Abstract
Background:
Occupational exposure to animal production is associated with chronic bronchitis symptoms; however, few studies consider associations with chronic obstructive pulmonary disease (COPD). We estimated associations between animal production activities and prevalence of self-reported COPD among farmers in the Agricultural Health Study.
Methods:
During a 2005–2010 interview, farmers self-reported information about: their operations (i.e., size, type, number of animals, insecticide use), respiratory symptoms, and COPD diagnoses (i.e., COPD, chronic bronchitis, emphysema). Operations were classified as small or medium/large based on regulatory definitions. Farmers were classified as having a COPD diagnosis, chronic bronchitis symptoms (cough and phlegm for ≥3 months during 2 consecutive years), or both. Polytomous logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI).
Results:
Of 22,491 participating farmers (median age: 59years), 922 (4%) reported a COPD diagnosis only, 254 (1%) reported a diagnosis and symptoms, and 962 (4%) reported symptoms only. Compared to raising no commercial animals, raising animals on a medium/large operation was positively associated with chronic bronchitis symptoms with (OR: 1.59; 95% CI: 1.16, 2.18) and withouta diagnosis (OR: 1.69; 95% CI: 1.42, 2.01). Ever use of multiple organophosphates, carbaryl, lindane, and permethrin were positively associated with chronic bronchitis symptoms.
Conclusion:
Animal production work, including insecticide use, was positively associated with chronic bronchitis symptoms; but not consistently with COPD diagnosis alone. Our results support the need for further investigation into the role of animal production-related exposures in the etiology of COPD and better respiratory protection for agricultural workers.
Keywords: Epidemiology, Occupational health, Environmental health, Pesticide
1. Introduction
Chronic obstructive pulmonary disease (COPD), a general term now used to refer to chronic bronchitis, emphysema, and other causes of airway obstruction, is an important cause of morbidity and mortality in the United States. COPD arises from an enhanced chronic inflammatory response in the airways to particles or gases (Maitra and Kumar, 2007). Although smoking is the most important risk factor for COPD, 15–20% of COPD in the United States may be related to occupational exposures, with this proportion being 30–48% for never smokers (Balmes et al., 2003; Trupin et al., 2003; Wurtz et al., 2015).
Elevated rates of COPD have been observed for farmers, especially those in the animal production industry (NIOSH, 2007). Workers involved in food animal production are exposed to respiratory irritants including organic dusts, gases and chemicals (ATS, 1998; Radon et al., 2002a; O’Shaughnessy et al., 2012; Senthilselvan et al., 2011). The concentration of irritants varies by characteristics of the production environment, with the highest concentrations often found in industrial animal production facilities where large numbers of animals are raised in confinement (ATS, 1998; Radon et al., 2002a; O’Shaughnessy et al., 2012; Senthilselvan et al., 2011). Organic dust and endotoxin levels in animal production facilities are associated with increased prevalence of COPD manifestations including chronic cough and phlegm, chronic bronchitis, and airway obstruction (ATS, 1998; Senthilselvan et al., 2011). Evidence also suggests a link between pesticide exposure and COPD, (de Jong et al., 2014; Hoppin, 2014; Hoppin et al., 2007; Sprince et al., 2000) including insecticides commonly used to control pests in the animal production environment (Hoppin et al., 2007).
Despite recognition of a link between exposures in the animal production environment and chronic bronchitis symptoms, assessment of the association between occupational exposure to animal production and manifestations of the category of disease referred to as COPD has been limited and is necessary to understand the potential burden of COPD in agricultural populations and relevant occupational exposures (Monso et al., 2004). To examine this, we estimated associations between occupational exposure to animals and types of insecticides potentially used in animal production, and self-reported chronic bronchitis symptoms and COPD diagnoses among farmers participating in the Agricultural Health Study (AHS).
2. Methods
2.1. Study population
The AHS enrolled 52,394 private pesticide applicators (mostly farmers), hereafter referred to as “farmers,” when they presented for a restricted use pesticide license in Iowa and North Carolina during 1993–1997 (Alavanja et al., 1996) (Fig. 1). This analysis is restricted to the 24,171 farmers who responded to the 2005–2010 follow-up interview, during which information about respiratory symptoms was solicited from all participants. Previous publications have described AHS participation over time (Hoppin et al., 2012; Rinsky et al., 2017; Montgomery et al., 2010), and the implications of restriction to the 2005–2010 follow-up interview participants (46% of farmers who enrolled) (Rinsky et al., 2017).
Fig. 1.
Participation in interviews and report of animal production and pesticide use information by phase in the Agricultural Health Study and the current analysis.
From the 2005–2010 population, we excluded 683 farmers because of missing COPD information and 997 farmers because of missing covariate data resulting in an analysis population of 22,491.
All farmers included in this analysis provided information on demographics, lifestyle, medical history and farming activities through an enrollment questionnaire (1993–1997). This analysis was approved by all relevant Institutional Review Boards. Participants indicated initial informed consent by completing the enrollment questionnaire. Questionnaires are available on the study web site (http://www.aghealth.nih.gov/collaboration/questionnaires.html).
2.2. Exposure assessment
We used self-reported information about raising animals and personal use of insecticides registered for use on or around animals as proxies for occupational exposure to the animal production environment. Fig. 1 illustrates information collected at each interview.
2.2.1. Animal production
At enrollment, farmers responded to the question “What are the major income producing crops and animals you are currently raising on your farm?” and “Last year, how many poultry/livestock were there on the farm?” Responses to the latter question were recorded in categories of the number of poultry and livestock. During the two follow-up interviews farmers were asked “What type of animals did you have” and “How many of each animal did you have” in the year prior to interview (1999–2003) and since the time of last interview (2005–2010). Differences in detail and reference points at each interview prohibited us from combining information across interviews; instead, we created variables indicating the presence and number of each animal type reported at each interview.
To characterize the scale of the operation, we applied US Environmental Protection Agency regulatory definitions for small, medium, and large concentrated animal feeding operations (CAFOs), which incorporate information about animal type and weight (U.S. EPA, 2012). Assuming animals were of mature weight, we categorized farmers into those working on: 1) an operation with no animals; 2) a small animal operation; or, 3) a medium/large animal operation. Small operations included those raising animals on pasture and possibly small industrial operations. Medium/large operations are likely to be industrial, raising animals in confinement, and, therefore, were considered as one category. We categorized operations based on each animal type separately and based on all animals on the property; results for the latter are presented here to account for production of multiple animal types on one property.
2.2.2. Insecticide use
We used responses to the question, “Have you applied insecticides to farm animals in the past 12 months?” asked at enrollment to assess general exposure to insecticides used on animals.
