Abstract
Background:
The Centers for Medicare and Medicaid Services (CMS) cover intensive behavioral therapy (IBT) for obesity. The efficacy, however, of CMS’ specific approach has never been evaluated in a randomized trial, as described here. The 1-year trial also assessed whether the addition to IBT of liraglutide 3.0 mg would significantly increase weight loss, and whether the provision of meal replacements would add further benefit.
Methods:
150 adults with obesity were randomized to: IBT-alone, providing 21 counseling visits; IBT combined with liraglutide (IBT-liraglutide); or IBT-liraglutide, combined for 12 weeks, with a 1000–1200 kcal/d meal-replacement diet (Multi-component). All participants received weekly IBT visits in month 1, every-other-week visits in months 2–6, and monthly sessions thereafter.
Results:
91% of participants completed 1 year, at which time mean (±SEM) losses for IBT-alone, IBT-liraglutide, and Muti-component participants were 6.1±1.3%, 11.5±1.3%, and 11.8±1.3% of baseline weight, respectively. Fully 44.0%, 70.0%, and 74.0% of these participants lost ≥5% of weight, respectively. The liraglutide-treated groups were superior to IBT-alone on both outcomes. Weight loss in all three groups was associated with clinically meaningful improvements in cardio-metabolic risk factors.
Conclusions:
The findings demonstrate the efficacy of IBT for obesity and the potential benefit of adding pharmacotherapy to this approach.
Keywords: weight management, lifestyle modification, pharmacologic therapy
INTRODUCTION
Two non-surgical interventions reliably induce a loss of 5–10% of initial body weight in persons with overweight or obesity (1). The first is a program of high intensity lifestyle modification (i.e., diet, physical activity, and behavior therapy), delivered in 14 or more counseling contacts in 6 months (1,2). This is the frequency of individual or group counseling recommended by the Guidelines for Management of Overweight and Obesity in Adults (2). It also is the frequency of brief (15 minute), individual counseling covered by the Centers for Medicare and Medicaid Services (CMS) (3). The U.S. Preventive Services Task Force recently reaffirmed its recommendation that clinicians screen all adults for obesity and offer those affected intensive behavioral counseling (4–6).
Medications for chronic weight management offer a second option for inducing a 5% or greater loss (7,8). The five medications currently approved by the Food and Drug Administration (FDA) are recommended as an adjunct to diet and exercise counseling and have been shown to increase mean 1-year weight losses (compared with the same counseling with placebo) by an average of 2.6 kg to 8.8 kg, depending on the medication used (9). In industry-sponsored trials, weight loss medications typically have been combined with moderate intensity lifestyle counseling (e.g., approximately monthly visits), potentially because of concerns that more intensive counseling could mask the comparative effects of medication (10). However, studies have shown that adding weight loss medication to high-intensity lifestyle modification produces mean losses that are approximately equal to the sum of the two separate interventions, suggesting additive benefits (11,12).
The present randomized controlled trial tested a treatment model for primary care practitioners (PCPs), including physicians and nurse practitioners, to provide intensive behavioral therapy (IBT) on the schedule covered by CMS -- weekly, brief in-person lifestyle counseling visits the first month, followed by every-other-week visits the next 5 months, approximating 14–15 contacts over 6 months (3). [This number of counseling visits is similar to that provided by registered dietitians in the first 6 months of the Diabetes Prevention Program (13).] Patients who lose ≥3 kg at month 6 are eligible for additional monthly visits through month 12. The specific schedule and length (15 minutes) of counseling visits proposed by CMS has never been tested in a randomized controlled trial in which PCPs provided IBT, as required by CMS for coverage.
Mean 1-year weight losses achieved with this approach were compared with those of two other interventions that included the same background of IBT, provided by the same PCPs. Participants in a second group received IBT combined with liraglutide 3.0 mg/d, a glucagon-like peptide-1 (GLP-1) receptor agonist approved for chronic weight management (7,14,15). A meta-analysis found that the addition of liraglutide 3.0 mg/day to approximately monthly lifestyle counseling increased weight loss by approximately 5.2 kg compared to the same counseling with placebo (9). Based on findings of the Diabetes Prevention Program (DPP), we anticipated that participants in the present study who received IBT alone would lose a mean of 5% of baseline weight at 1 year, which would be approximately doubled by the addition of liraglutide 3.0 mg/day (9,13). Participants in a third group received IBT, liraglutide, and the addition, for 12 weeks, of a portion-controlled diet that provided 1000–1200 kcal/d. Meal replacements - including liquid shakes, meal bars, and prepared entrees – increase weight loss by approximately 3–5% in 12 weeks, compared with consumption of an isocaloric diet comprised of conventional foods (16,17). This study assessed whether the provision of a portion-controlled diet would increase weight loss further when added to IBT plus liraglutide.
METHODS
Trial Design and Setting
This was a single-site, open-label, parallel-group design randomized trial, conducted at an academic medical center, whose institutional review board approved the study protocol (ClinicalTrials.gov number, ). The trial was supported by an investigator-initiated study award from Novo Nordisk. The company had no role in the design, execution, analysis, or reporting of the study, which was conceived by the first author. The last author analyzed the data, the first author wrote the initial draft of the manuscript, and all authors contributed to study implementation and the final draft. We used an open-label design to test IBT as it is delivered in clinical practice, without placebo. In addition, the efficacy of liraglutide 3.0 mg/day, compared with placebo, has been demonstrated in numerous double-blind, randomized trials (15,18,19), reducing the need for another such study.
Participants
Eligibility criteria included: ages 21–70 years; body mass index (BMI) of 30–55 kg/m2; prior lifetime weight-loss effort with diet and exercise (before considering anti-obesity medication) (20); and agreement to participate for 1 year. Exclusion criteria included: personal or family history of medullary thyroid cancer or multiple endocrine neoplasia syndrome; types 1 or 2 diabetes; renal, hepatic, or recent cardiovascular disease; blood pressure ≥160/100 mm Hg; medications that substantially affect body weight (e.g., corticosteroids); substance abuse; current major depression, suicidal ideation, or history of suicide attempts; bariatric surgery; use of weight loss medications or products, as well as weight loss ≥ 4.5 kg in past 3 months; and pregnancy/lactation. Anti-depressant medications were permitted, except for those associated with marked weight gain (e.g., paroxetine) or loss (e.g., bupropion).
Procedures
Participants were recruited by print and radio announcements, and referrals from the medical center’s affiliated primary care practices. Applicants completed a phone screen with a research coordinator. Those who appeared eligible completed an in-person screening visit with a psychologist, who fully described the trial’s nature and requirements, obtained applicants’ written informed consent, and assessed their eating and physical activity (21), as well as mood (22). Eligible participants next met with a study physician or nurse practitioner (NP) who completed a medical history, physical examination, electrocardiogram, and blood draw. Participants were informed of laboratory results within 72 hours and, if still eligible, were scheduled for their randomization visit.
