified hybridization probe (see Figure 2B). The probe was designed to bring a quencher into proximity to the fluorescein moiety on the ssDNA product. Upon hybridization, the quencher molecule reduces the fluorescence intensity by ~90%. The probe only hybridizes to ssDNA and so this demonstrates that the fluorescein modified product was single stranded. As a negative control, we also tested the dsDNA PCR product. The double-stranded PCR product was unable to hybridize to the quencher probe. Its fluorescence remained virtually unchanged.
Aptamers synthesized by PCR purified by co-polymerization and electrophoresis
High purity ssDNA is produced by our SSG technique. We purified the single-stranded DNA product from PCR by two methods: SSG by co-polymerization and electrophoresis, and the biotin-avidin immobilization technique described elsewhere (i.e. Ploysciences Technical data sheet 753) (17, 18). Single stranded aptamer was also generated by chemical synthesis. Figure 3A shows ssDNA aptamer generated by 3 methods. Lane 1 is a ladder for size comparison. Lane 2 shows the ssDNA generated by PCR with an acrydite primer followed by co-polymerization with acrylamide and electrophoresis. Only one band is visible at the correct size for ssDNA. Lane 3 is the ssDNA generated by PCR with a biotinylated primer followed by purification with avidin-coated magnetic beads. Lane 4 is chemically synthesized DNA with the same sequence. Purified ssDNA aptamer from SSG by co-polymerization and electrophoresis shows high ssDNA purity.
Figure 3: Purity and functionality of aptamers derived from this and other techniques.
(A) Native gel analysis shows the purity of SSG by co-polymerization and electrophoresis, SSG by avidin coated beads, and chemical synthesis. (B) Flow cytometry analysis shows the functionality of aptamers derived by SSG by co-polymerization and electrophoresis (blue) and chemical synthesis (red).
This procedure produced functional aptamer. We produced a previously described aptamer against lysozyme by PCR with modified primers. The “Clone 1” anti-lysozyme aptamer was originally produced in the Ellington laboratory. It was originally selected in RNA(19). Other groups report that the DNA sequence also acts as an aptamer(20, 21). In this study, both PCR and chemically synthesized DNA resulted in functional aptamer. Based on these and other experiments in our hands, we can conclude that this is one of the few, exceptional cases where RNA and its parent DNA sequence both bind the target. This is a strange and coincidental result; most aptamers do not function when translated directly to a different chemistry. These results corroborate the many instances in the literature that indicate that the Clone 1 anti-lysozyme aptamer is a special case.
Flow cytometry analysis shows that the generated ssDNA aptamer is functional. Figure 4B shows lysozyme-coated beads exposed to random DNA, the chemically synthesized aptamer against lysozyme or the PCR-generated aptamer against lysozyme. Both the PCR and chemically synthesized aptamers show strong binding to the lysozyme-coated beads. The random DNA shows no significant association to the lysozyme beads. This indicates that the interaction is specific for the aptamer sequence. Uncoated beads show no fluorescence with or without the aptamer. This indicates that the binding is specific to the protein.
Figure 4: PAGE analysis shows that added heat is required for successful SSG.
(A) A fluorescence image shows the gel after 10 min of electrophoresis. Schematic at right shows the contents of each well and band. (B) Fluorescence diagrams of the same gel after applying heat followed by 20 minutes of further electrophoresis. Heat releases all of the long DNA.
Necessity of heat to release DNA from the well
When purifying the ssDNA aptamer from the PCR product, it was necessary to heat the gel to allow the DNA to electrophorese (see Materials and Methods). We used horizontal PAGE to investigate why this was necessary. Without the application of heat, the desired ssDNA PCR product was retained in the well (despite 7M urea and the voltage gradient).
Our first hypothesis was that the retention was due to the 80 bases of hybridization in the PCR product. To test this, we reduced the complementarity to 23 bases. We annealed Clone 1 aptamer to a 23nt long acrydite-modified primer (denoted AC-Clone1-P2 in Figure 4A–B). SSG by co-polymerization and electrophoresis was then attempted. Despite the relatively short 23 bases of hybridization, significant quantities of the aptamer DNA were retained in the well (see Figure 4A, bottom lane). This indicated that our hypothesis was incorrect: short hybridization (which should be easily denatured in 7M urea) cannot account for the retention in the well.
