Abstract
Background:
Heart failure (HF) is a major global health problem. Clinical trials test efficacy, effectiveness, and safety of novel and emerging therapies in HF. We sought to determine the salient features of ongoing interventional clinical trials in HF.
Methods and Results:
We accessed the ClinicalTrials.gov registry of the National Institutes of Health (NIH) and the International Clinical Trials Registry Platform of the World Health Organization on January 1, 2017, and extracted pertinent information on current HF clinical trials for systematic review. Of 794 HF trials that met our inclusion criteria, almost one-half (49.1%) evaluated clinical end points and one-third (32.8%) examined imaging end points as primary outcomes. One-fourth (24.8%) were industry sponsored and one-third (35.6%) were university sponsored. The NIH and other United States federal agencies funded only 14 trials (1.8% of all trials; 10.7% of trials in the US). Among 536 HF trials with specified left ventricular ejection fraction status, 434 (81.0%) focused on HF with reduced ejection fraction (HFrEF) and only 102 (19.0%) trials targeted HF with preserved ejection fraction (HFpEF).
Conclusions:
Ongoing HF trials are predominantly sponsored by nongovernmental funding agencies. Although HFpEF occurs as commonly as HFrEF in the community, the number of clinical trials targeting HFpEF is substantially lower compared with HFrEF.
Keywords: Clinical trials, heart failure
INTRODUCTION
Heart failure (HF) is a leading cause of morbidity and mortality.1,2 It is estimated that ~6.5 million Americans have HF, with nearly 960,000 new cases being added annually.1 HF accounted for ~$30 billion in health care costs in the United States in 2012, and the costs are projected to double by 2030.2 HF with preserved ejection fraction (HFpEF) accounts for one-half of the HF burden.3 Several classes of drugs, including beta-blockers,4–6 angiotensin-converting enzyme (ACE) inhibitors,7,8 angiotensin receptor blockers (ARBs),9 aldosterone antagonists,10,11 and angiotensin receptor–neprilysin inhibitor,12 have been proven to improve outcomes in patients with HF with reduced ejection fraction (HFrEF). Similarly, device therapies, such as implantable cardioverter defibrillators and cardiac resynchronization, have shown a definite survival benefit in HFrEF.13 However, no therapies to date have shown such clear mortality benefit in HFpEF. In recent randomized trials targeting HFpEF, phosphdiesterase-5 inhibitors,14 aldosterone antagonists,15,16 ARBs,17–19 and long-acting nitrates20 failed to show improvements in hard clinical end points. Therefore, research efforts to identify new therapeutic approaches for patients with HFpEF are essential to improve outcomes.
Clinical trials are pivotal to develop novel therapies for disease. As such, examining the current focus of ongoing HF clinical trials improves awareness of upcoming therapies and interventions as well as identifies understudied aspects to prioritize resource allocation. In the present investigation, we examined the profile of ongoing interventional clinical trials in HF, determined the proportion of ongoing clinical trials focused on HFpEF versus HFrEF, and highlighted upcoming therapies for HFpEF.
METHODS
The International Committee of Medical Journal Editors requires that all clinical trials be enrolled in a public registry before patient enrollment if they are to be considered for publication. ClinicalTrials.gov, established by the Department of Health and Human Services through the National Institutes of Health (NIH),21 and the International Clinical Trials Registry Platform (ICTRP), maintained by the World Health Organization (WHO),22 are publicly available web-based registries that provide information on currently registered trials throughout the world. Clinical trial databases from the European Union, Africa, and 13 countries (Australia, Brazil, China, South Korea, India, Cuba, Germany, Iran, Japan, Thailand, The Netherlands, Peru, and Sri Lanka) are presently listed as primary registries in the WHO registry network. Thus, information available in these registries is a comprehensive resource to understand the focus of ongoing clinical trials.
Data Collection
We accessed the ClinicalTrials.gov registry and the ICTRP website on January 1, 2017, to identify and conduct a systematic review of ongoing interventional clinical trials in HF according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses Protocols (PRISMA-P).23 In the ClinicalTrials.gov registry, we used the “advanced search” option with the search term “heart failure.” We excluded trials of “unknown status” (trial passed completion date with recruitment status not verified within the past 2 years) and focused our search with the use of the selections “open studies” (currently recruiting participants), “interventional” (participants assigned to receive ≥1 intervention(s) [or no intervention]), and “adult (18–65) and senior (66+)” age groups. Complete definitions of all key search fields used are listed in the Appendix.21 Because similar advanced search options were not offered on the ICTRP search portal, we used the standard search option with the search term “heart failure” and applied the above exclusions during manual review. “Interventional” trials were defined as trials that were investigating a novel drug, medical device, procedure, vaccine, or other product.