To assess specific insecticide use, we examined personal use of insecticides belonging to major insecticide classes formerly or currently registered for use on animals or in and around animal production facilities. We limited our analysis to insecticides used by at least 1% of the cohort. With these criteria, we evaluated ever use of 18 insecticides including nine organophosphates, one carbamate, one organochlorine, and seven pyrethroids. Twelve of the evaluated insecticides have been used in or around animal confinement areas, while the remaining six are members of the same insecticide classes but were not approved for animal use.
For insecticides asked about on the enrollment questionnaire, and based on personal use information provided at each interview (Supplementary Table 1), AHS researchers created lifetime personal ever and days of use variables for each pesticide at each interview as described previously (Hoppin et al., 2012). The lifetime exposure variables created following the 2005–2010 interview represent the most complete lifetime exposure variables available for this analysis. We categorized lifetime days of use into three categories (never users, users reporting ≤median days of use, users reporting > median days of use) based on the distribution among the study population.
2.3. Outcome assessment
During the 2005–2010 interview, farmers were asked, “Have you ever been diagnosed with” chronic bronchitis, emphysema, and COPD, in three separate questions (Supplementary Table S2). Farmers also reported whether they typically cough, bring up phlegm, and the duration of each of these symptoms. COPD encompasses multiple conditions with chronic cough and phlegm being the defining symptoms (ATS, 2004). Physician diagnosis of COPD can vary based on criteria used and requires the patient to present for care. To capture the potential variety of manifestations and severity in COPD among farmers, we used self-reported symptoms and diagnosis to define multiple disease categories, as has been done previously (ATS, 2004).
COPD diagnosis only: Physician’s diagnosis of COPD, chronic bronchitis, or emphysema; but, no report of symptoms meeting the classical definition of chronic bronchitis (cough and phlegm for ≥three months during two consecutive years (ATS, 2004)).
COPD-related diagnosis and chronic bronchitis symptoms: Physician’s diagnosis of COPD, chronic bronchitis, or emphysema; and, symptoms consistent with the classical definition of chronic bronchitis.
Chronic bronchitis symptoms only: Symptoms consistent with the classical definition of chronic bronchitis; but, no report of a physician’s diagnosis of COPD, chronic bronchitis or emphysema.
No COPD: Farmers who did not report a diagnosis or symptoms consistent with chronic bronchitis.
2.4. Statistical analysis
We evaluated prevalent COPD diagnosis and chronic bronchitis symptoms reported during the 2005–2010 interview. We were unable to evaluate disease incidence because diagnoses and symptoms were not collected from all farmers at enrollment, prohibiting our ability to confidently exclude prevalent cases and because the questions used to collect exposure information at each interview did not provide a clear temporal ordering of exposure and disease.
We examined the distribution of demographic and lifestyle characteristics reported at enrollment for all participants, and by disease status. We also examined the distribution of animal production and insecticide use at each interview overall and by disease status to identify temporal variation in animal production or insecticide use during the study period. Based on literature and directed acyclic graphs (DAG), (Greenland et al., 1999) we identified age, state, gender, education, and smoking as potential confounders of associations between animal production, insecticide use, and COPD outcomes. We saw no evidence of correlation among the insecticides to suggest potential confounding by correlated exposures.
To control for confounding we used stabilized inverse probability of exposure weights (IPEW), which create a “pseudo-population” where the distributions of confounding variables are similar across exposure groups. This is a form of direct standardization that results in no association between the exposure and confounders in the analysis population (Cole and Hernan, 2008). To derive exposure weights, we used linear, logistic, or polytomous logistic regression models for continuous, binary, and multi-level exposures, respectively, to estimate the predicted probability of exposure, conditional on the identified con- founders (categorized as shown in Table 1). We assigned each individual a weight equal to the inverse of the predicted probability that the person had his/her observed exposure. To stabilize the weights, we multiplied each weight by the marginal probability of the individual’s observed exposure status.
Table 1.
Distribution of demographic and lifestyle characteristics by COPD diagnosis and symptoms among 22,491 farmers participating in the 2005–2010 interview, Agricultural Health Study.
| No COPD | COPD diagnosis onlya | COPD diagnosis & chronic bronchitis symptomsb |
Chronic bronchitis symptoms onlyc |
|||||
|---|---|---|---|---|---|---|---|---|
| Participant characteristicsd | N = 20,353 | % | N= 922 | % | N= 254 | % | N= 962 | % |
| Age (2005–2010 interview) | ||||||||
| <50 years | 4540 | 22 | 69 | 7 | 13 | 5 | 195 | 20 |
| 50–59 years | 6295 | 31 | 177 | 19 | 49 | 19 | 295 | 31 |
| 60–69 years | 5090 | 25 | 286 | 31 | 72 | 28 | 224 | 23 |
| 70–79years | 3574 | 18 | 297 | 32 | 97 | 38 | 188 | 20 |
| 80+ years | 854 | 4 | 93 | 10 | 23 | 9 | 60 | 6 |
| State | ||||||||
| Iowa | 13,771 | 68 | 437 | 47 | 140 | 55 | 711 | 74 |
| North Carolina | 6582 | 32 | 485 | 53 | 114 | 45 | 251 | 26 |
| Gender | ||||||||
| Female | 565 | 3 | 33 | 4 | 7 | 3 | 16 | 2 |
| Male | 19,788 | 97 | 889 | 96 | 247 | 97 | 946 | 98 |
| Race/ethnicity | ||||||||
| White, non-Hispanic | 19,519 | 96 | 841 | 91 | 217 | 85 | 920 | 96 |
| Other | 477 | 2 | 26 | 3 | 11 | 4 | 11 | 1 |
| Missing | 357 | 55 | 26 | 31 | ||||
| Education | ||||||||
| < High school degree | 1441 | 7 | 138 | 15 | 49 | 19 | 77 | 8 |
| High school graduate/GED | 9330 | 46 | 448 | 49 | 129 | 51 | 458 | 48 |
| Some college | 5283 | 26 | 203 | 22 | 53 | 21 | 261 | 27 |
