Abstract
We report a newborn female with craniofacial malformations, bilateral anophthalmia, large abnormally shaped ears, short neck, small distal phalanges and nails, left diaphragmatic hernia, hypoplastic optic nerves, severe pulmonary hypoplasia, and an accessory spleen, and describe the autopsy findings. The infant expired at 18 h of life. The features were most consistent with Fryns syndrome although other conditions were considered including Matthew Wood syndrome. Anophthalmia, to our knowledge, has not been reported previously in Fryns syndrome; however, eye findings are common, particularly microphthalmia and cloudy cornea.
Keywords: bilateral anophthalmia, diaphragmatic hernia, Fryns syndrome
CASE REPORT
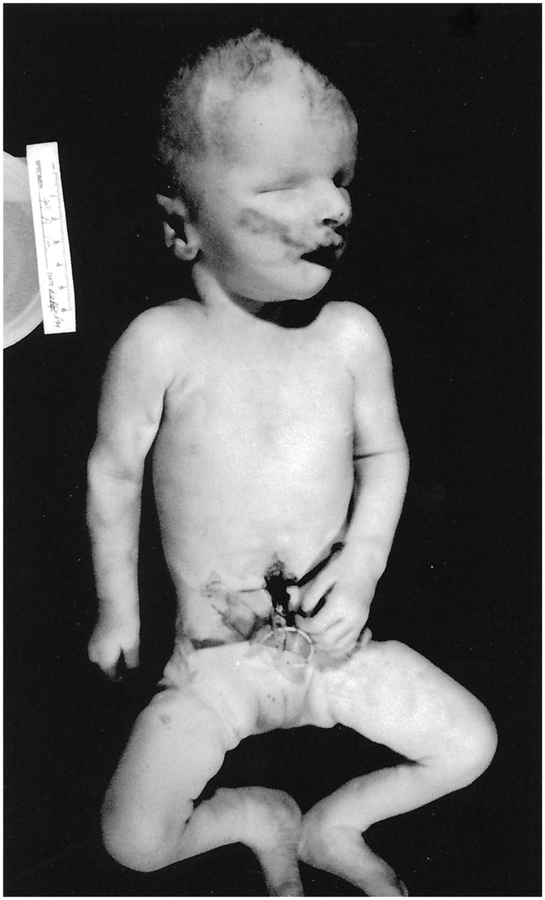
We describe a 2,600-g white female born to a G1P1 18-year-old mother and an unrelated father via cesarean section secondary to failure to progress. There was a normal ultrasound performed at 31 wk gestation. Height, weight, and head circumference were all at the 75th percentile at a gestational age of 35 wk. The infant presented with coarse facies, bilateral anophthalmia, hypertelorism, broad nasal bridge, large nose with anteverted nares, large abnormally shaped ears, microretrognathia, short neck with posterior nuchal folds, and small distal phalanges and nails (Fig. 1). Apgar scores were 1, 2, and 3 at 1, 5, and 10 min of life, respectively. Cardiac compressions, endotracheal intubation, and intravenous vasopressor support were initiated. On chest auscultation, breath sounds were absent on the left and decreased on the right, while the apical point of maximum intensity was displaced to the right of the sternum. The abdomen was scaphoid. A chest radiograph revealed a left diaphragmatic hernia. The infant expired at 18 h of life. Chromosome studies were interpreted as normal (46,XX).
Figure 1.
Frontal view of the patient at autopsy.