We downloaded all study fields from both databases in Extensible Markup Language format. Three investigators (N.V.P., A.J.K., and S.K.) reviewed these studies. All trial exclusions and outcome classifications were agreed to by at least 2 of the reviewers. We excluded trials if the primary condition being studied was not HF and if the primary purpose listed was diagnostic or basic science. We also excluded trials in which interventions being evaluated were behavioral therapies (eg, group therapies, surveys, etc), imaging modalities, physical therapy and rehabilitation (exercise therapy), or monitoring (eg, telehealth, device-based monitoring, nurse visit, etc), because our study was primarily designed to evaluate novel upcoming medical, surgical/device-based, and molecular therapies for HF. For all eligible studies, we extracted information on location, study design, trial phase, clinical setting, primary sponsor, primary outcome, therapeutic modality, and type of HF based on left ventricular ejection fraction (LVEF) status. This prespecified search strategy and review approach satisfied 15 out of the 17 checklist criteria recommended by PRISMA-P to avoid selective reporting and improve transparency, accuracy, completeness, and reproducibility.24
Clinical trials that required LVEF <50% as an inclusion criterion were grouped as “HFrEF” trials, trials that specified LVEF ≥50% were categorized as “HFpEF” trials, and investigations that did not have LVEF specified as an enrollment criterion were classified as “unspecified.” Primary outcomes listed in various trials were categorized into clinical (including hard end points such as mortality, HF hospitalization, myocardial infarction, and stroke and soft end points such as symptoms, quality of life, exercise capacity, peak oxygen consumption, and others, eg, medication adherence, heart rate and blood pressure variability, psychiatric well-being, etc), imaging (echocardiographic or other imaging parameter), laboratory (eg, troponin level, B-type natriuretic peptide level), and safety (eg, procedural complications, drug safety) end points. Imaging and laboratory outcomes were considered as surrogate end points. Interventions with pharmacologic agents and dietary supplements were grouped as medical therapies. Interventions focusing on device therapies, ablations, ventricular assist devices, renal denervation, respiratory devices, and other cardiac procedures were grouped as surgical therapies. Interventions involving stem cell therapy, gene therapy, and bone marrow aspirate infusions were grouped as molecular therapies.
Statistical Analysis
We summarized continuous variables as mean, median, interquartile range, and range and presented categorical variables as counts and percentages. We adapted the rworldmap package in R Studio to depict the number of ongoing interventional HF clinical trials by country on the world map.25 We constructed pie charts and clustered column charts with the use of Microsoft Excel for Mac 2017, version 15.30, and Venn diagrams with the use of Venn Diagram Plotter from Pacific Northwest National Laboratory, Richland, Washington, version 1.5.5228.29250, to illustrate key features of HF in ongoing clinical trials. We assessed normality of distribution of a continuous variable with the use of graphic methods, such as stem-and-leaf plot, box plot, histogram, normal probability plot, P-P plot, and Q-Q plot, summary statistics, such as skewness and kurtosis, and tests of normality, such as Kolmogorov-Smirnov test, and Shapiro-Wilk test. We used chi-square test, Wilcoxon rank sum test, and Fischer exact test as appropriate to compare differences between groups. We used SAS software version 9.4 to conduct all statistical analyses. We considered a P value of <.05 to indicate statistical significance.