| ≥College graduate | 4299 | 21 | 133 | 14 | 23 | 9 | 166 | 17 |
| Marital status | ||||||||
| Married/living as married | 17,769 | 87 | 842 | 92 | 224 | 89 | 855 | 89 |
| Other | 2566 | 13 | 78 | 8 | 28 | 11 | 103 | 11 |
| Missing | 18 | 2 | 2 | 4 | ||||
| Smoking status | ||||||||
| Never | 11,721 | 58 | 278 | 30 | 51 | 20 | 438 | 46 |
| Former | 6381 | 31 | 396 | 43 | 113 | 44 | 288 | 30 |
| Current | 2251 | 11 | 248 | 27 | 90 | 35 | 236 | 25 |
| Alcohol consumptione | ||||||||
| None | 6733 | 34 | 370 | 42 | 90 | 38 | 251 | 27 |
| Light drinker | 12,562 | 64 | 492 | 58 | 139 | 59 | 650 | 70 |
| Heavy drinker | 308 | 2 | 11 | 1 | 8 | 3 | 26 | 3 |
| Missing | 750 | 49 | 17 | 35 | ||||
| Size of farm (acres) | ||||||||
| Didn’t work on farm/none | 633 | 3 | 43 | 5 | 13 | 6 | 34 | 4 |
| <5 | 711 | 4 | 49 | 6 | 15 | 7 | 35 | 4 |
| 5–49 | 1804 | 10 | 143 | 17 | 32 | 14 | 74 | 8 |
| 50–199 | 3460 | 18 | 185 | 23 | 42 | 19 | 157 | 17 |
| 200–499 | 5499 | 29 | 204 | 25 | 55 | 24 | 287 | 32 |
| 500–999 | 4310 | 23 | 120 | 15 | 41 | 18 | 204 | 22 |
| > 1000 | 2550 | 13 | 75 | 9 | 27 | 12 | 119 | 13 |
| Missing | 1386 | 103 | 29 | 52 | ||||
| Early life exposure to farm environment (2005–2010 interview) | ||||||||
| Yes | 18,203 | 90 | 838 | 91 | 231 | 91 | 884 | 92 |
| No | 2110 | 10 | 82 | 9 | 23 | 9 | 75 | 8 |
| Missing | 40 | 2 | 0 | 3 | ||||
| Ever received doctor diagnosis of asthma (2005–2010 interview) | ||||||||
| Yes | 1086 | 5 | 253 | 28 | 85 | 34 | 109 | 11 |
| No | 19,256 | 95 | 666 | 72 | 167 | 66 | 850 | 89 |
| Missing | 11 | 3 | 2 | 3 | ||||
Reported diagnosis of chronic bronchitis, emphysema or COPD without symptoms.
Chronic cough and phlegm for more than three months in at least two consecutive years and a diagnosis of chronic bronchitis, emphysema, or COPD.
Chronic cough and phlegm for ≥ three months during two consecutive years without a reported diagnosis of chronic bronchitis, emphysema or COPD.
All characteristics were reported at enrollment unless otherwise specified.
Heavy drinkers reported consuming five or more drinks on the same occasion on each of five or more days in the past 30 days, light drinkers reported consuming at least one drink on at least one day during the past 12months but did not qualify as a heavy drinker (NIAAA, 2015).
We applied IPEW to polytomous logistic regression models to estimate standardized prevalence odds ratios (ORs). Each model included the exposure as the only explanatory variable. To account for within-subject correlation induced by weighting, we used a robust variance estimator to estimate standard errors and 95% confidence intervals (CIs) (Cole and Hernan, 2008). We used previously described methods to evaluate the appropriateness of IPEW (Cole and Hernan, 2008). We present results estimated using untruncated IPEW because these weights demonstrated means close to one with few extreme values (Supplementary Table S3).
Although the number of farmers raising animals and actively using insecticides declined during the study period, these patterns did not differ by COPD status and closely aligned with aging of the cohort and temporal trends in animal production and insecticide use in the United States (US EPA, 2011; US EPA, 2000; Pew Commission on Industrial Farm Animal Production, 2008). Further, estimates of associations with COPD status were similar for exposure variables defined at each interview with the most complete and detailed information available from the 2005–2010 interview. Consequently, we present only associations between COPD status and animal production reported during the 2005–2010 interview and the lifetime insecticide use variables.
We evaluated smoking (never, former, current), early-life exposure to farm animals (yes, no), and state of residence (Iowa, North Carolina) as potential effect-measure modifiers. Associations did not differ across these variables; consequently, we present only overall results.
2.5. Sensitivity analyses
We conducted several sensitivity analyses. We excluded farmers < 40 years old at enrollment (N = 6310) because these individuals may require longer follow-up to observe COPD. We also excluded farmers reporting a physician’s diagnosis of asthma (N = 1533), as asthma symptoms may overlap with symptoms of chronic bronchitis (Gibson and Simpson, 2009) and have been found to be related to use of pesticides (Hoppin et al., 2006; Henneberger et al., 2014) and work with animals (Hoppin et al., 2006; Henneberger et al., 2014; Omland, 2002). Additionally, we excluded farmers suspected to have COPD at enrollment (N = 849) based on reported age at diagnosis or symptom onset. Results of these analyses did not alter conclusions and are not presented here. Finally, to determine if an independent effect of insecticides existed, we adjusted insecticide ever-use models for animal production variables. All statistical analyses were conducted in SAS v9.3 (Cary, NC).
3. Results
Of the 22,491 farmers, a majority were male (97%) and ranged in age at the 2005–2010 interview from 27 to 97 years old (median age: 59 years). Forty-four percent of farmers were ever smokers and 13% were current smokers. Among participating farmers, 922 (4%) reported a COPD diagnosis only, 254 (1%) reported both a COPD diagnosis and chronic bronchitis symptoms, and 962 (4%) reported chronic bronchitis symptoms only (Table 1). The median age of disease onset was 52 years old (range: 18–90); the median age of onset was 55 years for diagnosis and 49 years for symptoms. Current or former smoking were more common among those reporting a COPD diagnosis or chronic bronchitis symptoms.
3.1. Animal exposures
Overall, raising animals was not positively associated with having a COPD diagnosis without chronic bronchitis symptoms with the exception of raising sheep/goats; raising hogs was inversely associated with a COPD diagnosis (Table 2). However, farmers raising hogs had greater odds of chronic bronchitis symptoms with and without a COPD diagnosis while those raising poultry and beef cattle had greater odds of chronic bronchitis symptoms only compared to farmers raising no animals. Farmers raising dairy cattle had greater odds of diagnosis and symptoms; however, this estimate is based on a small number of cases raising dairy cattle.
Table 2.