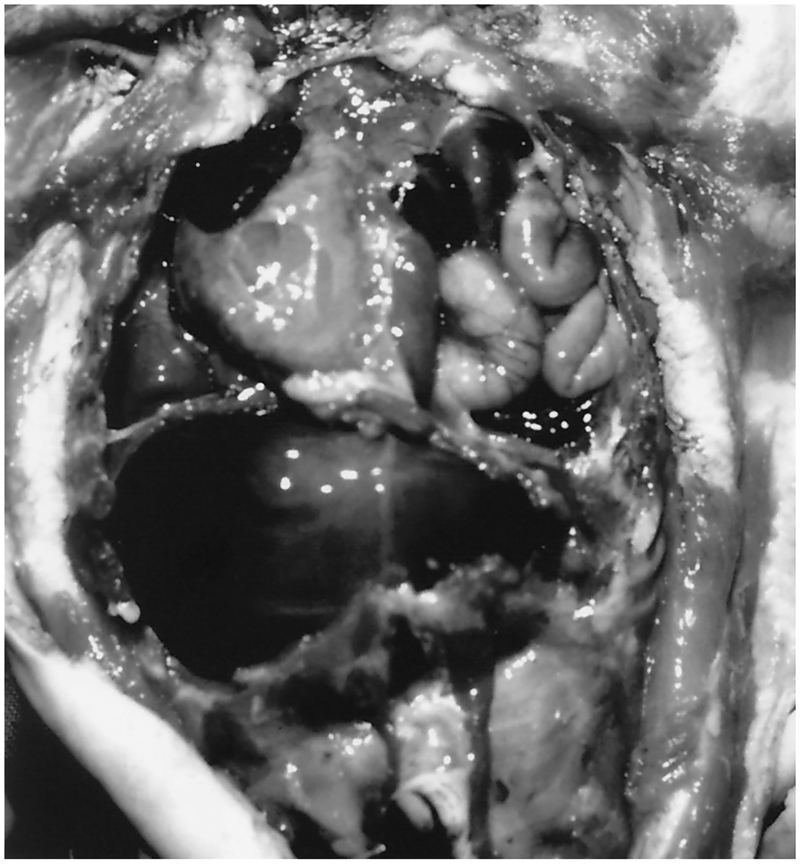
At autopsy, the left pleural cavity was found to contain herniated abdominal contents (Fig. 2) through a large diaphragmatic defect and included a misshapen stomach, spleen and accessory spleen, a portion of the liver, and all of the small and most of the large intestines. The abdominal cavity contained a small amount of serous fluid. The heart and thymus were deviated to the right. The thymus was hypoplastic and weighed 2 g (normal expected weight: 6.9 ± 4.5 g) [1]. The heart weighed 12 g (normal expected weight: 13.7 ± 3.6 g) [1] with normal anatomical configuration and normal valves. Pericardial effusion, persistent fetal circulation, large dilated pulmonary arteries, and bilateral ventricular hypertrophy were noted. The liver weighed 91 g (normal expected weight: 87.4 ± 30.6 g) [1] and had a smooth capsular surface. The liver showed deformation of the left hepatic lobe with protrusion of the lobe through a 5-cm circumference diaphragmatic septal defect. Microscopic views of the hepatic lobes were normal. The pancreas had normal lobation. There was severe pulmonary hypoplasia bilaterally with an atelectatic single-lobed left lung and an expanded two-lobed right lung. Microscopic view of the right upper lobe showed large prominent pulmonary arteries and bronchi, many of which were close to the pleural surface. The left lobe showed similar findings as well as marked hemorrhage, atelectasis, and pulmonary edema. The spleen weighed 5 g (normal expected weight: 7.2 ± 5.2 g) [1] with a smooth capsule. An accessory spleen measuring 1 × 2 × 1 cm was also observed.
Figure 2.
The chest and abdominal cavities at autopsy showing the left pleural space occupied with abdominal contents, including stomach, spleen, left hepatic lobe, and intestines. The heart and thymus were deviated to the right.
The genitourinary system was anatomically normal with a combined weight of 25 g for the right and left kidneys (normal expected weight: 20.1 ± 10.9 g) [1]. There were no renal masses or cysts observed. The bladder, uterus, and ovaries were grossly intact and of normal size. The microscopic studies were also normal. The right and left adrenal glands were normal (combined weight of 5.5 g with normal expected weight: 5.6 ± 2.8 g) [1]. There were no masses or cysts seen and the microscopic studies were normal.