RESULTS
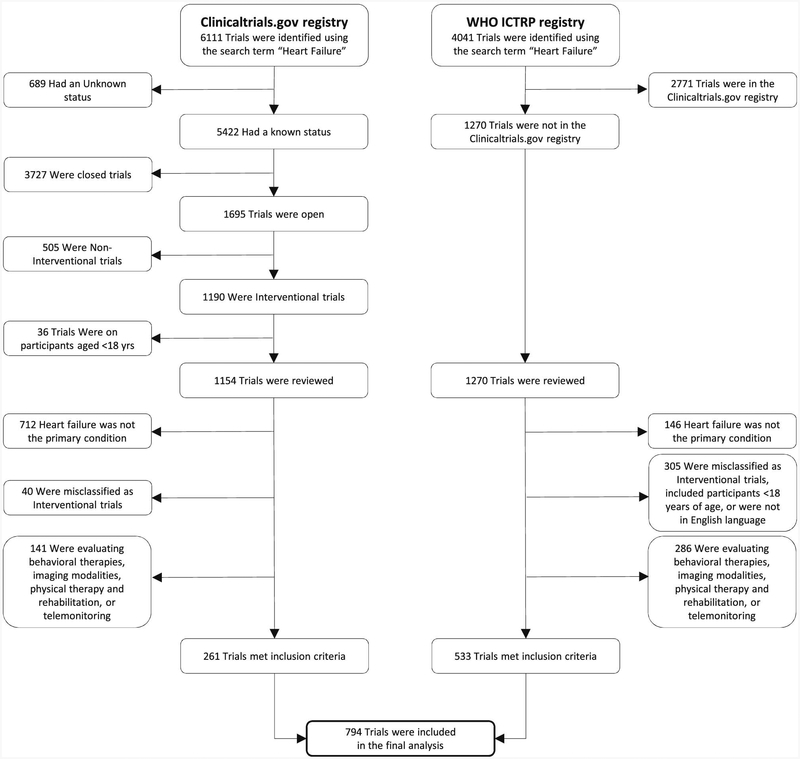
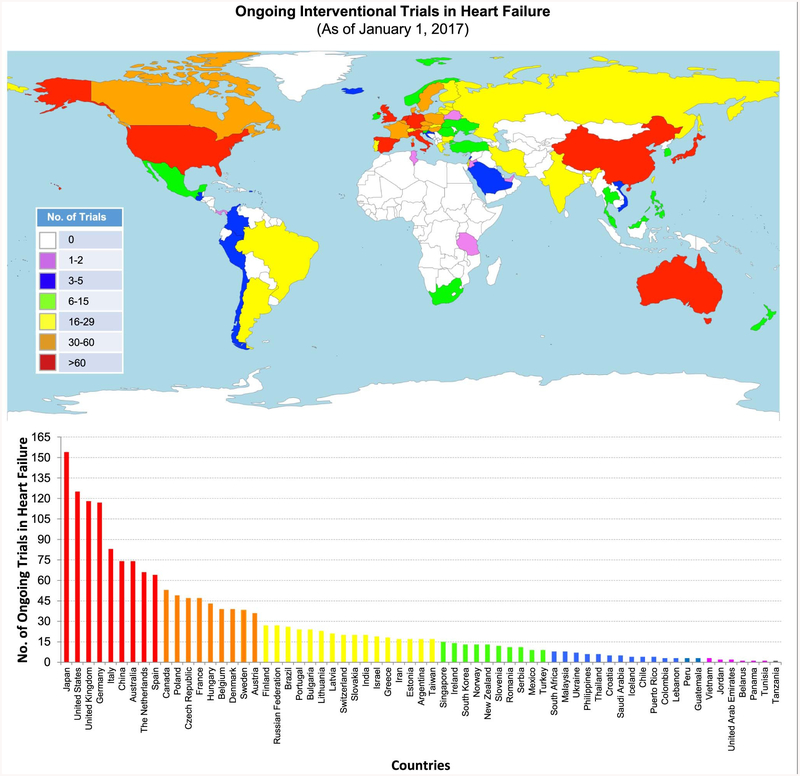
Of the 6,111 clinical trials identified with the search term “heart failure” in the ClinicalTrials.gov registry, we excluded 689 trials because of unknown status, 3,727 trials because recruitment was closed, 505 trials because the study type was not interventional, and 36 trials because participants belonged to the pediatric age group (Fig. 1). The remaining 1154 ongoing trials and an additional 1270 trials in ICTRP (excluding trials already found in the ClinicalTrials.gov registry) were manually reviewed. After applying the exclusion criteria described earlier, a total of 794 trials were eligible for final analysis. The status of all these trials were updated in the registries within 5 years before the date of our review (January 1, 2017). Based on data from 766 trials (96.5% of eligible trials) with information on participating countries, 9 countries (Japan, United States, United Kingdom, Germany, Italy, China, Australia, The Netherlands, and Spain) had ≥60 ongoing HF interventional trials (Fig. 2).
Fig. 1.
Strategy to identify ongoing interventional clinical trials in heart failure. WHO ICTRP, World Health Organization’s International Clinical Trials Registry Platform.
Fig. 2.
Global distribution of ongoing interventional clinical trials in heart failure, based on data from 794 interventional HF trials registered as of January 1, 2017, in ClinicalTrials.gov and the International Clinical Trials Registry Platform. The vertical bars are arranged according to descending order of the number of trials by country.