Associations between raising animals and COPD diagnosis and chronic bronchitis symptoms among 22,491 farmers participating in the 2005–2010 interview, Agricultural Health Study.
| COPD diagnosis onlya | COPD diagnosis & chronic bronchitis symptomsb | Chronic bronchitis symptoms onlyc | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No COPD | Cases | ORd | 95% CI | Cases | ORd | 95% CI | Cases | ORd | 95% CI | |||||
| N = 20353 | % | N = 922 | % | N = 254 | % | N = 962 | % | |||||||
| Type of Animal Raised | ||||||||||||||
| Hogs | 3208 | 16 | 62 | 7 | 0.72 | 0.58, 0.88 | 24 | 9 | 1.41 | 1.05, 1.89 | 184 | 19 | 1.25 | 1.06, 1.47 |
| Poultry/Eggs | 982 | 5 | 38 | 4 | 0.96 | 0.70, 1.32 | 9 | 4 | 1.02 | 0.57, 1.81 | 54 | 6 | 1.29 | 0.98, 1.70 |
| Beef cattle | 6906 | 34 | 246 | 27 | 0.95 | 0.82, 1.09 | 75 | 30 | 1.07 | 0.83, 1.39 | 376 | 39 | 1.29 | 1.13, 1.47 |
| Dairy cattle | 816 | 4 | 21 | 2 | 0.92 | 0.65, 1.31 | 9 | 4 | 1.63 | 0.98, 2.70 | 32 | 3 | 0.88 | 0.62, 1.25 |
| Sheep/Goats | 703 | 3 | 31 | 3 | 1.26 | 0.91, 1.75 | 3 | 1 | -e | 35 | 4 | 1.04 | 0.87, 1.24 | |
OR = odds ratio.
CI = confidence interval.
Reported diagnosis of chronic bronchitis, emphysema or COPD without symptoms.
Chronic cough and phlegm for more than three months in at least two consecutive years and a diagnosis of chronic bronchitis, emphysema, or COPD.
Chronic cough and phlegm for ≥ three months during two consecutive years without a reported diagnosis of chronic bronchitis, emphysema or COPD.
ORs were estimated using IPEW to address confounding. Weights were estimated using age at the 2005–2010 interview, state, gender, smoking status, and education categorized as shown in Table 1.
OR not calculated because there were < 5 exposed cases.
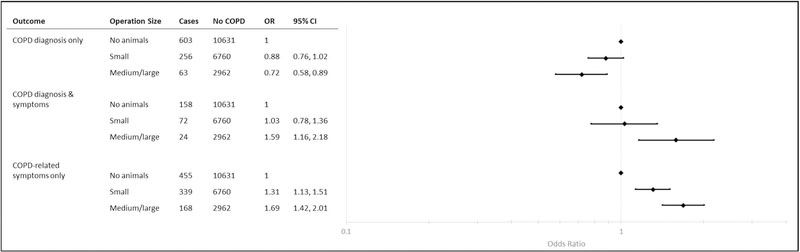
Reporting a COPD diagnosis without chronic bronchitis symptoms, was inversely associated with raising animals on a small and medium/ large operation. However, farmers working on small operations had 1.31 (95% CI: 1.13, 1.51) times the odds of COPD diagnosis and chronic bronchitis symptoms compared with farmers raising no animals. Working on a medium/large operation was positively associated with chronic bronchitis symptoms with (OR: 1.59; 95% CI: 1.16, 2.18) and without a diagnosis (OR: 1.69; 95% CI: 1.42, 2.01) (Fig. 2).
Fig. 2.
Associations between size of animal operationa and COPD status among 22,491 farmers participating in the 2005–2010 interview, Agricultural Health Study. aSize of animal production was determined using the number of animals produced on a farmer’s property and categorized using the regulatory definitions of CAFOs (U.S. EPA, 2012). Large and medium operations are likely to be raising animals in confinement.
3.2. Insecticide use
3.2.1. Insecticides ever approved for use on or around animals
Applying insecticides to animals or animal shelters in the year prior to enrollment was associated with a greater burden of chronic bronchitis symptoms with (OR: 1.39; 95% CI: 1.08, 1.78) and without (OR: 1.21; 95% CI: 1.05, 1.38) a COPD diagnosis (Table 3).
Table 3.
Associations between ever use of selected insecticides and COPD diagnosis and chronic bronchitis symptoms among 22,491 farmers participating in the 2005–2010 interview, Agricultural Health Study.
| No COPD | COPD diagnosis onlya | COPD diagnosis & chronic bronchitis symptomsb | Chronic bronchitis symptoms onlyc | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | ORd | 95% CI | Cases | ORd | 95% CI | Cases | ORd | 95% CI | ||||||
| Exposure | N = 20353 |
% | N = 922 |
% | N = 254 |
% | N = 962 |
% | ||||||
| Insecticide application to farm animals in past 12 monthse | 6524 | 32 | 221 | 24 | 1.03 | 0.90, 1.19 | 79 | 31 | 1.39 | 1.08, 1.78 | 348 | 36 | 1.21 | 1.05, 1.38 |
| Ever approved for use on or around animals | ||||||||||||||
| Organophosphates | ||||||||||||||
| Chlorpyrifos | 9488 | 47 | 375 | 41 | 0.96 | 0.84, 1.10 | 114 | 45 | 1.13 | 0.88, 1.44 | 475 | 49 | 1.12 | 0.98, 1.27 |
| Coumaphos | 1841 | 9 | 90 | 10 | 1.12 | 0.90, 1.40 | 28 | 11 | 1.30 | 0.88, 1.92 | 112 | 12 | 1.27 | 1.04, 1.57 |
| Diazinon | 7155 | 35 | 396 | 43 | 1.18 | 1.03, 1.35 | 110 | 43 | 1.42 | 1.10, 1.83 | 372 | 39 | 1.34 | 1.05, 1.72 |
| Dichlorvos | 2380 | 12 | 86 | 9 | 0.97 | 0.79, 1.20 | 34 | 13 | 1.26 | 0.88, 1.81 | 144 | 15 | 1.39 | 1.16, 1.66 |
| Malathion | 15135 | 74 | 673 | 73 | 1.03 | 0.88, 1.20 | 207 | 81 | 1.85 | 1.32, 2.60 | 749 | 78 | 1.22 | 1.05, 1.43 |
| Carbamates | ||||||||||||||
| Carbaryl | 12142 | 60 | 634 | 69 | 1.05 | 0.92, 1.20 | 180 | 71 | 1.28 | 0.99, 1.66 | 602 | 63 | 1.28 | 1.11, 1.47 |
| Pyrethroids | ||||||||||||||
| Permethrin (animals) | 3580 | 18 | 102 | 11 | 0.95 | 0.80, 1.14 | 41 | 16 | 1.35 | 1.00, 1.83 | 208 | 22 | 1.41 | 1.21, 1.65 |
| Pyrethrinsf | 318 | 2 | 11 | 1 | 0.81 | 0.44, 1.48 | 5 | 2 | 2.61 | 1.36, 5.02 | 15 | 2 | 1.05 | 0.62, 1.78 |
| Cyfluthrinf | 1648 | 8 | 33 | 4 | 0.79 | 0.60, 1.04 | 11 | 4 | 0.65 | 0.37, 1.15 | 84 | 9 | 1.11 | 0.88, 1.41 |
| Lambda Cyhalothrinf | 791 | 4 | 19 | 2 | 1.00 | 0.71, 1.41 | 1 | 0 | -d | 33 | 3 | 1.01 | 0.72, 1.42 | |
| Esfenvaleratef | 354 | 2 | 12 | 1 | 1.02 | 0.60, 1.71 | 3 | 1 | -g | 11 | 1 | 0.93 | 0.54, 1.58 | |
| Organochlorines | ||||||||||||||
| Lindane | 4762 | 23 | 218 | 24 | 1.07 | 0.91, 1.24 | 80 | 31 | 1.50 | 1.14, 1.96 | 304 | 31 | 1.48 | 1.29, 1.71 |