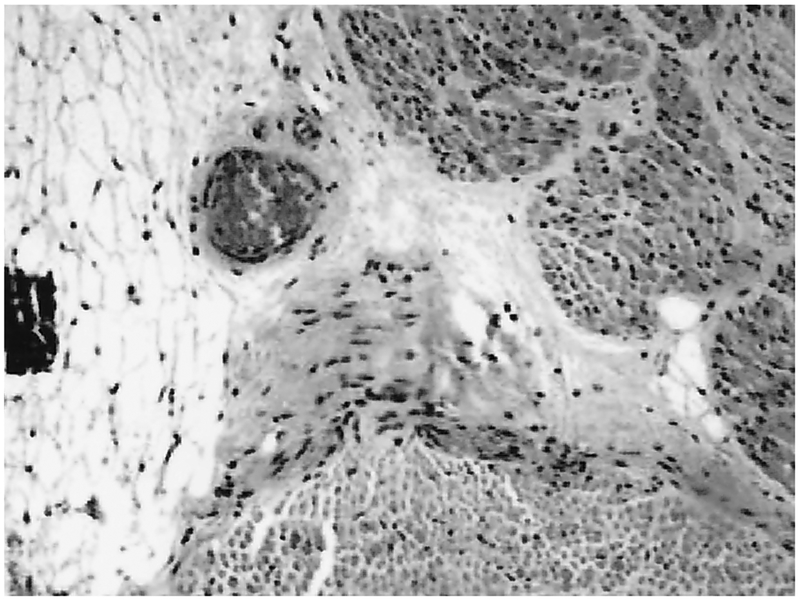
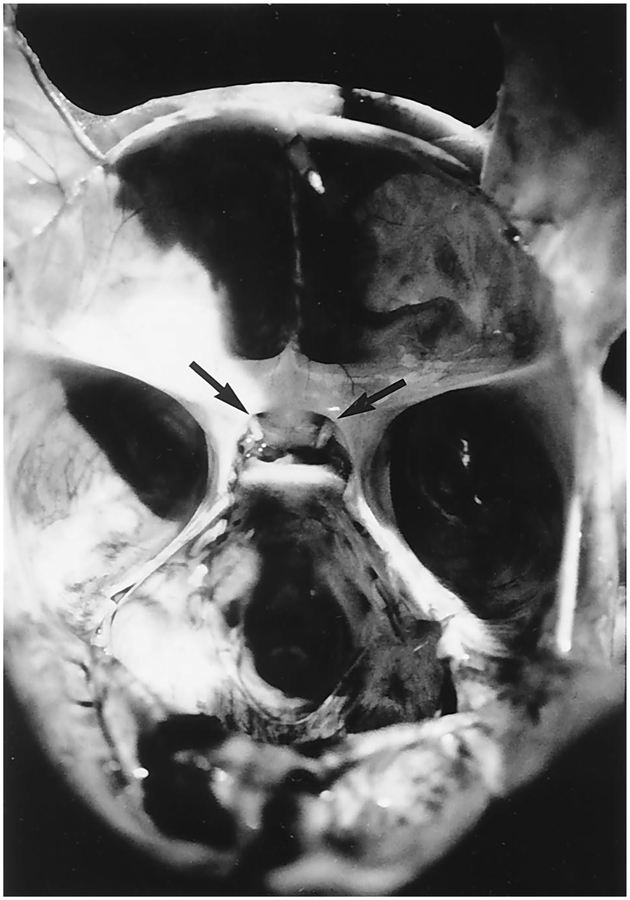
Postmortem gross eye examination showed absence of the globes bilaterally. Small amounts of fibroadipose tissue were removed for histology. Microscopic sections of the globes revealed disorganized tissue with foci of calcification, fragments of muscle, peripheral nerve and adipose tissue without distinctive eye elements (Fig. 3). The optic nerves were present bilaterally but were hypoplastic (Fig. 4). The other cranial nerves appeared to have normal proportional size. The central nervous system showed no gross defects of the gyri, sulci, falx cerebri, tentorium, or leptomeninges. The brain weighed 365 g after fixation (normal expected weight: 257 ± 45 g) [1]. Coronal sections of the cerebral hemispheres showed no focal lesions of the gray and white matter. The corpus callosum was intact and there was a cavum septi pellucidi. Microscopically, there were no distinctive diagnostic alterations. The cortical cytoarchitecture and the degrees of myelination in different areas were both consistent with gestational age. There were no specific lesions in the section of the hippocampus. The central gray nuclei showed normal neurons in the various nuclear groups without degenerative changes. The choroid plexi in the lateral and third ventricles were normal. There were no areas of calcification, heterotopias, or neoplasia. There were no malformations of the cerebellum or brainstem. Microscopic sections of the cerebellum showed normal retention of the external granular cell layer with a normal internal granular cell layer and intact lesions of the inferior olive, basis pontis, or dentate nucleus. The spinal cord was normal on gross and histological examinations.
Figure 3.
Microscopic sections of the globe revealed foci of calcification, fragments of muscle, peripheral nerve, and adipose tissue. There was no evidence of normal residual intrinsic tissue (hematoxylin-eosin original magnification ×100).
Figure 4.
Anatomic view of the hypoplastic optic nerve tract (arrows) bilaterally.