Trial Characteristics
A majority of the trials (n = 612; 77.1%) were randomized and about one-tenth (n = 88; 11.1%) were nonrandomized; data on randomization were missing in the remainder (n = 94; 11.8%). Among 315 trials (75.2% of trials in the ClinicalTrials.gov registry and 24.8% of trials in ICTRP) with data on the number of centers involved, about three-fifths were single-centered (n = 187; 59.4%) and two-fifths multicentered (n = 128; 40.6%). In 718 trials (90.4%) with information on estimated enrollment, the median projected sample size was 80 (interquartile range 40–210, range 3–8000).
Primary Sponsors
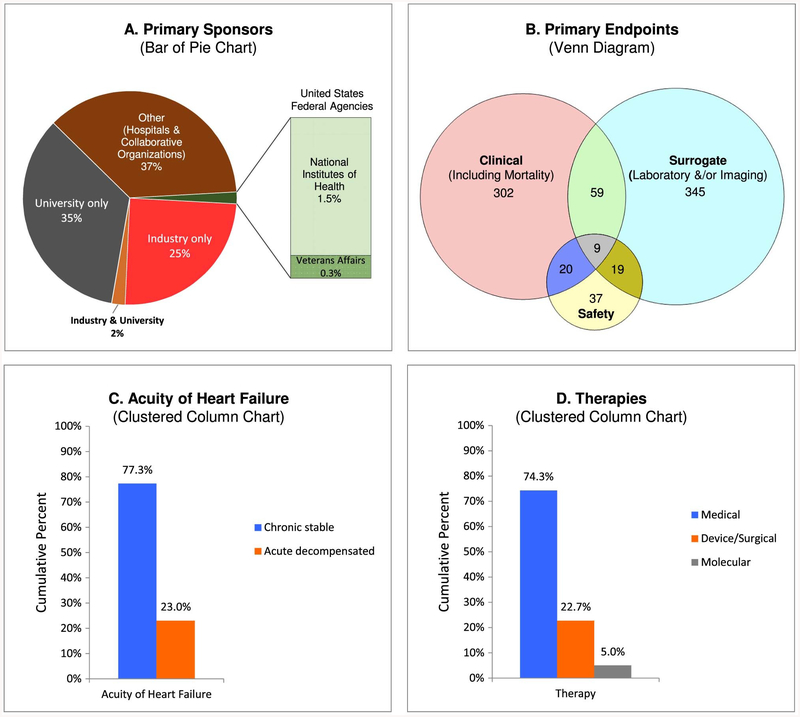
Ongoing trials in HF were mostly funded by nongovernmental sources, including industry only (n = 197; 24.8%), university only (n = 275; 34.6%), industry and university (n = 16; 2.0%), and “other,” consisting of individual hospitals or collaborative organizations (n = 292; 36.8%; Fig. 3A). A minority of trials were sponsored by US federal agencies (14 [1.8%] of 792 trials worldwide, 13 [10.7%] of 122 trials with US as a participating country). Of the 14 US government– funded trials, the NIH was the sole primary sponsor of only 2 trials and the Department of Veterans Affairs was recorded as the sole primary sponsor of only 1 trial; in the remaining 11 trials, industry (n = 2), university (n = 8), and both industry and university (n = 1) were coprimary sponsors.
Fig. 3.
Distribution of current interventional clinical trials in heart failure according to (A) primary sponsors, (B) primary end points, (C) acuity of heart failure, and (D) therapies. Percentages in panels C and D exceed 100% because of minor overlap in categories. Panel C includes 2 trials that enrolled both chronic stable and acute decompensated heart failure and excludes 11 trials that did not specify the acuity of heart failure. Panel D includes 13 trials that evaluated both medical and surgical therapies, 1 trial that examined both medical and molecular therapies, and 2 trials that considered both surgical and molecular therapies.
Phase of the Trial
Based on data from 217 trials (78.8% in ClinicalTrials.gov registry and 21.2% in ICTRP) with information on phase of the trial, about one-sixth (n = 35; 16.1%) were phase I (for dose ranging), about two-fifths (n = 93; 42.8%) were phase (to test efficacy and adverse effects), about one-fourth (n = 62; 28.6%) were phase III (to assess efficacy, effectiveness, and safety), and about another one-fourth (n = 57; 26.3%) were phase IV (post-marketing surveillance) studies. Of note, 17 trials were listed as both phase I and II trials and 13 trials as both phase II and III trials.