Randomization
Participants were randomly assigned to interventions, in equal numbers, using a computer-generated algorithm, operated by the study statistician (JST). Randomization used varying block sizes (i.e., 3, 6, or 9). The first randomization visit was on September 21, 2016 and the last outcome assessment was completed on May 23, 2018.
Interventions: Common Components
Participants in all three groups received the same 21 sessions of IBT, delivered on the schedule recommended by CMS: 4 initial weekly visits, followed by 10 every-other-week sessions (through month 6), followed by 7 additional visits, delivered every 4 weeks, through month 12. Departing from the CMS protocol, all participants were provided counseling in the second 6 months, regardless of whether they had lost ≥3 kg at month 6. (This was done principally for statistical purposes, to maintain an approximately equal number of participants in the three groups at the primary outcome assessment at month 12. Counseling sessions lasted 15 minutes and were delivered following a detailed protocol (23), adapted from the DPP (13). Participants who weighed <113.6 kg (250 lb) were prescribed a diet of 1200–1499 kcal/d, comprised of conventional foods, with approximately 15–20% kcal from protein, 20–35% from fat, and the remainder from carbohydrate. Those ≥ 113.6 kg were prescribed 1500–1800 kcal/d. Participants were instructed to record their food and calorie intake daily, using apps (e.g., MyFitnessPal) or paper diaries (24). They were provided lists of breakfast, lunch, and dinner options (of conventional foods) to be used, as in prior studies (13,25), if they had trouble selecting their meals. Participants were instructed to engage in low-to-moderate intensity physical activity (principally walking) 5 days/week, gradually building to ≥180 minutes/week by week 24 (24). This increased to ≥225 minutes/week from weeks 25–52, consistent with targets for weight loss maintenance (25). Treatment sessions included examining participants’ weight change since the last visit, reviewing calorie intake and physical activity for the most recent week, and discussing a new topic from the behavior-change curriculum (23). All participants also had seven brief (5 minutes) medical visits over the year (i.e., weeks 1, 4, 8, 16, 24, 40, and 52) to review vital signs and any health concerns. These visits were included principally to monitor liraglutide-treated participants but also were provided to the IBT-alone participants to maintain consistency of treatment contact.
IBT-alone.
Participants in this group received the intervention as described above. No supplemental treatment was provided.
IBT-liraglutide.
These participants received the same program of lifestyle counseling as those in IBT-alone. However, starting at week 1, they also were prescribed liraglutide as a once-daily self-administered, subcutaneous injection (14). A study physician or NP taught participants to inject in their abdomen, thigh, or upper arm. To reduce the likelihood of gastrointestinal symptoms (e.g., nausea), the medication was initiated at 0.6 mg/day for 1 week and increased by 0.6 mg/day in weekly intervals until 3.0 mg/day was achieved. Medical staff helped participants develop a medication schedule to facilitate adherence.
Multi-component.
These participants received the same treatment as those in IBT-liraglutide, with one exception. At week 4, they were prescribed, for 12 weeks, a 1000–1200 kcal/day diet that provided four servings daily of a liquid shake (Health Management Resources, HMR; 160 kcal per shake) and an evening meal of a frozen food entrée (250–300 kcal), with a serving of fruit and salad (24). As with liraglutide, all HMR products were provided free of charge; participants were responsible for purchasing frozen food entrees and other foods.
Interventionists
IBT was delivered by a physician and two NPs, as well as two RDs who worked incident to the PCPs, as permitted by CMS (26). (Incident coverage requires PCPs to be physically present in the primary care setting at the time when ancillary providers, such as RDs, deliver in-person counseling.) One NP and one RD had previously provided IBT; the three other clinicians had not, but had offered weight-management advice. Before treatment, all interventionists received 4–6 hours of instruction in delivering IBT and were certified after satisfactorily conducting two role-play visits (23,24). Monthly individual supervision of 30–60 minutes was provided thereafter. Interventionists counseled the same participants at each visit. The physician and two NPs treated a total of 90 participants and the RDs the remaining 60.
Outcomes
The study’s primary outcome was mean percentage reduction in baseline body weight at week 52. Weight was measured by certified staff at randomization and weeks 24 and 52 using a digital scale (Tanita BWB800). (It also was measured for clinical purposes at all IBT visits to provide participants feedback on their progress.) Waist circumference and blood pressure, as well as fasting glucose, insulin, triglycerides, C-reactive protein, and lipids, were measured on the same three occasions, using standardized methods described previously (23,24). Quality of life (27) and symptoms of depression (28) also were assessed.
Statistical Analyses
Preliminary analyses examined baseline differences between randomized groups on demographic and other variables. Mean percentage reduction in baseline weight at week 52 in the intention-to-treat (ITT) population was compared using repeated measures linear mixed-effects models (for continuous outcomes). With 50 participants per treatment arm, the study had 80% power to detect a 4.5 percentage-point difference in weight change between IBT-alone vs IBT-liraglutide and between IBT-alone vs Multi-component, the study’s two primary a priori comparisons. Holm’s procedure (29) was used to adjust for multiple comparisons and to identify differences in at least one of the two contrasts at p=0.025. The percentages of participants who lost ≥5%, ≥10%, and ≥15% of baseline weight were analyzed using logistic regression; participants who did not complete the assessments were categorized as not having achieved the categorical losses. These and other secondary outcomes, including changes in body weight at week 24, were examined using p<0.05.
RESULTS
Participants’ Baseline Characteristics
Participants were 119 (79.3%) women and 31 men (total N=150) with a mean (±SD) age of 47.6±11.8 years, weight of 108.4±17.5 kg, and BMI of 38.4±4.9 kg/m2 (Table 1). Ninety-eight percent had completed high school or more; 54.0% self-identified as non-Hispanic white, 44.7% as black, and 6.7% as Hispanic. Groups differed significantly at baseline on only physical-related quality of life, which was controlled for in relevant analyses (Table 1).
Table 1.
Participants’ characteristics at randomization.