DISCUSSION
Several conditions were considered in the differential diagnosis. When searching a commonly used computer genetic database of a catalogue of autosomal dominant, autosomal recessive, and X-linked phenotypes (On-line Mendelian Inheritance in Man) with the key word “diaphragmatic hernia,” 40 entries were found [2]. Isolated diaphragmatic hernia was the first entry listed, whereas Fryns syndrome was listed second. From this list of 40 conditions, only Fryns syndrome included the additional features seen in our patient. Three entries were found when diaphragmatic hernia and hypoplastic distal phalanges were searched. The three entries were Fryns syndrome, rhizomelic chondrodysplasia punctata, and Simpson-Golabi-Behmel syndrome.
Another condition considered in the differential diagnosis was Matthew Wood syndrome. This condition is very rare with only six reported cases, and is characterized by pulmonary agenesis, microphthalmia, diaphragmatic defect, and intrauterine growth retardation [3–5]. Anophthalmia and pulmonary hypoplasia have also been reported in this syndrome [3,5]. A stillborn male reported by Spear et al. [5] with this condition had a normal brain by gross examination. Our patient did not have intrauterine growth retardation but had other multiple congenital anomalies including an abnormal face, optic nerve hypoplasia, an accessory spleen, and distal limb defects not reported in Matthew Wood syndrome.
Cornelia de Lange syndrome is also similar in many regards, but is characterized by uterine growth retardation, while overgrowth syndromes, such as Marshall Smith syndrome and Beckwith-Wiedemann syndrome can have overlapping features [6]. Zellweger syndrome (cerebro-hepato-renal syndrome) can also be included in the differential diagnosis [7]. Roberts syndrome is distinguished by phocomelia and can share some commonalities with Fryns syndrome, but typically lacks lung hypoplasia, diaphragmatic hernia, and coarse facial features [8].
Fryns syndrome is the most frequent nonchromosomal multiple congenital anomaly syndrome associated with congenital diaphragmatic hernia, accounting for 4–10% of those cases. First reported in 1979 in two stillborn sisters presenting with cloudy corneae, diaphragmatic defects, and distal limb deformities, the syndrome since has been described with a high degree of variable phenotypic expression in over 50 literature cases [7–9]. Fryns syndrome has been described as a true malformation syndrome with a major nonspecific disturbance of a midline field and, possibly, disruption of lateral embryological structures [10,11]. Diaphragmatic hernia, which is frequently secondary to a postero-lateral diaphragmatic defect, and lung hypoplasia have been recorded in 80% of affected patients, but the exact pathogenesis is controversial [7,10]. Prenatal diagnosis of Fryns syndrome has been reported with echographic evidence of polyhydramnios and congenital diaphragmatic hernia in affected individuals. One retrospective study of 34 cases of Fryns syndrome reported abnormal ultrasound findings in 15 cases [12]. Major findings of Fryns syndrome include diaphragmatic hernia, hypoplastic lungs, coarse facies with macrostomia, eye anomalies, microretrognathia, dysmorphic ears, and distal digital hypoplasia [6]. In addition to the above, malformations include anomalies of the axial skeleton, and the central nervous, cardiovascular, gastrointestinal, and urogenital systems [13]. Prenatal overgrowth and polyhydramnios are often present, while cystic hygroma has been implicated in several instances.
Inheritance of Fryns syndrome is autosomal recessive with a 25% recurrence rate [14]. The specific gene defect is unknown. The incidence may be as high as 1 in 12,000 births with an approximate male:female ratio of 1.25:1 [13], but the estimated frequency seems high in view of the relatively few subjects reported in the literature. The syndrome may be underrecognized and underreported. Patients with Fryns syndrome generally present with a normal karyotype, although the collection of birth defects may resemble syndromes with chromosomal abnormalities. One common chromosome example is trisomy 18 (Edwards syndrome) with its characteristic findings of craniofacial anomalies, camptodactyly, polyhydramnios, and multiple visceral anomalies including diaphragmatic hernia [7]. Other disorders bearing resemblance, include partial trisomy 3 at bands q21–qter, mosaic trisomy 9, mosaic tetrasomy 12p (Pallister-Killian syndrome), terminal deletion at bands 6q, tandem duplication of 1q24–31.2, and ring chromosome 15 [6,7].