Among 62 phase III trials, about one-half (n = 29; 46.8%) were sponsored by industry and the rest by university (n = 18; 29.0%), other groups (individual hospitals & collaborative organizations; n = 17; 27.4%), and Veterans Affairs (n = 1; 1.6%). Of note, 3 phase III trials were cosponsored by industry and university. There were no NIH-funded ongoing phase III HF interventional trials.
Primary Outcomes
About one-half of the trials (n = 390, 49.1%) studied clinical end points as primary outcomes; this included just over one-tenth of the trials (n = 89; 11.2%) that evaluated mortality (Fig. 3B). Almost one-third of the trials (n = 260; 32.8%) examined imaging parameters and about one-fourth (n = 213; 26.8%) evaluated change in laboratory markers; a small fraction (n = 41; 5.2%) of the trials considered both of these surrogate end points. Finally, about one-tenth (n = 85; 10.7%) considered safety risks as primary outcomes. Among 390 trials evaluating clinical primary outcomes, about one-third (n = 139; 35.5%) examined hard clinical end points and almost four-fifths (n = 308; 79.1%) soft clinical end points; the overlap in these categories comprised 57 trials (14.6%) that considered both hard and soft end points.
Acuity of Heart Failure
Chronic stable HF was the focus of a majority of the trials (n = 605; 77.3%) and acute decompensated HF in about one-fourth (n = 180; 23.0%) (Fig. 3C). Both patients with chronic stable HF and those with acute decompensated HF were eligible for enrollment in a very small fraction (n = 2; 0.3%) of the trials. The acuity of HF was not specified in 11 trials.
Therapeutic Strategies
The primary intervention modality was medical therapy (n = 590; 74.3%) (Fig. 3D). Surgical/device therapy, including but not limited to cardiac resynchronization therapy, renal denervation, and ventricular assist devices, was the major focus of about one-fourth of the trials (n = 180; 22.7%). Molecular therapies consisting of stem cell and gene therapies were being examined in 40 trials (5.0%). A small number of trials simultaneously studied the impact of more than 1 therapeutic strategy (medical and surgical in 13 trials, medical and molecular in 1 trial, and surgical and molecular in 2 trials).
Industry- Versus Nonindustry-Sponsored Trials
Compared with nonindustry-sponsored trials, industry-sponsored trials had a larger projected sample size (P < .0001) and more commonly assessed imaging outcomes (P = .002), safety end points (P < .0001), surgical/device therapies (P = .036), and patients with HFrEF (P < .001; Table 1). Proportions of industry- and nonindustry-sponsored trials evaluating clinical outcomes, laboratory end points, and molecular therapies were similar (all P ≥ .49).
Table 1.
Characteristics of Interventional Clinical Trials According to Primary Sponsor and Type of Heart Failure, %
| Primary Sponsor | Type of Heart Failure* | |||||
|---|---|---|---|---|---|---|
| Industry (n=216) | Non-Industry (n=578) | P Value | HFrEF (n= 434) | HFpEF (n= 102) | P Value | |
| Estimated Enrollment,† median, (IQR) | 140 (50–450) | 70 (40–170) | <.0001‡ | 100 (45–240) | 60 (40–147) | <.0001‡ |
| Clinical Setting§ | ||||||
| Chronic stable | 75.9 | 77.8 | .58 | 85.8 | 86.6 | .84 |
| Acute decompensated | 24.1 | 22.6 | .66 | 14.7 | 13.4 | .75 |
| Primary Outcome | ||||||
| Clinical | 48.1 | 49.5 | .74 | 45.2 | 42.2 | .58 |
| Laboratory | 26.9 | 26.8 | .99 | 25.3 | 27.5 | .66 |
| Imaging | 24.1 | 36.0 | .002 | 36.4 | 47.1 | .047 |
| Safety | 21.8 | 6.6 | <.0001 | 11.1 | 4.9 | .061 |
| Therapy | ||||||
| Medical | 71.3 | 75.4 | .24 | 65.0 | 85.3 | <.0001 |
| Surgical/Device | 27.8 | 20.8 | .036 | 29.7 | 13.7 | .001 |
| Molecular | 4.2 | 5.4 | .49 | 7.8 | 2.0 | .029‖ |
| Primary Sponsor | ||||||
| Industry | − | − | − | 32.5 | 19.6 | .011 |
| Non-industry | − | − | − | 67.5 | 80.4 | |
| Type of Heart Failure | ||||||
| HFrEF | 65.3 | 50.7 | <.001 | − | − | − |
| HFpEF | 9.3 | 14.2 | .064 | − | − | − |
| HF with Unspecified EF | 25.5 | 35.1 | .010 | − | − | − |
Percentages in clinical setting, primary outcome, and therapy may exceed 100% because of overlap. HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; IQR, interquartile range; HF, heart failure; EF, ejection fraction.