| Characteristic | IBT-alone (N = 50) | IBT-liraglutide (N = 50) | Multi-component (N = 50) | Total (N = 150) |
|---|---|---|---|---|
| Sex (female), N (%) | 39 (78%) | 42 (84%) | 38 (76%) | 119 (79.3%) |
| Race, N (%) | ||||
| Black | 22 (44%) | 23 (46%) | 22 (44%) | 67 (44.7%) |
| White | 27 (54%) | 27 (54%) | 27 (54%) | 81 (54.0%) |
| Multiracial or other | 1 (2%) | 0 (0%) | 1 (2%) | 2 (1.3%) |
| Ethnicity (Hispanic), N (%) | 3 (2%) | 3 (6%) | 4 (8%) | 10 (6.7%) |
| Age (years) | 49.5 ± 11.0 | 45.2 ± 12.3 | 48.0 ± 11.9 | 47.6 ± 11.8 |
| Weight (kg) | 105.8 ± 14.7 | 107.8 ± 17.9 | 111.7 ± 19.4 | 108.4 ± 17.5 |
| Height (cm) | 166.8 ± 7.3 | 167.3 ± 8.8 | 169.5 ± 9.1 | 167.8 ± 8.5 |
| BMI (kg/m2) | 38.0 ± 4.3 | 38.5 ± 5.4 | 38.8 ± 5.0 | 38.4 ± 4.9 |
| Waist circumference (cm) | 116.7 ± 11.6 | 116.7 ± 10.4 | 120.1 ± 11.8 | 117.8 ± 11.3 |
| Systolic BP (mm Hg) | 139.1 ± 14.6 | 135.2± 12.3 | 138.7 ± 13.1 | 137.7 ± 13.4 |
| Diastolic BP (mm Hg) | 77.5 ± 9.7ab | 73.9 ± 7.6a | 78.4 ± 10.5b | 76.6 ± 9.5 |
| Heart rate (BPM) | 82.0 ± 14.2 | 79.3 ± 12.7 | 81.8 ± 15.5 | 81.0 ± 14.2 |
| Total cholesterol (mg/dL) | 197.2 ± 33.3 | 192.6 ± 35.2 | 188.1 ± 38.1 | 192.6 ± 35.5 |
| HDL cholesterol (mg/dL)* | 59.3 ± 14.8 | 60.0 ± 14.1 | 53.6 ± 13.0 | 57.6 ± 14.2 |
| LDL cholesterol (mg/dL) | 116.6 ± 30.2 | 112.2 ± 29.5 | 112.8 ± 32.0 | 113.9 ± 30.5 |
| Triglycerides (mg/dL) | 106.4 ± 43.6 | 102.1 ± 56.1 | 108.6 ± 52.6 | 105.7 ± 50.8 |
| C-Reactive protein (mg/L) | 5.9 ± 6.0 | 6.5 ± 5.7 | 7.9 ± 9.2 | 6.8 ± 7.1 |
| Hemoglobin A1c | 5.7 ± 0.3 | 5.6 ± 0.3 | 5.7 ± 0.4 | 5.7 ± 0.3 |
| Fasting glucose (mg/dL) | 90.8 ± 10.2 | 88.3 ± 8.1 | 89.0 ± 8.4 | 89.4 ± 8.9 |
| Fasting insulin | 9.9 ± 5.7 | 9.2 ± 5.3 | 9.5 ± 5.5 | 9.5 ± 5.5 |
| HOMA-IR | 2.3 ± 1.6 | 2.0 ± 1.2 | 2.1 ± 1.2 | 2.1 ± 1.3 |
| Depression symptoms (PHQ-9) | 4.9 ± 3.6 | 4.4 ± 4.0 | 5.4 ± 4.0 | 4.8 ± 3.9 |
| Short-Form 36 | ||||
| Physical component summary | 46.7 ± 7.7a | 50.6 ± 5.7b | 47.6 ± 8.6ab | 48.3 ± 7.5 |
| Mental component summary | 50.9 ± 7.6 | 49.9 ± 10.1 | 46.6 ± 9.8 | 49.1 ± 9.4 |
Values shown are N (%) or means ± standard deviations. Note: BMI = body mass index; BP = blood pressure; BPM = beats per minute; HDL = high-density lipoprotein; LDL = low-density lipoprotein; HOMA-IR = homeostatic model assessment of insulin resistance; PHQ-9 = Patient Health Questionnaire.
P > 0.05 for all comparisons between treatment groups, except as noted.
For diastolic blood pressure and the Short-Form 36, physical component summary score, values with different superscripts (a vs b) differ significantly from each other at p <0.05. (Values that share a superscript do not differ significantly.)
There was a significant omnibus effect for HDL cholesterol (p = .04), but no two treatment groups differed significantly in pairwise comparisons using Tukey’s tests.
Retention
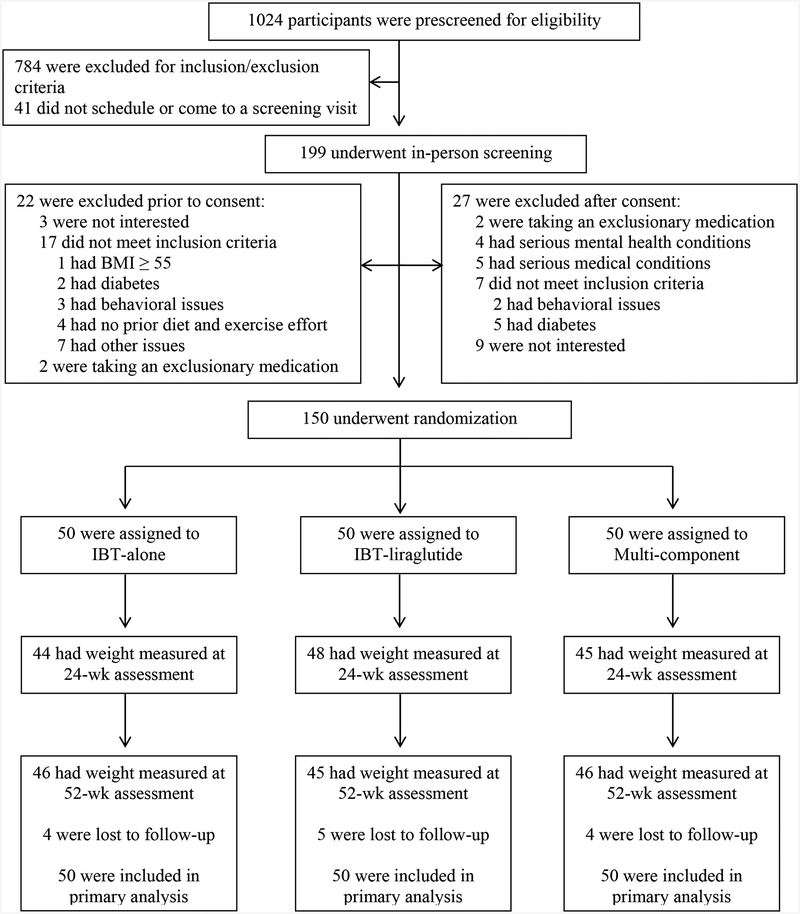
Figure 1 shows the progression of participants through the study. More than 91% of participants provided a 52-week measurement of weight. Missed visits at this time resulted from 13 individuals who were lost to follow-up.
Figure 1.
CONSORT diagram showing screening, randomization, and assessment of study participants. Weight was measured at week 52 on 137 of 150 (91.3%) participants, and all participants were included in the intention-to-treat analyses.