| Never approved for use on or around animals | ||||||||||||||
| Organophosphates | ||||||||||||||
| Parathion | 3258 | 16 | 203 | 22 | 1.06 | 0.89, 1.27 | 59 | 23 | 1.41 | 1.04, 1.90 | 176 | 18 | 1.29 | 1.10, 1.52 |
| Phorate | 7163 | 35 | 262 | 28 | 0.84 | 0.73, 0.97 | 100 | 39 | 1.24 | 0.96, 1.60 | 387 | 40 | 1.01 | 0.88, 1.16 |
| Fonofos | 4421 | 22 | 148 | 16 | 0.90 | 0.77, 1.06 | 62 | 24 | 1.20 | 0.90, 1.61 | 249 | 26 | 1.05 | 0.89, 1.22 |
| Terbufos | 7984 | 39 | 292 | 32 | 1.07 | 0.94, 1.22 | 99 | 39 | 1.22 | 0.95, 1.57 | 400 | 42 | 1.00 | 0.87, 1.14 |
| Pyrethroids | ||||||||||||||
| Permethrin (crops) | 3117 | 15 | 140 | 15 | 1.29 | 1.09, 1.52 | 33 | 13 | 1.18 | 0.85, 1.63 | 136 | 14 | 0.99 | 0.83, 1.19 |
| Tefluthrinf | 692 | 3 | 18 | 2 | 0.77 | 0.51, 1.16 | 6 | 2 | 1.08 | 0.56, 2.10 | 42 | 4 | 0.95 | 0.66, 1.37 |
OR = odds ratio.
CI = confidence interval.
Reported diagnosis of chronic bronchitis, emphysema or COPD without symptoms.
Chronic cough and phlegm for more than three months in at least two consecutive years and a diagnosis of chronic bronchitis, emphysema, or COPD.
Chronic cough and phlegm for ≥three months during two consecutive years without a reported diagnosis of chronic bronchitis, emphysema or COPD.
ORs were estimated using IPEW to address confounding. Weights were estimated using age at the 2005–2010 interview, state, gender, smoking status and education categorized as shown in Table 1.
Reported at enrollment.
Use information from the 2005–2010 interview only.
OR not calculated because there were < 5 exposed cases.
Of the 12 insecticides ever approved for use on animals, eight were associated with at least one COPD-related outcome. The only insecticide associated with all three COPD-related categories was diazinon. Otherwise, use of the following insecticides was associated with increased chronic bronchitis symptoms with and without a COPD diagnosis: coumaphos, diazinon, malathion, carbaryl, permethrin (animals), and lindane. Ever use of dichlorvos was associated with greater odds of chronic bronchitis symptoms alone. Personal use of pyrethrins was associated with greater odds of chronic bronchitis symptoms and a COPD diagnosis; however, this was based on a small number of cases reporting use. Results remained similar, though less precise, when controlling for types of animals raised (Supplementary Table S4).
When lifetime days of use were modeled, personal use of couma- phos, dichlorvos, and permethrin (animals) was associated with elevated odds of chronic bronchitis symptoms with or without a COPD diagnosis; for all, the largest OR was observed at the highest levels of use (Supplementary Table S5). Similar patterns remained when estimates were adjusted for types of animals raised (Supplementary Table S6).
3.2.2. Insecticides never approved for use on or around animals
Of the six insecticides never approved for use on animals, three were associated with at least one COPD-related outcome. Parathion was positively associated with chronic bronchitis symptoms with or without COPD diagnosis. Phorate was inversely associated with COPD diagnosis alone, while permethrin (for crops) was positively associated with COPD diagnosis in the absence of chronic bronchitis symptoms.
When lifetime days of use were considered, permethrin (crops) was associated with COPD diagnosis irrespective of chronic bronchitis symptoms (Supplementary Table S5). Similar patterns remained when estimates were adjusted for types of animals raised (Supplementary Table S6).
4. Discussion
Occupational exposure to animal production has been previously linked to COPD-related manifestations including short-term decline in pulmonary function (ATS, 1998; Monso et al., 2004; Omland, 2002), symptoms of respiratory irritation (ATS, 1998; Senthilselvan et al., 2007), and increased risk of cough and phlegm (ATS, 1998; Omland, 2002). Among AHS participants, we observed evidence to support a link between animal production work, including exposure to animals and insecticide use, and a greater burden of chronic bronchitis symptoms whether a COPD diagnosis was reported or not; however, we observed only limited evidence of associations between animal production work and COPD diagnoses independent of chronic bronchitis symptoms.
Raising hogs, poultry, and beef cattle were associated with a greater burden of chronic bronchitis symptoms. Farmers producing animals on medium/large operations likely using industrial production methods had approximately 60% greater odds of chronic bronchitis symptoms irrespective of a COPD diagnosis. Researchers have previously found greater prevalence of chronic bronchitis (ATS, 1998; Melbostad et al., 1997; Mauny et al., 1997; Chaudemanche et al., 2003; Gainet et al., 2007) and cough and phlegm (ATS, 1998; Senthilselvan et al., 2007; Chaudemanche et al., 2003) among farmers and farm workers involved in production of hogs, poultry, and cattle (mostly dairy), mainly in industrial settings. The respiratory effects of occupational exposure to beef cattle production have not been well studied but researchers have documented similar levels of organic dust and endotoxin in cattle feedlots as levels found in hog confinement facilities (Von Essen et al., 2010; McEachran et al., 2015).