Based on 536 (67.5%) of 794 of trials with left ventricular ejection fraction cutoff specified as an enrollment criterion.
Based on 718 (90.4%) of 794 of trials with data on estimated enrollment.
Two-sided P value was computed with the use of Wilcoxon rank sum test because the data were not normally distributed (mean 325, median 80, skewness 5.6, kurtosis 35.7, Kolmogorov-Smirnov P <.010, and Shapiro-Wilk P <.0001).
Excludes 11 trials where acuity of heart failure was not specified.
Two-sided P value was computed with the use of Fischer exact test of independence because the expected numbers were small.
HFpEF Versus HFrEF
Among 536 (67.5%) of 794 ongoing HF trials with LVEF specified as an enrollment criterion, HFrEF was the focus of 434 (81.0%) and HFpEF was targeted in only 102 (19.0%). The projected sample size was higher in HFrEF than in HFpEF trials (P < .0001) (Table 1). HFpEF trials were more commonly examining medical therapies (P < .0001) and less often evaluating surgical/device interventions (P ≤ .001). Of note, 2 HFpEF trials were examining molecular therapies: allogenic-derived cells () and transendocardial CD34+ cell therapy ().
Therapeutic Approaches for HFpEF
In addition to usual classes of medications, such as beta-blockers, ACE inhibitors, ARBs, and aldosterone antagonists, some of the novel drug therapy groups being evaluated for HFpEF include phosphodiesterase inhibitors, vasopressor receptor 2 antagonists, dipeptidyl peptidase 4 inhibitors, and angiotensin receptor–neprilysin inhibitors (Supplemental Table 1). Eleven trials were examining surgical/device therapies for HFpEF; of those, 2 were assessing the effect of sympathetic modulation through renal denervation, 3 the effect of positive airway pressure therapy, 2 the safety of Corolla elastic left ventricular implant placed via a transapical approach to improve diastolic function by increasing left ventricular elasticity, 2 the effect of vagal nerve stimulation, and 1 each the efficacies of rate adaptive atrial pacing and inspiratory muscle training.
DISCUSSION
Principal Findings
This investigation provides a comprehensive profile of ongoing clinical trials in HF and draws focus on the paucity of interventional studies in HFpEF compared with HFrEF. Although HFpEF constitutes almost one-half of new-onset HF in the community and has no proven beneficial therapies, it is the primary focus of only one-fifth of the currently ongoing HF clinical trials. A second important finding of our study is that nongovernmental agencies are the primary sponsors of most HF trials and funding support from governmental agencies is limited. NIH agencies and the Department of Veterans Affairs currently fund only ~1.8% of all ongoing interventional HF clinical trials and about one-tenth of the trials with the US as a participating country. Another notable finding is that only one-half of the clinical trials have a clinical end point as a primary outcome.
HFpEF Versus HFrEF
HF substantially increases the risk of death and illness and accounts for a large portion of health care costs, with HFpEF contributing to at least one-half of the HF burden.1 Early registry data indicated that post–hospital discharge mortality and rehospitalization rates were similar in HFpEF and HFrEF,26 but recent data shows that HFpEF is associated with a lower risk of death than HFrEF,27,28 and it is important to note that HFpEF patients are older and have a greater burden of noncardiovascular comorbidities that primarily determine their prognosis.28,29 This may partly explain the significantly higher number of outpatient and emergency room visits compared with HFrEF patients which adds to greater use of health care resources.30 Therefore, the relative lack of ongoing HFpEF trials is worrisome.