Weight Loss
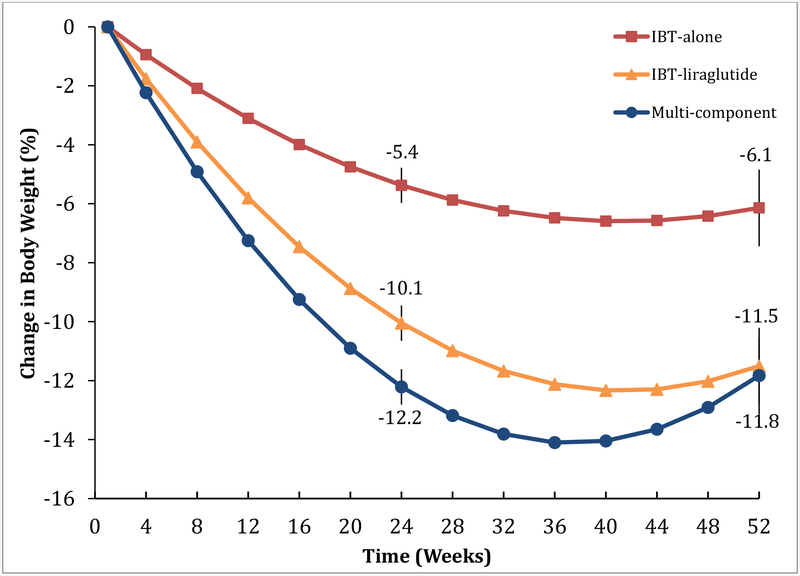
At week 52, the IBT-alone, IBT-liraglutide, and Multi-component groups achieved mean (±SEM) reductions in baseline weight of 6.1±1.3%, 11.5±1.3%, and 11.8±1.3%, respectively (Figure 2 and Table 2). Both liraglutide-treated groups lost significantly more weight than IBT-alone. The IBT-liraglutide and Multi-component interventions did not differ significantly at week 52, but did at week 24, when losses were 10.1±0.6% and 12.2±0.6%, respectively (Table 2).
Figure 2.
Estimated mean percentage reduction in baseline weight over 52 weeks in the intention-to-treat-population (N=150, with 50 participants in each treatment group). P values for pairwise comparisons at weeks 24 and week 52 are available in Table 2.
Table 2.
Estimated mean percent reduction in baseline weight, weight loss (kg), and change in body mass index at weeks 24 and 52 in the intention-to-treat population (N=150).
| Variable | IBT-alone (N = 50) | IBT-liraglutide (N = 50) | Multi-component (N = 50) | P value | ||
|---|---|---|---|---|---|---|
| IBT-liraglutide vs. IBT-alone | Multi-component vs. IBT-alone | Multi-component vs. IBT-liraglutide | ||||
| Change in weight (%) | ||||||
| Week 24 | −5.4 ± 0.6 | −10.1 ± 0.6 | −12.2 ± 0.6 | <0.001 | <0.001 | 0.017 |
| Week 52 | −6.1 ± 1.3 | −11.5 ± 1.3 | −11.8 ± 1.3 | 0.005 | 0.003 | 0.863 |
| Change in weight (kg) | ||||||
| Week 24 | −5.8 ± 0.8 | −10.6 ± 0.8 | −13.6 ± 0.8 | <0.001 | <0.001 | 0.007 |
| Week 52 | −6.6 ± 1.3 | −12.2 ± 1.3 | −13.3 ± 1.3 | 0.004 | 0.001 | 0.561 |
| Change in body mass index | ||||||
| Week 24 | −2.0 ± 0.3 | −3.8 ± 0.3 | −4.7 ± 0.3 | <0.001 | <0.001 | 0.016 |
| Week 52 | −2.3 ± 0.5 | −4.3 ± 0.5 | −4.6 ± 0.5 | 0.003 | 0.001 | 0.687 |
Values shown are estimated marginal means (± SEM) for the intention-to-treat population (N = 150)
Categorical weight losses.
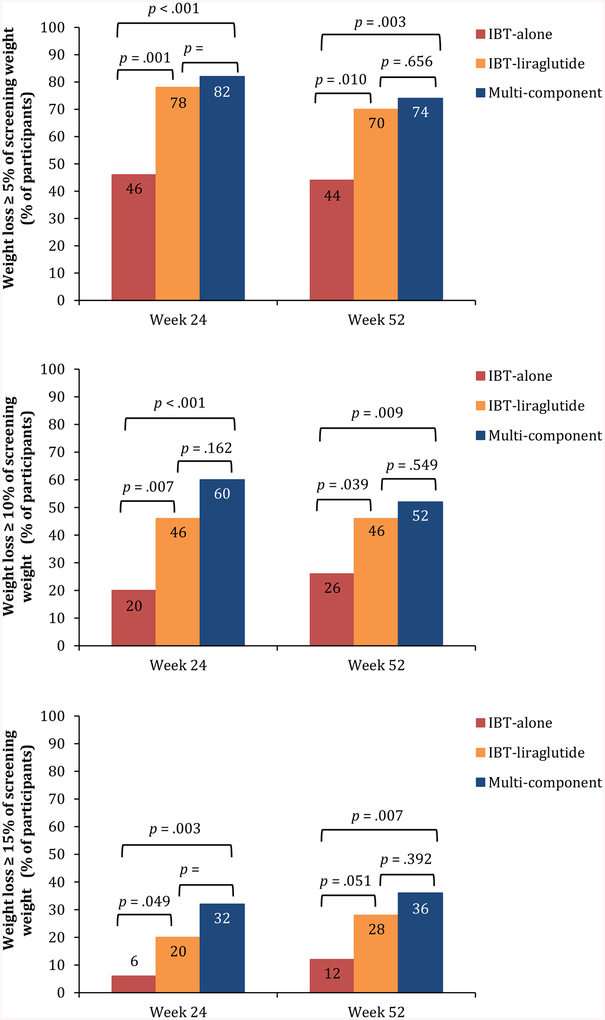
At week 52, 44% of IBT-alone participants lost ≥5% of baseline weight, 26% lost ≥10%, and 12% lost >15% (Figure 3). (The categories are overlapping, such that the 44% who lost ≥5% includes those who lost ≥10% and ≥15%.) Among participants in each of the liraglutide-treated groups, 70% or more lost ≥5% of baseline weight, 46% or more lost ≥10%, and ≥28% lost ≥15% of baseline weight (Figure 3). Significantly more IBT-liraglutide and Multi-component participants met the 5% and 10% categorical weight losses than did those who received IBT-alone. Significantly more Multi-component than IBT-alone participants also achieved the 15% weight loss criterion. The IBT-liraglutide and Multi-component groups did not differ significantly on any of the categorical losses at week 52 or at week 24 (Figure 3).
Figure 3.
Panel A shows the percentage of participants in each group in the ITT population (N=150) who lost 5% or more of baseline weight at week 24 and week 52. (Participants with missing weights were assumed not to have met the categorical loss.) Panels B and C show the percentages of participants who lost ≥10% and ≥15% of baseline weight, respectively. Percentages are cumulative; the percentage of participants, for example, that lost ≥5% of baseline weight includes the percentage that lost 10% or more.
Weight loss ≥3 kg.