Historically, the prognosis in Fryns syndrome has been poor with the overwhelming number of affected pregnancies ending in stillbirth, perinatal demise, or severe-to-profound mental retardation [13,15]. Perinatal death is almost exclusively due to pulmonary hypoplasia, whereas the etiology of neurologic impairment is unknown, perhaps secondary to perinatal hypoxia, congenital central nervous system defects, or other contributing factors [16]. Of interest, the oldest known patient with Fryns syndrome, who was severely mentally handicapped, died in status epilepticus at 15 years of age [15]. Moreover, three patients have been described recently with only mild developmental delay, which may be reflective of milder syndromic phenotypic expression, especially regarding the diaphragmatic defect, lung hypoplasia, cardiac anomalies, and/or central nervous system involvement [14,16]. Our patient presented with most features recognized in this syndrome and, in addition, had bilateral anophthalmia which has not previously been reported, although microphthalmia and cloudy cornea occur.
REFERENCES
- 1.Stocker JT, Dehner LP (eds) (2001) Appendix 29. Means and standard deviations of weights and measurements of liveborn infants by gestational age Pediatric Pathology. 2nd ed, Lippincott Williams & Wilkins, Philadelphia, pp 1440–1441. [Google Scholar]
- 2.On-line Mendelian Inheritance in Man (OMIM). Catalogue of Autosomal Dominant, Autosomal Recessive, and X-linked Phenotypes (http://www.ncbi.nlm.nih.gov/omim). 2002.
- 3.Berkenstadt M, Lev D, Achiron R, Rosner M, Barkai G. Pulmonary agenesis, microphthalmia, and diaphragmatic defect (PMD): new syndrome or association? Am J Med Genet 1999;86:6–8. [PubMed] [Google Scholar]
- 4.Seller MJ, Davis TB, Fear CN, Flinter FA, Ellis I, Gibson AG. Two sibs with anophthalmia and pulmonary hypoplasia (the Matthew-Wood syndrome). Am J Med Genet 1996; 62:227–229. [DOI] [PubMed] [Google Scholar]
- 5.Spear GS, Yetup P, Beyerlein RA. Bilateral pulmonary agenesis and microphthalmia. Am J Med Genet Suppl 1987;3:379–382. [DOI] [PubMed] [Google Scholar]
- 6.Wymersch DV, Favre R, Gasser B. Use of three-dimensional ultrasound to establish the prenatal diagnosis of Fryns syndrome. Fetal Diagn Ther 1996;11:335–340. [DOI] [PubMed] [Google Scholar]
- 7.Sheffield JS, Twickler DM, Timmons C, Land K, Harrod M, Ramus RM. Fryns syndrome: prenatal diagnosis and pathologic correlation. J Ultrasound Med 1998;17:585–589. [DOI] [PubMed] [Google Scholar]
- 8.Fryns JP, Moerman F, Goddeeris P, Bossuyt C, Van den Berghe H. A new lethal syndrome with cloudy corneae, diaphragmatic defects and distal limb deformities. Hum Genet 1979;50:65–70. [DOI] [PubMed] [Google Scholar]
- 9.Vargas JE, Cox GF, Korf BR. Discordant phenotype in monozygotic twins with Fryns syndrome. Am J Med Genet 2000;94:42–45. [DOI] [PubMed] [Google Scholar]
- 10.Bartsch O, Meinecke P, Kamin G. Fryns syndrome: two further cases without lateral diaphragmatic defects. Clin Dysmorphol 1995;4:352–358. [DOI] [PubMed] [Google Scholar]
- 11.Fryns JP. Scalp defects in Fryns syndrome. Genet Couns 1998;9:153–154. [PubMed] [Google Scholar]
- 12.Pellissier MC, Philip N, Potier A, et al. Prenatal diagnosis of Fryns syndrome. Prenat Diagn 1992;12:299–303. [DOI] [PubMed] [Google Scholar]
- 13.Pinar H, Carpenter MW, Abuelo D, Singer DB. Fryns syndrome: a new definition. Pediatr Pathol 1994;14:467–478. [DOI] [PubMed] [Google Scholar]
- 14.Van Hove JLK, Spiridigliozzi GA, Heinz R, McConkie-Rosell A, Iafolla AK, Kahler SG. Fryns syndrome survivors and neurologic outcome. Am J Med Genet 1995;59:334–340. [DOI] [PubMed] [Google Scholar]
- 15.Dingens M, Fryns JP. Hematometra and sudden death after status epilepticus in the adolescent female with Fryns syndrome. Genet Couns 1999;10:329–330. [PubMed] [Google Scholar]
- 16.Langer JC, Winthrop AL, Whelan D. Fryns syndrome: a rare familial cause of congenital diaphragmatic hernia. J Pediatr Surg 1994;29:1266–1267. [DOI] [PubMed] [Google Scholar]