Challenges in Designing HFpEF Trials
First, the mechanisms for development of HFpEF remain poorly understood, perhaps because of lack of suitable nonhuman animal models that simulate pathologic features of human HFpEF (diastolic dysfunction).31 Second, HFpEF patients have multiple comorbidities, such as hypertension, diabetes mellitus, chronic kidney disease, obesity, metabolic syndrome, and sleep apnea, which potentiate a state of chronic inflammation. The phenotypic heterogeneity seen in HFpEF patients restricts selection of appropriate patients and pertinent end points for clinical trials. Specific phenotypic expression of HFpEF resulting from the preponderance of any of these comorbidities may partly explain the failure to demonstrate statistical benefit of a particular intervention on a heterogenous group of HFpEF patients in earlier clinical trials.32 Finally, HFpEF patients are predominantly managed by primary care practitioners rather than cardiologists, and a much lower proportion of HFpEF than HFrEF patients are enrolled in major HF registries.26,28 These factors may, in turn, pose difficulties for enrolling an appropriate subset of HFpEF patients in clinical trials. Justifiably, the Food and Drug Administration has recently highlighted the urgent need to develop novel therapies for HFpEF.33 Given the paucity of ongoing interventional HFpEF trials, research stimulus funds from both government and nongovernment sources would be pivotal in this effort.
Clinical Versus Surrogate End Points
Our findings indicate that about one-half of the current clinical trials considered surrogate outcomes as primary end points. The advantages of using surrogate end points include smaller required sample size, shorter duration of follow-up, and reduced cost of the trial. However, translation of these outcomes into hard end points concerning survival and quality of life may not be straightforward.34 For example, in the Vasodilator–Heart Failure II trial, increase in LVEF from baseline to 13 weeks was significantly greater in the hydralazine–isosorbide dinitrate arm compared with the enalapril arm. However, patients in the enalapril group had significantly better long-term (3-year) survival.35 Therefore, all therapeutic end points may not be affected similarly by a certain treatment at a given length of follow-up. Clinicians should be aware of these nuances and be cautious when interpreting results of trials with small sample sizes and short follow-ups that use surrogate primary outcome measures.
Decompensated HF
Admissions for acute decompensated HF account for a sizable portion of health care costs.36 HFpEF patients hospitalized for acute decompensated HF are at an increased risk of death, compared with HFpEF patients with a non-HF hospitalization.37 Therefore, it is encouraging to note that almost one-fourth of the ongoing interventional HF trials are focused on acute decompensated HF. In this context, it is worth noting the difficulties of studying the effect of specific interventions on acute decompensated HF in clinical trial settings. First, acute HF is a heterogeneous syndrome with numerous precipitating factors, such as hypertensive emergencies, acute coronary syndrome, arrhythmias, infections, etc. This poses challenges in defining selection criteria for these trials and developing a common therapy for various forms of decompensated HF. Second, acute HF patients frequently present with multiple organ system dysfunction which limits enrollment in trials. Third, the acuity of illness in decompensated HF may prompt physicians to treat the patient aggressively with the use of conventional therapies for HF, providing a limited time window for an agent to demonstrate its efficacy. Finally, translating short-term effectiveness of interventions in acute decompensated HF to long-term improvements in overall cardiovascular outcomes may be difficult to evaluate in a clinical trial setting.
Study Strengths and Limitations
The strengths of the present study include a systematic and comprehensive evaluation of ongoing interventional clinical trial data on HF across multiple international clinical trial registries. Nonetheless, we acknowledge several limitations. First, we excluded HF trials examining telemonitoring and exercise therapy. Recent trials of pulmonary artery pressure monitoring38 and cardiac rehabilitation39 have revealed beneficial effects in reducing HF hospitalizations. However, our primary objective was to focus on emerging medical, surgical/device, and molecular therapies for HF. Second, all registry information is provided and updated by the sponsor of the clinical trial or the principal investigator. Data on number of centers involved and phase of the trial were not listed in about two-thirds of the eligible interventional HF clinical trials. Almost one-third of the registered trials did not specify LVEF status in the registry database. Even among trials with specified LVEF cutoff as an enrollment criterion (eg, LVEF <45% or <50%) the available information was not adequate to examine HFrEF using the more contemporary LVEF value of ≤40% or to identify the increasingly recognized borderline or intermediate category of HF with midrange LVEF of >40% but <50%.40,41 Whether ongoing trials have sufficient statistical power to conduct stratified analyses according to these LVEF categories of interest is not known. Third, some trials with “university” listed as the primary sponsor in the registry database could have concurrent secondary funding from industry sources that were not noted.
Conclusion
The paucity of ongoing clinical trials in HFpEF despite the similar community burden of HFpEF and HFrEF is a cause for concern. Our findings highlight a pressing need to allocate more resources, particularly from government funding agencies, for novel and high-priority investigations that target HFpEF.
Supplementary Material
HIGHLIGHTS.
Although HF with preserved ejection fraction (HFpEF) occurs as commonly as HF with reduced ejection fraction (HFrEF) in the community, only about one-fifth (19%) of the trials are focused on HFpEF.