At week 24, 56% of IBT-alone participants lost ≥3 kg, the CMS criterion for receiving additional monthly counseling through week 52. Significantly more IBT-liraglutide (86%; p=0.002) and Multi-component participants (90%; p<0.001) met this criterion than did those who received IBT-alone (with no significant differences between the two liraglutide-treated groups). At 1 year, 52%, 78%, and 82% of participants in the three groups, respectively, lost ≥3 kg, with same pattern of significant differences between groups. Of the 22 IBT-alone participants (44%) who did not lose ≥3 kg at week 24, 3 participants did so at week 52 with the additional counseling visits provided between months 7 and 12. Five participants, however, who had lost ≥ 3 kg at week 24, did not maintain this loss at week 52
Attendance and Effect of Interventionist
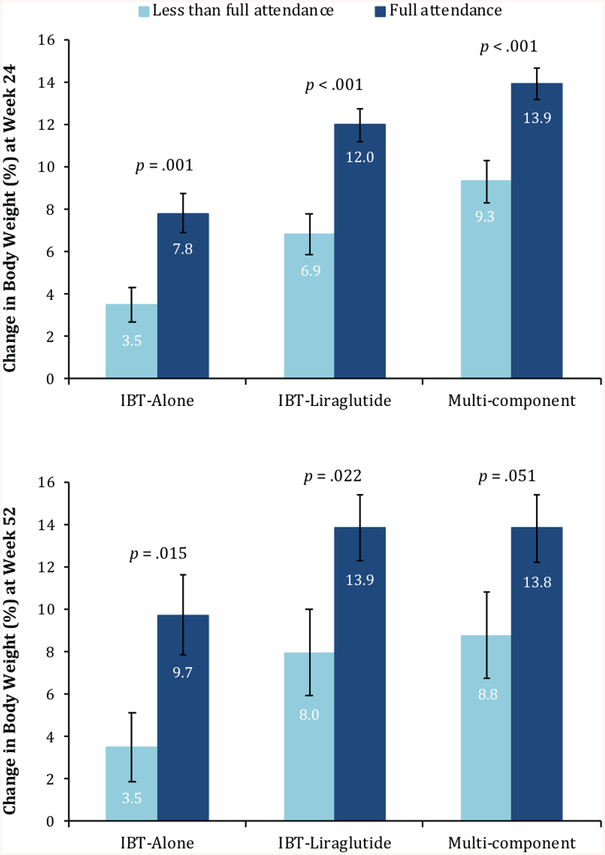
Participants in the IBT-alone, IBT-liraglutide, and Multi-component interventions attended a mean (±SD) of 72.4±35.1%, 91.2±16.8%, and 89.0±22.6% of 21 scheduled counseling visits, respectively. Attendance was significantly lower in IBT-alone than in the two other groups (p=.011 and p=.016, respectively), which did not differ significantly from each other. Figure 4 shows that, in each of the three treatment groups, greater visit attendance was generally associated with greater weight loss. For example, participants in the IBT-alone group who attended 100% of treatment visits lost a mean of 9.7% of initial weight, compared with 3.5% for those who attended an average of 54% of possible visits.
Figure 4.
The figure shows mean weight losses for the three groups in the intention-to-treat population (N=150) at weeks 24 and 52 based on attendance of IBT visits. Forty percent (n=20) of IBT-alone, 60% (n=30) of IBT-liraglutide, and 58% (n=29) of Multi-component participants attended all 21 treatment visits (p=.09 for difference among groups). The remaining 71 participants who did not have “full attendance” completed a mean of 14.0±6.6 visits (66.7±31.2% of possible visits). Among participants without “full attendance,” the 30 IBT-alone participants attended a mean of 11.3±7.3 visits (54.0±34.7%), the 20 IBT-liraglutide participants attended 16.4±4.3 sessions (78.1±20.6%), and the 21 Multi-component participants attended 15.5±6.1 sessions (73.9±28.9%). The IBT-alone group differed significantly from IBT-liraglutide (p=0.017) but not from the Multi-component group (p=0.053).
Analyses across the three treatment groups revealed no significant differences in mean (±SEM) 52-week weight losses among participants treated by the physician/NPs vs the RDs (9.8±1.0% vs 9.9±1.3%, respectively). Mean weight losses also did not differ significantly between the two experienced and three novice interventionists (10.2±1.5% vs 9.7±1.0%, respectively).
Changes in Cardiometabolic Risk Factors and Quality of Life
All three interventions produced clinically meaningful improvements at week 52 in systolic and diastolic blood pressure, heart rate, low-density lipoprotein (LDL) cholesterol, triglycerides, and depression, although differences between groups were not statistically significant (Table 3). By contrast, significantly greater improvements were observed in one or both of the liraglutide-treated groups in 52-week changes in waist circumference, high-density lipoprotein (HDL) cholesterol, C-reactive protein, fasting glucose, hemoglobin A1c, and mental health (28). The liraglutide-treated groups, compared with IBT-alone, had significantly smaller decreases in heart rate at week 24 but not 52.
Table 3.
Changes in CVD risk factors and other secondary outcomes at weeks 24 and 52, as measured from randomization.