We then tested the hypothesis that this retention phenomenon was dependent on length. We annealed a 5’-acrydite modified 19nt long oligonucleotide (denoted AC-DNA in Figure 4A–B) to a 19nt long, fully complementary, fluorescein-modified strand (denoted F-DNA* in Figure 4A–B). Despite similar hybridization length, the short DNA was easily purified from its complement in a denaturing gel without application of heat. Virtually all of the 19nt, fluorescein-modified mobile strand enters the gel (Figure 4A, middle lane). In order to show that the acrydite-modified 19-mer was retained, a strand modified with both fluorescein and acrydite (denoted AC-DNA-F in Figure 4A–B) was also co-polymerized into the well (top lane) and was successfully retained. (see materials and methods, Table 1 for sequence and nomenclature details).
After the first 20 minutes of electrophoresis (without heating), it was clear that much of the aptamer-length DNA was not mobile (despite a short hybridization length). In order to release aptamer-length DNA from the polyacrylamide, it was necessary to heat the gel. We heated running buffer to near boiling in a microwave and then poured it over the gel in the region of the wells. Electrophoresis was then continued for 20 more minutes (see Figure 4B). The aptamer band became mobile. This new band had the same mobility as the first, indicating that this was the same species. Likewise, this was the same sample of DNA that yielded only a single band in Figure 3A. This experiment demonstrates the necessity of heating the gel in order to release all long ssDNA from the polymer. The retention of DNA in the well is not due to hybridization, but likely due to entanglement in the polymer matrix.
Horizontal PAGE for SSG and integrated electroelution
The use of a horizontal gel allows for electroelution to be carried out rather than cutting and extracting the product band. The use of a second “extraction” comb (for additional wells for electroelution and extraction) in horizontal PAGE made SSG and electroelution of DNA faster. We used Inkscape software to design a separate comb for electroelution that was deeper and wider than the loading comb. The design was laser cut in acrylic with a CO2 laser cutter (see Figure 5A–B).
Figure 5: Demonstration of electro-elution technique using a set of “extraction wells” molded into a horizontal polyacrylamide gel.
(A) Digital photograph shows the original sample comb and our adapted injection and extraction combs as well as the BioRad gel tray for which they were designed. (B) Digital photograph shows the extraction and injection combs in the tray for molding. (C) A digital fluorescence image shows the separation of product and primer from a PCR amplified aptamer pool. (D) A digital fluorescence image shows the same gel after further separation with the product poised to enter the extraction wells for recovery.
Typically, polyacrylamide gel electrophoresis is carried out in a vertical gel between glass plates. It is not possible to cast extraction wells in a vertical gel because of the glass plates. Vertical gels are cast between glass plates to exclude oxygen during polymerization. To cast a horizontal PAGE gel, it was necessary to exclude oxygen during polymerization by other means. We carried out the polymerization in a bag filled with nitrogen gas (or argon) to exclude oxygen. The gel was cast with the two combs and electrophoresis was carried out per standard horizontal gel electrophoresis procedures.
Figure 5C shows a horizontal gel for SSG by co-polymerization and electrophoresis with electroelution. Lanes one through five (from top) are an aptamer pool. The pool was amplified using acrydite and fluorescein primers. The acrydite product was retained in the well. The fluorescein labeled product is clearly visible under blue LED illumination. We allowed the primer to run through the extraction wells and out the other side of the gel (see Figure 5D). The product bands could then be observed approaching the extraction wells under a blue light transilluminator in real time. When the product bands entered the wells, they were aspirated with a pipette. The process takes approximately 1 hour. Precipitation and recovery then proceeded as per published aptamer selection protocols. This alleviated the need for a separate strand separation step or an overnight extraction of the gel.