The observed associations between personal use of insecticides and chronic bronchitis symptoms support mounting evidence that use of insecticides may either play a role in the etiology or exacerbation of COPD-related manifestations or serve as a marker of other relevant exposures. General use of pesticides has been linked with chronic bronchitis in a case-control study in Lebanon (Salameh et al., 2006) and reduced pulmonary function and higher prevalence of airway obstruction among two population-based cohorts in The Netherlands (de Jong et al., 2014). Among rural residents of Beijing, China, use of insecticides was associated with twice the odds of cough and phlegm production (Zhang et al., 2002). Similar to our results for farmers who reported directly applying insecticides to farm animals, farmers in the Iowa Farm Family Health and Hazard Surveillance Project who applied insecticides to livestock had twice the odds of phlegm than farmers who did not apply insecticides, but who raised livestock (Sprince et al., 2000).
Most previous work investigating COPD-related outcomes and animal production lacks information on type, frequency and duration of pesticides used (Hoppin, 2014). Using the AHS, we were able to quantify associations between COPD-related outcomes and specific insecticides. Use of insecticides ever approved for use on or around animals demonstrated multiple positive associations with chronic bronchitis symptoms. However, many of these insecticides are also used for purposes other than treating animals or their enclosures (North Carolina Cooperative Extension Service, 2014). We also found that ever use of several of the insecticides never approved for use on or around animals were associated with greater odds of chronic bronchitis symptoms, COPD diagnosis, or both. Therefore, the associations reported here cannot be attributed solely to animal production. Additional work with detailed personal exposure information is necessary to better elucidate the interrelationships between animal exposures and insecticide use on COPD.
With the exception of permethrin, pyrethroids were not consistently associated with increased odds of chronic bronchitis symptoms or COPD diagnosis. Use of pyrethroids has increased since the 1970s while the use of the more acutely toxic organophosphates has declined (US EPA, 2013). Because pyrethroid use is more common, the potential respiratory health effects of exposure should be examined.
Associations were not consistently observed between raising animals, insecticide use and COPD diagnosis independent of chronic bronchitis symptoms. It is possible that the biological response to animal production exposures may manifest in chronic bronchitis symptoms but not lead to a COPD diagnosis. This hypothesis aligns with what has been observed for other dusty trades (Becklake, 1985). Limitations in our exposure assessment may also explain some of the discrepancy. Presence of animals on a property is a crude proxy for personal exposure to respiratory irritants in animal production. Additional information is necessary to understand each farmers’ potential exposures including lifetime duration and intensity of animal production work and details about the production environment including age of animals, confinement practices, waste management systems, and insecticide use. Future work would be strengthened by including this information and objective measures of irritants, and considering the interaction between such exposures. It is also possible that exposure misclassification operated differently in analyses of COPD-related diagnosis and symptoms. For example, if farmers with a COPD diagnosis limited personal exposure to animal production, a healthy-worker survivor effect would be stronger in analysis of diagnosis than symptoms (Chenard et al., 2007; Radon et al., 2002b; Thelin and Hoglund, 1994).
To capture the full burden of COPD-related outcomes, we considered both diagnoses and symptoms consistent with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for COPD, which includes symptoms as a key aspect of defining disease severity (GOLD, 2011). The prevalence of COPD-related diagnosis among AHS farmers was similar to the age-adjusted prevalence in the general population (6%) (Iowa: 5%; North Carolina: 7%), (Kosacz et al., 2012) despite a lower prevalence of smoking compared to the general population (CDC, 2010). The prevalence of chronic bronchitis symptoms was lower than estimates of chronic bronchitis symptoms from other US studies of animal confinement workers (7–25%) (ATS, 1998; Omland, 2002). Varying approaches to measure COPD burden may explain some of these differences. For example, when spirometry is used, COPD prevalence among individuals involved in animal production (17%) (Monso et al., 2004) and the general public (10–20%) (Tilert et al., 2013) is consistently greater than what we found here. This is not surprising, as reliance on self-report is known to result in an underestimate of COPD compared with spirometry (Trupin et al., 2003; Mannino et al., 2002; Halbert et al., 2006; Barr et al., 2002). Although spirometry or clinical confirmation of case status is useful in confirming COPD cases, spirometry also identifies those with subclinical obstruction. Reliance on self-reported diagnosis and symptoms does not exclude those who have not accessed care to receive spirometry or a diagnosis and may be more representative of clinically relevant disease; especially in populations with limited access to care (Barr et al., 2002).
Our analysis was restricted to farmers responding to the 2005–2010 interview. Restriction was necessary because COPD-related outcomes were not available for all participants prior to this interview. We previously examined the impact of non-participation in the 2005–2010 interview on estimation of exposure-disease associations (Rinsky et al., 2017). The previous analysis suggested that animal production, use of insecticides, and COPD-related outcomes are not strongly associated with response to the 2005–2010 interview and that effect estimates generated from 2005–2010 respondents should be similar to those that would have been generated from the full cohort had complete information been available (Rinsky et al., 2017; Daniel et al., 2012; Westreich, 2012).
In this analysis, we were unable to establish the temporal ordering of exposure and COPD outcomes, and therefore could not directly assess incident disease. Our differential findings for those with COPD diagnoses without chronic bronchitis symptoms, suggest that diagnosed workers may be more likely to have changed their agricultural practices. Future work, with detailed information about timing of exposure and disease onset is needed to directly assess COPD risk in populations working in animal production. The AHS allows researchers to evaluate the potential health risks of agricultural exposures by comparing exposed farmers to unexposed farmers rather than the general population. However, AHS participants were recruited on the basis of being private pesticide applicators and therefore, few participants are truly unexposed to all pesticides. When multiple pesticides are independently associated with disease risk, having no clear unexposed group limits the ability to observe associations (Rose, 1985). Because the AHS includes only pesticide applicator farm owners and operators, these results may not be generalizable to all animal operation operators, or to farm workers who may experience a different intensity and duration of exposure to the animal production environment.
Several design elements of the AHS strengthened this analysis. The large sample size and extensive information about demographics and lifestyle factors allowed for confounding control and exploration of effect modification by important covariates. The ability to consider modification by smoking revealed that the observed associations were present among never, former, and current smokers alike. Because the AHS continues to follow farmers who change farming activities or even cease farm work, concerns common to cross-sectional studies regarding exclusion of farmers who have left work are reduced. In addition, although there are limitations to our exposure assessment, the AHS provides one of the most detailed assessments of personal insecticide use available.
These results provide more evidence that animal production work and personal use of specific insecticides are associated with a greater burden of chronic bronchitis symptoms. The results support the call for further research into the role of occupational exposures, specifically animal production work, in the etiology of COPD manifestations and the need to provide better protections for farmers and agricultural workers. This analysis also demonstrates the need for prospective studies of farmers and farm workers in the United States to be conducted with improved information on personal exposure to animal production and other farming activities. Addressing these limitations will allow the scientific and farming communities to better understand effects of exposure to animal production and insecticide use on respiratory health.