| Characteristic | IBT-alone (N = 50) | IBT-liraglutide (N = 50) | Multi-component (N = 50) | Total (N = 150) |
|---|---|---|---|---|
| Systolic BP (mm Hg) | ||||
| Week 24 | −13.6 ± 2.1 | −12.0 ± 2.1 | −15.4 ± 2.1 | −13.7 ± 1.2*** |
| Week 52 | −14.1 ± 2.1 | −13.3 ± 2.1 | −15.3 ± 2.1 | −14.2 ± 1.2*** |
| Diastolic BP (mm Hg)° | ||||
| Week 24 | −3.2 ± 1.2 | −3.4 ± 1.2 | −4.5 ± 1.2 | −3.7 ± 0.7*** |
| Week 52 | −3.0 ± 1.2 | −2.9 ± 1.2 | −3.5 ± 1.2 | −3.2 ± 0.7*** |
| Heart rate (BPM) | ||||
| Week 24 | −9.5 ± 2.0a | −3.6 ± 2.0b | −5.5 ± 2.0ab | −6.1 ± 1.2 |
| Week 52 | −7.4 ± 2.0 | −5.3 ± 2.0 | −9.7 ± 2.0 | −7.5 ± 1.2*** |
| Waist circumference (cm) | ||||
| Week 24 | −5.2 ± 1.1a | −9.9 ± 1.1b | −12.9 ± 1.1b | −9.3 ± 0.7 |
| Week 52 | −6.5 ± 1.3a | −11.1 ± 1.3b | −12.6 ± 1.3b | −10.1 ± 0.8 |
| Total cholesterol (mg/dL) | ||||
| Week 24 | −12.2 ± 3.6 | −13.9 ± 3.5 | −16.8 ± 3.6 | −14.3 ± 2.0*** |
| Week 52 | −7.0 ± 3.5 | −9.7 ± 3.6 | −10.0 ± 3.5 | −8.9 ± 2.0*** |
| HDL cholesterol (mg/dL)° | ||||
| Week 24 | −2.5 ± 1.3 | −1.2 ± 1.2 | −1.1 ± 1.3 | −1.6 ± 0.7* |
| Week 52 | −1.3 ± 1.3a | 3.0 ± 1.3b | 2.0 ± 1.3ab | 1.2 ± 0.7 |
| LDL cholesterol (mg/dL) | ||||
| Week 24 | −8.7 ± 3.1 | −9.3 ± 3.0 | −11.9 ± 3.0 | −10.0 ± 1.8*** |
| Week 52 | −3.3 ± 3.1 | −9.6 ± 3.1 | −9.4 ± 3.1 | −7.4 ± 1.8*** |
| Triglycerides (mg/dL) | ||||
| Week 24 | −4.6 ± 5.8 | −14.9 ± 5.6 | −15.5 ± 5.7 | −11.8 ± 3.3*** |
| Week 52 | −16.3 ± 5.7 | −21.3 ± 5.8 | −14.4 ± 5.7 | −17.3 ± 3.3*** |
| C-Reactive protein (mg/L) | ||||
| Week 24 | −0.7 ± 0.7 | −1.2 ± 0.7 | −2.5 ± 0.7 | −1.4 ± 0.4 |
| Week 52 | −0.4 ± 0.7a | −2.0 ± 0.7ab | −3.0 ± 0.7b | −1.8 ± 0.4 |
| HbA1c | ||||
| Week 24 | −0.3 ± 0.04a | −0.4 ± 0.03b | −0.5 ± 0.03b | −0.4 ± 0.02 |
| Week 52 | −0.3 ± 0.03a | −0.5 ± 0.03b | −0.6 ± 0.03b | −0.5 ± 0.02 |
| Fasting glucose (mg/dL) | ||||
| Week 24 | −1.9 ± 1.3a | −4.4 ± 1.3ab | −6.6 ± 1.3b | −4.3 ± 0.8 |
| Week 52 | 0.01 ± 1.3a | −5.2 ± 1.3b | −5.7 ± 1.3b | −3.6 ± 0.8 |
| Fasting insulin | ||||
| Week 24 | −1.9 ± 0.7 | −0.8 ± 0.7 | −0.9 ± 0.7 | −1.2 ± 0.4** |
| Week 52 | −1.5 ± 0.8 | −1.1 ± 0.8 | −1.5 ± 0.8 | −1.4 ± 0.5** |
| HOMA-IR | ||||
| Week 24 | −0.5 ± 0.2 | −0.3 ± 0.2 | −0.3 ± 0.2 | −0.4 ± 0.1** |
| Week 52 | −0.4 ± 0.2 | −0.3 ± 0.2 | −0.4 ± 0.2 | −0.4 ± 0.1** |
| Depression symptoms (PHQ-9) | ||||
| Week 24 | −1.5 ± 0.6 | −3.0 ± 0.6 | −2.9 ± 0.6 | −2.6 ± 0.3*** |
| Week 52 | −1.8 ± 0.6 | −1.9 ± 0.6 | −1.5 ± 0.6 | −1.8 ± 0.4*** |
| Short-Form 36 | ||||
| Physical component summary° | ||||
| Week 24 | 4.8 ± 1.0 | 2.3 ± 1.0 | 4.4 ± 1.0 | 4.0 ± 0.6 |
| Week 52 | 4.4 ± 1.0 | 2.1 ± 1.0 | 3.4 ± 1.0 | 3.4 ± 0.6 |
| Mental component summary | ||||
| Week 24 | 0.8 ± 1.3a | 4.4 ± 1.3ab | 6.9 ± 1.3b | 4.3 ± 0.8 |
| Week 52 | 0.8 ± 1.3a | 4.5 ± 1.3b | 6.4 ± 1.3b | 4.1 ± 0.8 |
Values shown are means ± SE. Note: BP = blood pressure; BPM = beats per minute; HDL = high-density lipoprotein; LDL = low-density lipoprotein; HOMA-IR = homeostatic model assessment of insulin resistance; PHQ-9 = Patient Health Questionnaire.
Note: For each variable, the three treatment groups were compared using pair-wise comparisons.
Significant differences between groups at p <0.05 are denoted by different superscripts (a vs b) between the values. For example, at week 24, the 9.5 BPM decrease in heart rate for IBT-alone was significantly greater than the 3.6 BPM decrease in IBT-liraglutide. This is shown by the “a” superscript with −9.5±2.0a, compared with the b superscript for −3.6±2.0b. Values that share a superscript do not differ significantly. For example, neither group differed significantly from the 5.5 BPM decrease in the Multi-component group, which is marked “ab”.
For the comparison of each variable, the absence of any superscripts indicates that there were no significant differences among groups. In those cases, changes over time in the total sample (N=150) that were statistically significant are indicated using * p <0.05, ** p <0.01, and *** p < 0.001.
Note:
Scores at randomization were included as a covariate in these models to control for initial differences among the groups.
Adverse Events
Cases of nausea, constipation, upper respiratory infection, and gastroenteritis were 10 or more percentage points higher in both liraglutide-treated groups than in IBT-alone (Table 4). A total of six serious adverse events were reported, including asthma, bile duct stones, gastroenteritis, pneumonia, and wound infection, all of which resolved fully.
Table 4.
Adverse events with an incidence of 5% or more of patients in any treatment group, as well as all serious adverse events.
| Event | IBT-alone (N = 50) | IBT-liraglutide (N = 50) | Multi-component (N = 50) | |||
|---|---|---|---|---|---|---|
| N (%) | Events, N | N (%) | Events, N | N (%) | Events, N | |
| All Adverse Events | 30 (60%) | 63 | 45 (90%) | 164 | 47 (94%) | 171 |
| Adverse events ≥5% in any treatment group | 21 (42%) | 39 | 42 (84%) | 119 | 44 (88%) | 128 |
| Nausea | 4 (8%) | 4 | 18 (36%) | 21 | 13 (26%) | 17 |
| Constipation | 1 (2%) | 2 | 15 (30%) | 16 | 17 (34%) | 21 |
| Upper respiratory infection | 8 (16%) | 8 | 16 (32%) | 18 | 14 (28%) | 18 |
| Musculoskeletal injury | 6 (12%) | 7 | 6 (12%) | 6 | 12 (24%) | 12 |
| Gastroenteritis | 2 (4%) | 2 | 10 (20%) | 12 | 8 (16%) | 8 |
| Diarrhea | 2 (4%) | 2 | 6 (12%) | 9 | 7 (14%) | 7 |
| Vomiting | 3 (6%) | 3 | 6 (12%) | 8 | 5 (10%) | 6 |
| Gastroesophageal reflux disorder | 2 (4%) | 2 | 2 (4%) | 2 | 6 (12%) | 9 |
| Injection site irritation | 0 | 0 | 5 (10%) | 5 | 6 (12%) | 6 |
| Fatigue | 0 | 0 | 4 (8%) | 4 | 5 (10%) | 6 |
| Sinusitis | 3 (6%) | 3 | 2 (4%) | 2 | 5 (10%) | 5 |
| Knee pain | 2 (4%) | 2 | 2 (4%) | 2 | 4 (8%) | 4 |
| Lower back pain | 2 (4%) | 2 | 1 (2%) | 1 | 4 (8%) | 5 |
| Abdominal pain | 0 | 0 | 4 (8%) | 4 | 2 (4%) | 2 |
| Headache | 1 (2%) | 1 | 3 (6%) | 3 | 1 (2%) | 1 |
| Tonsillopharyngitis | 1 (2%) | 1 | 3 (6%) | 3 | 0 | 0 |
| Depressed mood | 0 | 0 | 3 (6%) | 3 | 1 (2%) | 1 |
| All Serious Adverse Events | 2 (4%) | 2 | 0 | 0 | 3 (6%) | 4 |
| Asthma exacerbation | 1 (2%) | 1 | 0 | 0 | 0 | 0 |
| Bile duct stone | 0 | 0 | 0 | 0 | 1 (2%) | 1 |
| Gastroenteritis | 0 | 0 | 0 | 0 | 1 (2%) | 2 |
| Pneumonia | 0 | 0 | 0 | 0 | 1 (2%) | 1 |
| Wound infection | 1 (2%) | 1 | 0 | 0 | 0 | 0 |
Note: N (%) = number of participants (%), N events = number of events.