Conclusions
There are several methods by which one can isolate ssDNA from dsDNA. Methods for purifying ssDNA from PCR products include induced mobility shift(22), biotin-avidin bead immobilization(18), selective digestion with a DNase(23), and asymmetric addition of PCR primers(24). These methods vary in cost, purity and scalability. An induced mobility shift(22) in one primer can cause problems in amplification (e.g. PEG-modified primers may perform less well in PCR) enzymatic digestion with a DNAse(23) or chemical breakage of one strand will leave impurities (as well as introducing another step to the overall process). Asymmetric addition of PCR primers(24) will leave a significant quantity of dsDNA in the product and is highly prone to amplification errors. The most popular method is to extract the unwanted, biotinylated strand using avidin-coated microspheres(18). This method has several pitfalls. Unreacted primer occupies the avidin sites on the beads. The biotin-avidin interaction will break down at the melting temperature of long, double-stranded DNA(25). This contributes to significant dsDNA impurities. Bead based methods have significant costs: $1.8/nMol for avidin beads plus $.50/nMol for the biotin primer. SSG by co-polymerization and electrophoresis requires only acrydite primer which costs $.8 per nMol. The immobile phase, polyacrylamide, is very inexpensive and scalable. Electrophoretic mobility is a second dimension of purification inherent in the technique. Both the acrydite modified strand and any residual primer are removed from a PCR reaction in a single step.
We have shown that this can be used to purify a functional aptamer. It could also be used to purify an ssDNA pool in the course of DNA aptamer selection. It may also find application in generating single-stranded DNA for affinity probes or the backbone for DNA origami(3). PCR production of DNA has much higher sequence fidelity than chemical synthesis. In applications such as DNA computation/DNA circuitry(26), high sequence fidelity is critical(7). This technique may find application in these DNA nanotechnology applications as well.
Acknowledgments
This work was funded by the National Institutes of Health (EUREKA, 1-R01-GM094933), The Welch Foundation (F-1654), a National Security Science and Engineering Faculty Fellowship (FA9550-10-1-0169), and support from the National Institute of General Medical Sciences (P20-GM104420).
Footnotes
Competing interests statement
The authors have declared that no competing interests exist.
References
- 1.Cho EJ, Lee J-W, and Ellington AD. 2009. Applications of Aptamers as Sensors. Annu. Rev. Anal. Chem 2:241–264. [DOI] [PubMed] [Google Scholar]
- 2.Tang H, Deschner R, Allen P, Cho Y, Sermas P, M aurer A, Ellington AD, and Willson CG. 2012. Analysis of DNA-Guided Self-Assembly of Microspheres Using Imaging Flow Cytometry. J. Am. Chem. Soc 15245–15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothemund PWK 2006. Folding DNA to create nanoscale shapes and patterns. Nature 440:297–302. [DOI] [PubMed] [Google Scholar]
- 4.Allen PB, Arshad SA, Li B, Chen X, and Ellington A. 2012. DNA circuits as amplifiers for the detection of nucleic acids on a paperfluidic platform. Lab. Chip 72:2951–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iida K, and Nishimura I. 2002. Gene Expression Profiling by DNA Microarray Technology. Crit. Rev. Oral Biol. Med 73:35–50. [DOI] [PubMed] [Google Scholar]
- 6.Olah MJ, and Stefanovic D. 2011. Multivalent Random Walkers — A Model for Deoxyribozyme Walkers In DNA Computing and Molecular Programming, Cardelli L, and Shih W, eds. (Springer; Berlin Heidelberg: ), pp. 160–174. [Google Scholar]