Ongoing HF clinical trials are predominantly sponsored by nongovernmental funding agencies.
Only about one-half of all ongoing HF clinical trials have a clinical end point as the primary outcome.
Our findings highlight a pressing need to allocate more resources, particularly from government funding agencies, for novel and high-priority investigations that target HFpEF to improve outcomes.
ACKNOWLEDGMENTS
Dr. Kenchaiah was partly supported by the intramural research program of the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), grant number Z99 HL999999, and the Translational Research Institute, grant numbers UL1TR000039 and KL2TR000063 through the NIH National Center for Research Resources and National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
DISCLOSURES
None.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at doi:10.1016/j.cardfail.2018.02.006
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. , American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. , American Heart Association Advocacy Coordinating Committee, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 4.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349–55. [DOI] [PubMed] [Google Scholar]
- 5.CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 6.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–7. [PubMed] [Google Scholar]
- 7.Investigators SOLVD. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992;327:669–77. [DOI] [PubMed] [Google Scholar]
- 9.Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial—the losartan heart failure survival study ELITE II. Lancet 2000;355:1582–7. [DOI] [PubMed] [Google Scholar]
- 10.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–17. [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–21. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg I, Gillespie J, Moss AJ, Hall WJ, Klein H, McNitt S, et al. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation 2010;122:1265–71. [DOI] [PubMed] [Google Scholar]
- 14.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- 16.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013;309:781–91. [DOI] [PubMed] [Google Scholar]
- 17.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–67. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet 2003;362:777–81. [DOI] [PubMed] [Google Scholar]
- 19.Parthasarathy HK, Pieske B, Weisskopf M, Andrews CD, Brunel P, Struthers AD, et al. A randomized, double-blind, placebo-controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction. Eur J Heart Fail 2009;11:980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015;373:2314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinicaltrials.gov. Available at: https://clinicaltrials.gov. Accessed January 1, 2017.
- 22.International Clinical Trials Registry Platform. Available at: http://www.who.int/entity/ictrp/en/. Accessed January 1, 2017.
- 23.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015:g7647. [DOI] [PubMed] [Google Scholar]
- 25.South A. rworldmap: a new R package for mapping global data. R J 2011;3:35–53. [Google Scholar]
- 26.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J Am Coll Cardiol 2007;50:768–77. [DOI] [PubMed] [Google Scholar]
- 27.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 2012;33:1750–7. [DOI] [PubMed] [Google Scholar]
- 28.Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC heart failure long-term registry. Eur J Heart Fail 2017;19:1574–85. [DOI] [PubMed] [Google Scholar]
- 29.Lund LH, Donal E, Oger E, Hage C, Persson H, Haugen-Löfman I, et al. Association between cardiovascular vs non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fraction. Eur J Heart Fail 2014;16:992–1001. [DOI] [PubMed] [Google Scholar]
- 30.Nichols GA, Reynolds K, Kimes TM, Rosales AG, Chan WW. Comparison of risk of re-hospitalization, all-cause mortality, and medical care resource utilization in patients with heart failure and preserved versus reduced ejection fraction. Am J Cardiol 2015;116:1088–92. [DOI] [PubMed] [Google Scholar]
- 31.Conceição G, Heinonen I, Lourenço AP, Duncker DJ, Falcão-Pires I. Animal models of heart failure with preserved ejection fraction. Neth Heart J 2016;24:275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015;131:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, et al. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail 2014;2:97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zannad F, Garcia AA, Anker SD, Armstrong PW, Calvo G, Cleland JG, et al. Clinical outcome end points in heart failure trials: a European Society of Cardiology Heart Failure Association consensus document. Eur J Heart Fail 2013;15:1082–94. [DOI] [PubMed] [Google Scholar]
- 35.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, et al. A comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–10. [DOI] [PubMed] [Google Scholar]
- 36.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, Scientific ADHERE Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 37.Carson PE, Anand IS, Win S, Rector T, Haass M, Lopez-Sendon J, et al. The hospitalization burden and post-hospitalization mortality risk in heart failure with preserved ejection fraction: results From the I-PRESERVE Trial (Irbesartan in Heart Failure and Preserved Ejection Fraction). JACC Heart Fail 2015;3:429–41. [DOI] [PubMed] [Google Scholar]
- 38.Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, et al. , CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet 2016;387:453–61. [DOI] [PubMed] [Google Scholar]
- 39.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. , HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure—HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 41.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.