DISCUSSION
This study has two principal findings, the first which was that 21 brief sessions of IBT, delivered by PCPs, induced clinically meaningful weight loss at 1 year. The second was that the addition to IBT of liraglutide 3.0 mg/day nearly doubled the mean weight loss produced by behavioral counseling alone. These results have important implications for the management of obesity in primary care practice, as recommended by the U.S. Preventive Services Task Force (4,5).
This is the first randomized controlled trial of which we are aware to test the efficacy of IBT for obesity, as largely modeled on the treatment approach covered by CMS. Participants in the IBT-alone group lost a mean 6.1% of baseline weight at 1 year, and 44% lost ≥5% of body weight, a common criterion of clinically meaningful weight loss (2). Participants who attended all counseling visits lost an average of 9.7% of baseline weight, compared with 3.5% for those with lower attendance (i.e., attended 54.0% of possible visits), confirming the positive relationship between these variables reported in prior observational studies of IBT (30,31).
The frequency and duration of treatment visits proposed by CMS was based largely on findings of systematic reviews of randomized trials (32,33), many of which included group, behavioral weight loss of 60–90 minutes, often considered the standard of care (2). Few if any interventions, whether for groups or individuals, provided visits <30 minutes, raising questions about CMS’ proposed 15-minute sessions. However, CMS’ recommendation of frequent visits in the first 6 months (i.e., 14–15 sessions) is consistent with findings of systematic reviews (32) and treatment guidelines (2) and resembles the 16 sessions of individual counseling provided in the first 6 months of the DPP (13). Participants in that study lost a mean of approximately 7 kg at both 6 and 12 months, results only marginally better than those from the present study (i.e., 5.8 and 6.6 kg at weeks 24 and 52, respectively), which used an abbreviated version of the DPP protocol, previously implemented in a primary care setting (23,34). CMS does not provide or recommend a specific weight loss protocol, instead encouraging clinicians to follow a more general 5-A approach to diet and activity modification (i.e., assess, advise, agree, assist, and arrange) (4,35). We believe that the use of a structured behavioral weight loss protocol (e.g, DPP) will increase the likelihood of clinically meaningful weight reduction with CMS-covered IBT (35), a hypothesis that needs to be tested.
The addition to IBT of liraglutide 3.0 mg/d increased mean 1-year weight loss from 6.1% to 11.5% of initial weight, confirming the additive effects of behavior therapy and pharmacotherapy for obesity (11,12). Fully 70% of IBT-liraglutide participants lost ≥5% of baseline weight, and 46% lost ≥10%, values higher than those in trials of liraglutide 3.0/mg combined with less intensive counseling (9,15). The greater weight loss in these participants, compared with IBT-alone, translated into significantly greater 1-year improvements in waist circumference, HDL cholesterol, and mental health (27). Significantly greater improvements also were observed in glucose and HbA1c, results that likely were attributable to liraglutide’s independent effects on glucose metabolism (14). Liraglutide was generally well tolerated, with adverse events similar to those reported previously (9,14,15).
The addition of a 12 week meal-replacement diet to IBT-liraglutide significantly increased weight loss at week 24, but not by as much as expected (37), and the benefit was not maintained at week 52. We previously showed in a randomized trial that the combination of the weight loss medication sibutramine and high-intensity group behavioral weight loss counseling, delivered with a 1200–1500 kcal/d diet of conventional foods, produced a 10.4% reduction in initial weight at 1 year. The addition of a 1000 kcal/d meal-replacement diet to this regimen for the first 16 weeks increased mean 1-year weight loss to 16.5% (37). Participants in the meal-replacement group challenged each other, by their large weekly weight losses, to adhere strictly to the 1000 kcal/d diet. Our clinical impression is that the individual counseling in the present study did not facilitate the same robust adherence to the meal-placement diet as did the social support and healthy competition engendered by group counseling (37).
This study’s strengths include high participant engagement and retention rates. Potential limitations include the absence of a usual-care group, which was not included because of consistent evidence that it would yield a 1-year weight loss of 1–2% (23,38). The study also did not include principally older adults (≥65 years), as covered by the Medicare benefit, and the trial was not conducted in a primary care setting. In addition, by design, participants were provided 21 sessions of IBT regardless of whether they lost ≥3 kg at month 6. The favorable results from this well controlled efficacy study await replication in larger pragmatic trials that include greater numbers of older adults (i.e., the target for CMS) and are conducted in primary care settings.
The present findings show that physicians and NPs can provide effective IBT for obesity, facilitating weight losses comparable to those produced by RDs. Further study, however, is needed to determine the minimum instruction that PCPs in non-specialty practices would require to provide such care and at what financial and personal costs to their already busy practice schedules.36 Most physicians do not have the training, time, or financial incentive to provide intensive behavioral counseling (36). CMS allows ancillary health professionals (e.g., RDs) to provide IBT if working incident to PCPs. However, PCPs must be physically present when ancillary counseling is provided (3,26). Such restrictions likely have contributed to IBT’s low utilization rate (<1%) among eligible CMS beneficiaries (39). We encourage CMS to change the model by which IBT is delivered to include RDs, health counselors, and other trained interventionists (2) as eligible primary providers. This modification, as well as covering group treatment and validated phone- and digitally-delivered counseling (2,40) could greatly increase access to IBT, while substantially reducing the cost of this important care.
What is already known about this subject?
Intensive behavioral therapy (IBT) for obesity, as well as medications approved for chronic weight management, both reliably produce mean weight losses of 5% or more of initial weight at 1 year.