- 7.Chen X, Briggs N, McLain JR, and Ellington AD. 2013. Stacking nonenzymatic circuits for high signal gain. Proc. Natl. Acad. Sci 770:5386–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson DG, Benders GA, Andrews-Pfannkoch C, Denisova EA, Baden-Tillson H, Zaveri J, Stockwell TB, Brownley A, et al. 2008. Complete Chemical Synthesis, Assembly, and Cloning of a Mycoplasma genitalium Genome. Science 379:1215–1220. [DOI] [PubMed] [Google Scholar]
- 9.Kenney M, Ray S, and Boles TC. 1998. Mutation typing using electrophoresis and gel-immobilized Acrydite probes. BioTechniques 25:516–521. [DOI] [PubMed] [Google Scholar]
- 10.Liedl T, Dietz H, Yurke B, and Simmel F. 2007. Controlled Trapping and Release of Quantum Dots in a DNA-Switchable Hydrogel. Small 3:1688–1693. [DOI] [PubMed] [Google Scholar]
- 11.MacLean JL, Morishita K, and Liu J. 2013. DNA stabilized silver nanoclusters for ratiometric and visual detection of Hg2+ and its immobilization in hydrogels. Biosens. Bioelectron 48:82–86. [DOI] [PubMed] [Google Scholar]
- 12.Soontornworajit B, Zhou J, Shaw MT, Fan T-H, and Wang Y. 2010. Hydrogel functionalization with DNA aptamers for sustained PDGF-BB release. Chem. Commun 46:1857–1859. [DOI] [PubMed] [Google Scholar]
- 13.Kenney M, Ray S, and Boles TC. 1998. Mutation typing using electrophoresis and gel-immobilized Acrydite probes. BioTechniques 25:516–521. [DOI] [PubMed] [Google Scholar]
- 14.M itra RD, and Church GM. 1999. In situ localized amplification and contact replication of many individual DNA molecules. Nucleic Acids Res. 27:e34–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, and Ruffner DE. 1996. Modified crush-and-soak method for recovering oligodeoxynucleotides from polyacrylamide gel. BioTechniques 21:820–822. [DOI] [PubMed] [Google Scholar]
- 16.Damase TR, Stephens D, Spencer A, and Allen PB. 2015. Open source and DIY hardware for DNA nanotechnology labs. J. Biol. Methods 2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang C, Li D, Zhang G, Li H, Shao N, Liang Z, Zhang L, Lu A, and Zhang G. 2015. Comparison of the methods for generating single-stranded DNA in SELEX. The Analyst 740:3439–3444. [DOI] [PubMed] [Google Scholar]
- 18.Kuo T-C 2005. Streamlined method for purifying single-stranded DNA from PCR products for frequent or high-throughput needs. BioTechniques 38:100, 702. [DOI] [PubMed] [Google Scholar]
- 19.Kirby R, Cho EJ, Gehrke B, Bayer T, Park YS, Neikirk DP, McDevitt JT, and Ellington AD. 2004. Aptamer-Based Sensor Arrays for the Detection and Quantitation of Proteins. Anal. Chem 76:4066–4075. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez MC, Kawde A-N, and Wang J. 2005. Aptamer biosensor for label-free impedance spectroscopy detection of proteins based on recognition-induced switching of the surface charge. Chem. Commun. Camb. Engl 4267–4269. [DOI] [PubMed] [Google Scholar]
- 21.Cheng AKH, Ge B, and Yu H-Z. 2007. Aptamer-based biosensors for label-free voltammetric detection of lysozyme. Anal. Chem 79:5158–5164. [DOI] [PubMed] [Google Scholar]
- 22.Pagratis NC 1996. Rapid Preparation of Single Stranded DNA from PCR Products by Streptavidin Induced Electrophoretic Mobility Shift. Nucleic Acids Res. 24:36452–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avci-Adali M, Paul A, Wilhelm N, Ziemer G, and Wendel HP. 2009. Upgrading SELEX Technology by Using Lambda Exonuclease Digestion for Single-Stranded DNA Generation. Molecules 15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gyllensten UB, and Erlich HA. 1988. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc. Natl. Acad. Sci. U. S. A 85:7652–7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmberg A, Blomstergren A, Nord O, Lukacs M, Lundeberg J, and Uhlén M. 2005. The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. ELECTROPHORESIS 26:501–510. [DOI] [PubMed] [Google Scholar]
- 26.Yin P, Choi HMT, Calvert CR, and Pierce NA. 2008. Programming biomolecular self-assembly pathways. Nature 451:318–322. [DOI] [PubMed] [Google Scholar]