Supplementary Material
Acknowledgements
The authors thank the participants of the Agricultural Health Study for their continued support and participation and Stuart Long for assistance with data analysis. The authors also thank Dr. Steve Wing for his guidance in study design, interpretation of results and drafting of this manuscript.
JLR was supported by the National Institute for Occupational Safety and Health award no. T42OH00867302. This work was supported [in part] by the intramural research program of the National Institutes of Health, the National Institute of Environmental Health Sciences (Z01-ES049030) and the National Cancer Institute (Z01-CP010119). This analysis used AHS data release P1REL0907.00.00 and REL0905.00. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health or the National Institutes for Health.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2019.02.049.
References
- Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, Pennybacker M, Rothman N, Dosemeci M, Bond AE, Blair A, 1996. The agricultural health study. Environ. Health Perspect. 104 (4), 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society, 1998. Official Conference Report of the American Thoracic Society. Respiratory health hazards in agriculture. Am. J. Respir. Crit. Care Med. 158 (5 Pt 2), S1–S76. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society, 2004. Standards for the Diagnosis and Management of Patients With COPD. [Google Scholar]
- Balmes J, Becklake M, Blanc P, Henneberger P, Kreiss K, Mapp C, Milton D, Schwartz D, Toren K, Viegi G, Environmental and Occupational Health Assembly, ATS, 2003. American Thoracic Society statement: occupational contribution to the burden of airway disease. Am. J. Respir. Crit. Care Med. 167 (5), 787–797. [DOI] [PubMed] [Google Scholar]
- Barr RG, Herbstman J, Speizer FE, Camargo CA Jr., 2002. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am. J. Epidemiol. 155 (10), 965–971. [DOI] [PubMed] [Google Scholar]
- Becklake MR, 1985. Chronic airflow limitation: its relationship to work in dusty occupations. Chest 88 (4), 608–617. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2010. State-specific prevalence of cigarette smoking and smokeless tobacco use among adults—United States, 2009. Morb. Mortal. Wkly Rep. 59 (43), 1400–1406. [PubMed] [Google Scholar]
- Chaudemanche H, Monnet E, Westeel V, Pernet D, Dubiez A, Perrin C, Laplante JJ, Depierre A, Dalphin JC, 2003. Respiratory status in dairy farmers in France; cross sectional and longitudinal analyses. Occup. Environ. Med. 60 (11), 858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenard L, Senthilselvan A, Grover VK, Kirychuk SP, Lawson JA, Hurst TS, Dosman JA, 2007. Lung function and farm size predict healthy worker effect in swine farmers. Chest 131 (1), 245–254. [DOI] [PubMed] [Google Scholar]
- Cole SR, Hernan MA, 2008. Constructing inverse probability weights for marginal structural models. Am. J. Epidemiol. 168 (6), 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel RM, Kenward MG, Cousens SN, De Stavola BL, 2012. Using causal diagrams to guide analysis in missing data problems. Stat. Methods Med. Res. 21 (3), 243–256. [DOI] [PubMed] [Google Scholar]
- de Jong K, Boezen HM, Kromhout H, Vermeulen R, Postma DS, Vonk JM, 2014. Pesticides and other occupational exposures are associated with airway obstruction: the LifeLines cohort study. Occup. Environ. Med. 71 (2), 88–96. [DOI] [PubMed] [Google Scholar]
- Kosacz NM, Punturieri A, Croxton TL, Ndenecho MN, Kiley JP, Weinmann GG, Wheaton AG, Ford ES, Presley-Cantrell LR, Croft JB, Giles WH, 2012. Chronic obstructive pulmonary disease among adults - United States, 2011. MMWR Morb. Mortal. Wkly Rep. 61 (46), 938–943. [PubMed] [Google Scholar]
- Gainet M, Thaon I, Westeel V, Chaudemanche H, Venier AG, Dubiez A, Laplante JJ, Dalphin JC, 2007. Twelve-year longitudinal study of respiratory status in dairy farmers. Eur. Respir. J. 30 (1), 97–103. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Simpson JL, 2009. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 64 (8), 728–735. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease, 2011. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Revised 2011. [Google Scholar]
- Greenland S, Pearl J, Robins JM, 1999. Causal diagrams for epidemiologic research. Epidemiology 10 (1), 37–48. [PubMed] [Google Scholar]
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM, 2006. Global burden of COPD: systematic review and meta-analysis. Eur. Respir. J. 28 (3), 523–532. [DOI] [PubMed] [Google Scholar]
- Henneberger PK, Liang X, London SJ, Umbach DM, Sandler DP, Hoppin JA, 2014. Exacerbation of symptoms in agricultural pesticide applicators with asthma. Int. Arch. Occup. Environ. Health 87 (4), 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, 2014. Pesticides and respiratory health: where do we go from here? Occup. Environ. Med. 71 (2), 80. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Umbach DM, London SJ, Lynch CF, Alavanja MC, Sandler DP, 2006. Pesticides and adult respiratory outcomes in the agricultural health study. Ann. N. Y. Acad. Sci. 1076, 343–354. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Valcin M, Henneberger PK, Kullman GJ, Umbach DM, London SJ, Alavanja MC, Sandler DP, 2007. Pesticide use and chronic bronchitis among farmers in the agricultural health study. Am. J. Ind. Med. 50 (12), 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Long S, Umbach DM, Lubin JH, Starks SE, Gerr F, Thomas K, Hines CJ, Weichenthal S, Kamel F, Koutros S, Alavanja M, Beane Freeman LE, Sandler DP, 2012. Lifetime organophosphorous insecticide use among private pesticide applicators in the Agricultural Health Study. J. Expo. Sci. Environ. Epidemiol. 22 (6), 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A, Kumar K, 2007. Chapter 12. The Lung In: Aster JC, Robbins SL, Kumar V, Abbas AK (Eds.), Robbins Basic Pathology, 9th Edition Elsevier/Saunders, Philadelphia, PA, pp. 459–515. [Google Scholar]
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC, 2002. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. Morb. Mortal. Wkly. Rep. Surveill. Summ. 51 (6), 1–16. [PubMed] [Google Scholar]
- Mauny F, Polio JC, Monnet E, Pernet D, Laplante JJ, Depierre A, Dalphin JC, 1997. Longitudinal study of respiratory health in dairy farmers: influence of artificial barn fodder drying. Eur. Respir. J. 10 (11), 2522–2528. [DOI] [PubMed] [Google Scholar]