What does this study add?
This randomized controlled trial tested a model of IBT for obesity covered by the Centers for Medicare and Medicaid Services (CMS) and found that it induced a mean 6.1% reduction in initial body weight at 1 year. The addition to IBT of the weight loss medication liraglutide 3.0 mg/day increased mean weight loss to 11.5%; the further addition of meal replacements (for 12 weeks) to IBT and liraglutide increased weight loss to only 11.8%.
Acknowledgements
This trial was supported by an Investigator Initiated Study award to the University of Pennsylvania (on behalf of Dr. Wadden) from Novo Nordisk. The authors thank Drs. Cate Nojiri and Anthony Fabricatore at Novo Nordisk for coordinating administration of the award, as well as Breanna D’Antonio, Callie Fisher, and Emilie Pinkasavage at the University of Pennsylvania’s Center for Weight and Eating Disorders for assistance with data management.
A de-identified data set will be made available to external investigators (upon request to the first author), once the research team has completed its analysis and reporting of secondary findings from the study. This is expected to be approximately two years after the publication of this report.
This study was supported by an Investigator-Initiated Study award from Novo Nordisk to the University of Pennsylvania (TAW).
Dr. Wadden reports serving on advisory boards for Novo Nordisk and Weight Watchers Inc. Dr. Berkowitz serves as a consultant to Eisai Pharmaceutical, and Dr. Chao has consulted with Shire Pharmaceutical. Drs. Alamuddin and Tronieri serve as consultants to Novo Nordisk.
Footnotes
Trial Registration: ClinicalTrials.gov number,
References
- 1.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017;376(3):254–266. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MD, Ryan DH, Donato SM, et al. Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/America Heart Association Task Force on practice guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on practice guidelines. Based on a systematic review from the obesity expert panel, 2013. Obesity (Silver Springs) 2014;22:Suppl 2:S5–S39. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Medicare and Medicaid Services. Decision memo for intensive behavioral therapy for obesity (CAG-00423N). 2015. (http://www.cms.gov/medicare-coverage-database/details/ncadecisionmemo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253).
- 4.Moyer VA, U.S. Preventive Services Task Force. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(5):373–378. [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force Recommendation Statement. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults. JAMA 2018;320(11):1163–1171. [DOI] [PubMed] [Google Scholar]
- 6.LeBlanc ES, Patnode CD, Webber EH, Redmond N, Rushkin M, O’Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320(11):1172–1191. [DOI] [PubMed] [Google Scholar]
- 7.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. [DOI] [PubMed] [Google Scholar]
- 8.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 2016;22:Suppl 3:1–203. [DOI] [PubMed] [Google Scholar]
- 9.Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA 2016;315(22):2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phelan S, Wadden TA. Combining behavioral and pharmacological treatments for obesity. Obes Res. 2002;10(6): 560–74. [DOI] [PubMed] [Google Scholar]
- 11.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–2120. [DOI] [PubMed] [Google Scholar]
- 12.Craighead LW, Stunkard AJ, O’Brien RM. Behavior therapy and pharmacotherapy for obesity. Arch Gen Psychiatry 1981;38(7):763–68. [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liraglutide (rDNA origin) injection [package insert]. Plainsboro, NJ: Novo Nordisk, Inc.; 2017. [Google Scholar]
- 15.Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373(1):11–22. [DOI] [PubMed] [Google Scholar]
- 16.Heymsfield SB, van Mierlo CA, van der Knaap HC, et al. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes 2003;27(5):537–549. [DOI] [PubMed] [Google Scholar]
- 17.Metz JA, Stern JS, Kris-Etherton P, et al. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: impact on cardiovascular risk reduction. Arch Int Med 2000;160(14):2150–2158. [DOI] [PubMed] [Google Scholar]
- 18.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012;36(6):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015;314(7):687–699. [DOI] [PubMed] [Google Scholar]
- 20.National Heart, Lung, and Blood Institute and North American Association for the Study of Obesity. Practical guide to the identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: National Institutes of Health, 2000, p. 25. [Google Scholar]
- 21.Wadden TA, Foster GD. Weight and lifestyle inventory (WALI). Obesity (Silver Springs) 2006;14 (Suppl 2):99S–118S. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 23.Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw Tronieri J, Wadden TA, Berkowitz RI, et al. A randomized trial of lorcaserin and lifestyle counseling for maintaining weight loss achieved with a low-calorie diet. Obesity (Silver Spring) 2018;26(2):299–309. [DOI] [PubMed] [Google Scholar]
- 25.Look AHEAD Research Group, Wadden TA, West DS, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14(5):737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Medicare and Medicaid Services. Services and supplies incident to a physician’s professional services: Conditions. 42 CFR § 410.26. 2011. (http://www.gpo.gov/fdsys/pkg/CFR-2011-title42-vol2/pdf/CFR-2011-title42-vol2-sec410-26.pdf).
- 27.Ware J, Sherbourne C. The MOS 36-item short-form health survey (SF-36): conceptual framework and item selection. Med Care 1992;30(6):473–483. [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- 30.Treviño RP, Piña C, Fuentes JC, Nuñez M. Evaluation of Medicare’s intensive behavioral therapy for obesity: The BieneStar experience. Am J Prev Med 2018;54(4):497–502. [DOI] [PubMed] [Google Scholar]
- 31.Thabault PJ, Burke PJ, Ades PA. Intensive behavioral treatment weight loss program in an adult primary care practice. J Am Assoc Nurse Pract 2016;28(5):249–257. [DOI] [PubMed] [Google Scholar]
- 32.Leblanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155(7):434–447. [DOI] [PubMed] [Google Scholar]
- 33.Brown T, Avenell A, Edmunds LD, et al. Systematic review of long-term lifestyle interventions to prevent weight gain and morbidity in adults. Obes Rev 2009;10(6):627–638. [DOI] [PubMed] [Google Scholar]
- 34.Tsai AG, Wadden TA, Rogers MA, Day SC, Moore RH, Islam BJ. A primary care intervention for weight loss: results of a randomized controlled pilot study. Obesity 2010;18(8):1614–1618. [DOI] [PubMed] [Google Scholar]
- 35.Glasgow RE, Emont S, Miller DC. Assessing delivery of the five ‘As’ for patient-centered counseling. Health Promot Int 2006;21(3):245–255. [DOI] [PubMed] [Google Scholar]
- 36.Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA 2014;312(17):1779–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wadden TA, Berkowitz RI, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: a randomized trial. Arch Intern Med 2001;161(2):218–227. [DOI] [PubMed] [Google Scholar]
- 38.Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011;365(21):1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batsis JA, Bynum JP. Uptake of the Centers for Medicare and Medicaid obesity benefit: 2012–2013. Obesity (Silver Spring) 2016;24(9):1983–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas JG, Leahey TM, Wing RR. An automated internet behavioral weight-loss program by physician referral: a randomized controlled trial. Diabetes Care 2015;38(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]