- McEachran A, Blackwell B, Hanson J, Wooten K, Mayer G, Cox S, Smith P, 2015. Antibiotics, bacteria, and antibiotic resistance genes: aerial transport from cattle feed yards via particulate matter. Environ. Health Perspect. 123 (4), 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melbostad E, Wijnand E, Magnus P, 1997. Chronic bronchitis in farmers. Scand. J. Work Environ. Health 23 (4), 271–280. [DOI] [PubMed] [Google Scholar]
- Monso E, Riu E, Radon K, Magarolas R, Danuser B, Iversen M, Morera J, Nowak D, 2004. Chronic obstructive pulmonary disease in never-smoking animal farmers working inside confinement buildings. Am. J. Ind. Med. 46 (4), 357–362. [DOI] [PubMed] [Google Scholar]
- Montgomery MP, Kamel F, Hoppin JA, Beane Freeman LE, Alavanja MC, Sandler DP, 2010. Effects of self-reported health conditions and pesticide exposures on probability of follow-up in a prospective cohort study. Am. J. Ind. Med. 53 (5), 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2015. Drinking levels defined Overview of Alcohol Consumption [cited 2015 February 18]. Available from: http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- National Institute for Occupational Safety and Health, 2007. Respiratory Disease in Agricultural Workers: Mortality and Morbidity Statistics. Accessed at: https://www.cdc.gov/niosh/docs/2007-106/pdfs/2007-106.pdf?id=10.26616/NI0SHPUB2007106. [DOI] [PubMed]
- North Carolina Cooperative Extension Service, 2014. North Carolina Agricultural Chemicals Manual. College of Agriculture and Life Sciences, North Carolina State University, Raleigh, N.C, pp. 67–180. [Google Scholar]
- Omland O, 2002. Exposure and respiratory health in farming in temperate zones—a review of the literature. Ann. Agric. Environ. Med. 9 (2), 119–136. [PubMed] [Google Scholar]
- O’Shaughnessy P, Peters T, Donham K, Taylor C, Altmaier R, Kelly K, 2012. Assessment of swine worker exposures to dust and endotoxin during hog load-out and power washing. The Annals of Occupational Hygiene 56 (7), 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Commission on Industrial Farm Animal Production, 2008. Putting Meat on the Table: Industrial Farm Animal Production in America. Pew Charitable Trusts and Johns Hopkins Bloomberg School of Public Health. Accessed at. https://www.pewtrusts.org/~/media/assets/2008/pcifap_exec-summary.pdf. [Google Scholar]
- Radon K, Danuser B, Iversen M, Monso E, Weber C, Hartung J, Donham K, Palmgren U, Nowak D, 2002a. Air contaminants in different European farming environments. Annals of Agricultural and Environmental Medicine 9 (1), 41–48. [PubMed] [Google Scholar]
- Radon K, Goldberg M, Becklake M, 2002b. Healthy worker effect in cohort studies on chronic bronchitis. Scand. J. Work Environ. Health 28 (5), 328–332. [DOI] [PubMed] [Google Scholar]
- Rinsky JL, Richardson DB, Wing S, Beard JD, Alavanja M, Beane Freeman LE, Chen H, Henneberger PK, Kamel F, Sandler DP, Hoppin JA, 2017. Non-response to a study follow-up interview does not always result in biased effect estimates: an example from the Agricultural Health Study. Am. J. Epidemiol. 186 (4), 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, 1985. Sick individuals and sick populations. Int. J. Epidemiol. 14 (1), 32–38. [DOI] [PubMed] [Google Scholar]
- Salameh PR, Waked M, Baldi I, Brochard P, Saleh BA, 2006. Chronic bronchitis and pesticide exposure: a case-control study in Lebanon. Eur. J. Epidemiol. 21 (9), 681–688. [DOI] [PubMed] [Google Scholar]
- Senthilselvan A, Chenard L, Ulmer K, Gibson-Burlinguette N, Leuschen C, Dosman JA, 2007. Excess respiratory symptoms in full-time male and female workers in large-scale swine operations. Chest 131 (4), 1197–1204. [DOI] [PubMed] [Google Scholar]
- Senthilselvan A, Beach J, Feddes J, Cherry N, Wenger I, 2011. A prospective evaluation of air quality and workers’ health in broiler and layer operations. Occup. Environ. Med. 68 (2), 102–107. [DOI] [PubMed] [Google Scholar]
- Sprince NL, Lewis MQ, Whitten PS, Reynolds SJ, Zwerling C, 2000. Respiratory symptoms: associations with pesticides, silos, and animal confinement in the Iowa Farm Family Health and Hazard Surveillance project. Am. J. Ind. Med. 38 (4), 455–462. [DOI] [PubMed] [Google Scholar]
- Thelin A, Hoglund S, 1994. Change of occupation and retirement among Swedish farmers and farm workers in relation to those in other occupations. A study of “elimination” from farming during the period 1970–1988. Soc. Sci. Med. 38 (1), 147–151. [DOI] [PubMed] [Google Scholar]
- Tilert T, Dillon C, Paulose-Ram R, Hnizdo E, Doney B, 2013. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Respir. Res. 14, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupin L, Earnest G, San Pedro M, Balmes JR, Eisner MD, Yelin E, Katz PP, Blanc PD, 2003. The occupational burden of chronic obstructive pulmonary disease. Eur. Respir. J. 22 (3), 462–469. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency, 2000. Profile of Agricultural Livestock Production Industry. Washington DC.
- U.S. Environmental Protection Agency, 2011. Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates. Washington, D.C. Accessed at. https://www.epa.gov/sites/production/files/2015-10/documents/market_estimates2007.pdf.
- U.S. Environmental Protection Agency, 2012. Chapter 2. AFOs and CAFOs.
- U.S. Environmental Protection Agency, 2013. Pyrethroids and Pyrethrins. Reevaluation: Review of Registered Pesticides. August 5, 2014, [cited 2015 January 28]. Available from: http://www.epa.gov/oppsrrd1/reevaluation/pyrethroids-pyrethrins.html.
- Von Essen S, Moore G, Gibbs S, Larson KL, 2010. Respiratory issues in beef and pork production: recommendations from an expert panel. J. Agromedicine 15 (3), 216–225. [DOI] [PubMed] [Google Scholar]
- Westreich D, 2012. Berkson’s bias, selection bias, and missing data. Epidemiology 23 (1), 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz ET, Schlunssen V, Malling TH, Hansen JG, Omland O, 2015. Occupational chronic obstructive pulmonary disease in a Danish population-based study. COPD 12 (4), 435–443. [DOI] [PubMed] [Google Scholar]
- Zhang LX, Enarson DA, He GX, Li B, Chan-Yeung M, 2002. Occupational and environmental risk factors for respiratory symptoms in rural Beijing, China. Eur. Respir. J. 20 (6), 1525–1531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.